Abstract
Our previous study suggested that increased cytoplasmic calcium (Ca) signals may mediate aluminum (Al) toxicity in yeast (Saccharomyces cerevisiae). In this report, we found that a yeast mutant, pmc1, lacking the vacuolar calcium ion (Ca2+) pump Ca2+-ATPase (Pmc1p), was more sensitive to Al treatment than the wild-type strain. Overexpression of either PMC1 or an anti-apoptotic factor, such as Bcl-2, Ced-9 or PpBI-1, decreased cytoplasmic Ca2+ levels and rescued yeast from Al sensitivity in both the wild-type and pmc1 mutant. Moreover, pretreatment with the Ca2+ chelator BAPTA-AM sustained cytoplasmic Ca2+ at low levels in the presence of Al, effectively making the cells more tolerant to Al exposure. Quantitative RT-PCR revealed that the expression of calmodulin (CaM) and phospholipase C (PLC), which are in the Ca2+ signaling pathway, was down-regulated under Al stress. This effect was largely counteracted when cells overexpressed anti-apoptotic Ced-9 or were pretreated with BAPTA-AM. Taken together, our results suggest that the negative regulation of Al-induced cytoplasmic Ca signaling is a novel mechanism underlying internal resistance to Al toxicity.
Introduction
Aluminum (Al) toxicity has been implicated as a major cause of severe loss of crops grown in acidic soils. In recent years, significant progress has been made in understanding the molecular mechanisms of Al toxicity and tolerance in the area of stress phytophysiology. Al targets multiple cellular sites, resulting in disruption of the structure and function of the cell wall, plasma membrane, cytoskeleton, signal transduction pathways, and calcium (Ca) uptake capability [1], [2].
Al exclusion and intracellular resistance are two important mechanisms that promote Al tolerance in plants. External mechanisms defined to date include the release of organic acid anions and phenolic compounds, increased pH in the rhizosphere, modifications in cell wall components, and redistribution and internalization of Al. Organic acid anions, such as citrate, oxalate, and malate, are secreted by roots in response to Al [2], [3]. Some of the genes involved in Al-induced organic acid secretion have been identified as ALMT1, HvAACT1 and SbMATE [4], [5], [6]. The expression of the Al-inducible Arabidopsis genes ALS1 and ALS3 or the rice genes STAR1, STAR2 and ART1 can confer Al tolerance [7], [8], [9], [10]. Internal resistance can be achieved by both complexation and sequestration. Al is taken up in the ionic form through a transporter and then chelated with organic anions to form a stable, non-phytotoxic complex. Finally, Al becomes sequestrated in the vacuoles in its chelated form [2]. Recently, Nrat1 (Nramp aluminum transporter 1) was identified as a plasma membrane-localized transporter that mediates sequestration of Al into vacuoles in rice [11]. It is important to note that internal resistance to Al can also be achieved through negative regulation of programmed cell death (PCD) [12], [13].
Apoptosis, a typical form of PCD, facilitates the rapid removal of potentially threatening or undesired cells and plays a central role in normal development and homeostasis of metazoan organisms [14], [15], [16]. As in metazoan cells, yeast apoptosis can be detected by typical hallmarks [17]. Bcl-2-family proteins and Bax inhibitor-1 (BI-1) are well known for their ability to respond to stress signals and protect cells against apoptosis [18], [19], [20]. Al is capable of inducing apoptosis in various cell types. Al-induced apoptosis can be inhibited by the up-regulation of Bcl-2 family members or BI-1 [12], [13], [21]. Al increases cytosolic calcium ion (Ca2+) levels in yeast, which can be decreased by overexpressing anti-apoptotic members [13]. It remains unknown, however, whether negative regulation of the Ca signals is a significant mechanism involved in internal resistance to Al toxicity.
Abiotic and biotic stresses induce cell death via the disruption of Ca homeostasis in yeast, plant, and animal cells [22], [23], [24]. The Ca2+, as a vital intracellular second messenger, governs countless cellular functions. To maintain basal levels of cytoplasmic Ca2+ under various and ever-changing conditions, cells have evolved mechanisms to carefully regulate Ca2+ entry and removal [25], [26], [27]. Al toxicity strongly affects intracellular Ca homeostasis, which is another mechanism that is hypothesized to cause Al injury [1], [13]. The sensitivity and response of cells to various stresses, including Al, is dependent on the ability of cells to adequately sequester Ca2+ into their internal stores [28], [29], [30]. Ca2+ is stored in the cell wall and in intracellular organelles, including the endoplasmic reticulum (ER), mitochondria, and vacuoles. In plant and yeast cells, the vacuole serves as the principal Ca2+ sequestration site and contains >95% of total cellular Ca2+ stores [31], [32], [33]. In yeast, this large Ca2+ store is maintained through the action of two specialized transporters: the high-affinity Ca2+-ATPase Pmc1p and the low-affinity Ca2+/H+ exchanger Vcx1p [25], [34]. The deletion of the PMC1 gene effectively decreases the ability of yeast cells to grow in high Ca2+ environments, whereas the deletion of VCX1 decreases tolerance to Ca2+ only slightly; these findings suggest that Pmc1p plays a more significant role in vacuolar Ca2+ sequestration.
Pmc1p has approximately 40% identity to mammalian plasma membrane Ca2+-ATPases (PMCAs) and functions as a P-type ion pump. Calcineurin activation by Calmodulin (CaM) and elevated cytosolic Ca2+ leads to increased expression of PMC1 [35]. CaM is a small acidic protein that contains four EF-hands and is one of the best characterized Ca2+ sensors [36]. In yeast, many transcriptional and translational events downstream of the Ca2+ signaling pathways are controlled by the Ca2+-mediated activation of CaM. CaM is essential for all eukaryotic life and participates in Ca2+-dependent stress response pathways through activation of CaM kinases Cmk1 and Cmk2 and the phosphatase calcineurin [29]. Phosphoinositide-specific phospholipase C (PLC) is responsible for the production of two second-messenger molecules, containing an activator of protein kinase C diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3), which in turn releases Ca from internal stores [37], [38].
Al can induce both PCD and Ca burst in yeast, and anti-apoptotic members can enhance Al tolerance coincident with decreased Ca signals [13]. Therefore, understanding the mechanisms through which cytoplasmic Ca2+ regulates Al stress will provide insights into the process of reducing Al toxicity in plants. To this end, we investigated the functional roles of Pmc1p, the Ca2+ chelator BAPTA-AM and anti-apoptotic members in modulating cytosolic Ca2+ and facilitating Al tolerance.
Materials and Methods
Yeast strains, media, and growth conditions
The budding yeast wild-type strain K601/W303-1A (Mata ade2-1 can1-100 his3-11,15 leu2-3112 trp1-1 ura3-1) and its isogenic derivative mutants K605 (pmc1::TRP1), K661 (vcx1△) and K609 (pmr1::HIS3) [39] were generously provided by Dr. Kyle W. Cunningham (Johns Hopkins University, Baltimore, MD, USA). Another isogenic derivative mutant K616 (pmr1::HIS3 pmc1::TRP1 cnb1::LEU2) [40] was generously provided by Dr. Michael G. Palmgren (University of Copenhagen, Denmark). Yeast cells were grown in YPD (1% yeast extract, 2% bacto-peptone, and 2% glucose), 1/2 SD/Gal-Raf/-Ura or 1/2 SD/Gal-Raf/-His (Clontech, Mountain View, CA, USA) supplemented with 2% agar. AlCl3 and CaCl2 stock solutions at concentrations of 1 M and 2.5 M, respectively, were filter sterilized and added to liquid medium at room temperature or to plates at <50°C. For the BAPTA-AM test, the cells were incubated with BAPTA-AM (Dojindo Laboratories, Kumamoto, Japan) in liquid medium for 30 min, and then other stresses were added.
Growth and survival tests
Cells were preincubated in the appropriate liquid medium and allowed to grow twice to the exponential phase. For growth assays, the concentration at OD600 was adjusted to 0.05, and then grown with shaking at 200 rpm at 30°C. For spot assays, the OD600 of each cell culture was adjusted to 1, and diluted in a 10-fold series (1∶1, 1∶10, 1∶100, 1∶1,000, 1∶10,000); aliquots (5 µL) of each dilution were spotted onto a 1/2 SD/Gal-Raf/-Ura or 1/2 SD/Gal-Raf/-His plate with or without treatment. Plates were incubated at 30°C for 3 days. Cell survival was evaluated by counting colony-forming units (cfu). The cultured cells were harvested at defined intervals, diluted to cell density of 0.0005 at OD600, and a 30 µL aliquot of each was plated onto YPD plates [13].
Constructs and transformation
The yeast-inducible expression vector pYES2 [40] was kindly provided by Prof. Lone Bækgaard (The Royal Veterinary and Agricultural University, Copenhagen, Denmark). To clone the entire coding region of PMC1, we designed primers to PMC1-P1 (5′-CCC AAG CTT ATG TCT AGA CAA GAC GAA AAT TC-3′) with a HindIII site and PMC1-P2 (5′-CGC GGA TCC TTA ATA AAA GGC GGT GGA-3′) with a BamHI site. The resulting fragment was inserted into the pYES2 vector, in order to express PMC1 under the control of the GAL1 promoter and generated the plasmid pYES2-PMC1. After confirmation of the fidelity of the constructs by sequencing, the wild-type and pmc1 mutant yeast strains were, respectively, transformed with plasmids pYES2-PMC1 and the pYES2 vector by the lithium acetate (LiAc)/polyethylene glycol method [41]. Transformants were selected for uracil prototrophy by plating on 1/2 SD/Glu/-Ura (Clontech) medium. Bcl-2, Ced-9 and PpBI-1 were inserted into the yeast-inducible expression vector pGilda, which expresses LexA fusion proteins [13]. The three constructs (pGilda-Bcl-2, pGilda-Ced-9, and pGilda-PpBI-1) or the empty vector (pGilda) were transformed into the wild-type yeast and the pmc1 mutant. Transformants were selected for histidine prototrophy by plating on 1/2 SD/Glu/-His (Clontech) medium.
RNA isolation, RT-PCR and quantitative RT-PCR
To examine the expression pattern of transformants, the overnight cultures were harvested. The frozen cells were mechanically disrupted using a ball mill [42]. Yeast total RNA was isolated using TRIzol reagent (Invitrogen, Mountain View, CA, USA). To eliminate genomic DNA contamination, an additional DNase treatment was performed with RNase-free DNase (Takara Bio Inc., Shiga, Japan). The extracted RNA was quantified using the BioPhotometer (Eppendorf, Hamburg, Germany). One microgram of total RNA was used for first-strand cDNA synthesis using a PrimeScript RT reagent kit (Takara) and following the manufacturer's instructions.
0.5 µL of cDNA was used as a template for the PCR amplification of target genes, and ACT1 was used as an internal control (27 cycles). PCR was performed with an initial incubation at 94°C for 5 min, followed by 27 cycles at 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s. The reaction was terminated by a final incubation at 72°C for 10 min. Primers for LexA are shown in Table 1.
Table 1. Primers used for RT-PCR.
| Gene | Primer sequences (5′-3′)a | Product size (bps) |
| LexA | F: GCA GGA AGA GGA AGA AGG GTT | 182 |
| R: AGT CAC CAT CCA TAA TGC CGA | ||
| ACT1 | F: TAC TCT TTC TCC ACC ACT GCT GA | 148 |
| R: CTT GAC CAT CTG GAA GTT CGT AG | ||
| PMC1 | F: GAG AAT CTG CCC CGA TGA AG | 164 |
| R: AGG CGG TGG ACT CTG GAC TA | ||
| CMD1 | F: CGC CCA GTG AAG CAG AAG T | 190 |
| R: AAC TCA GCG GCG GAG ATT A | ||
| CNB1 | F: CTT GCT GGA CGT ATA ATG GAG GT | 129 |
| R: GAA GGC GAA TCT TAA CTT TTC GT | ||
| PLC1 | F: GAA TGA TAC ATC GCC AAG CAG | 181 |
| R: AGA AAC TTC AGC ATC CCA TAT TG |
LexA primer pairs were used for semi-quantitative RT-PCR analysis. ACT1 primer pairs were used for both semi-quantitative and quantitative RT-PCR. PMC1, CMD1, CNB1, and PLC1 primer pairs were used for quantitative RT-PCR.
For quantitative RT-PCR, 0.4 µL of a 5-fold dilution of cDNA from each sample was used to analyze gene expression by the SYBR Premix Ex Taq (Takara). The cycling program was as follows: an initial cycle of 5 min at 95°C, followed by 45 cycles of 15 s at 95°C, 10 s at 60°C and 15 s at 72°C. Data were collected and analyzed by the real-time PCR system (Eppendorf realplex2). ACT1 was used as an internal control. Primers for PMC1, CMD1, CNB1 and PLC1 are shown in Table 1.
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) analysis
To visualize DNA strand breaks, cells were fixed in 4% formaldehyde in PBS (pH 7.4) then treated with lyticase (Sigma, St. Louis, MO, USA) and stained with fluorescein isothiocyanate-labeled TUNEL reagent (in situ cell death detection kit; Roche, Mannheim, Germany) [13], [43]. TUNEL fluorescence was examined at a 488-nm excitation and filtered at 525 nm. Photos were taken under a fluorescence microscope (VANOX-AH-1; Olympus).
Intracellular Ca2+ measurement
Yeast cells were grown in 1/2 SD/Gal-Raf/-Ura or 1/2 SD/Gal-Raf/-His medium with or without treatment for 6 h. After harvesting, cells were resuspended in PBS (pH 7.4) and vortexed briefly. For determination of intracellular Ca2+ levels, yeast cells were incubated in PBS (pH 7.4) at 37°C for 30 min with 10 µM Fluo-3-acetoxymethyl ester (Fluo-3/AM; Biotium, Hayward, CA, USA) prepared with a 1 mM stock solution in dimethyl sulfoxide. A non-cytotoxic detergent, pluronic F-127 (0.1%), was added to increase solubility of the Fluo-3/AM. Fluo-3 fluorescence was measured by FACSCalibur with 488-nm (blue) argon (Becton-Dickinson, San Jose, CA, USA) in the FL1 channel. Data acquisition was performed using CellQuest (3.1f) software and data analysis by ModFit LT (3.0) software (Variety Software House). 2,000 to 10,000 cells were measured for each analysis [13], [44].
Statistical Analysis
Data were calculated as the mean of results from at least three independent experiments or one representative result of parallel experiments. The Origin 8 program was used for calculation. Error bars represent standard deviation (SD).
Results
pmc1 mutant displayed increased sensitivity to Al-induced growth inhibition and Al-induced PCD
The previous investigations showed that the loss of PMC1 in yeast cells leads to a failure in pumping Ca2+ into the vacuole; thus, the cells failed to survive high Ca2+ stress [39]. To examine the roles of vacuolar Ca2+ transporters in Al toxicity, we tested the sensitivity of pmc1 and vcx1 mutants under a series of exogenous Ca2+ and aluminum ion (Al3+) treatments. As shown in Figure 1A, upon exposure to Ca2+ and Al3+, the pmc1 mutant was more sensitive than wild-type yeast and the vcx1 mutant. Because of the variant status of other mutants tested under normal conditions as well as their reduced sensitivity to Al treatment (Figure S1), we chose PMC1 as the best candidate for studying Al tolerance.
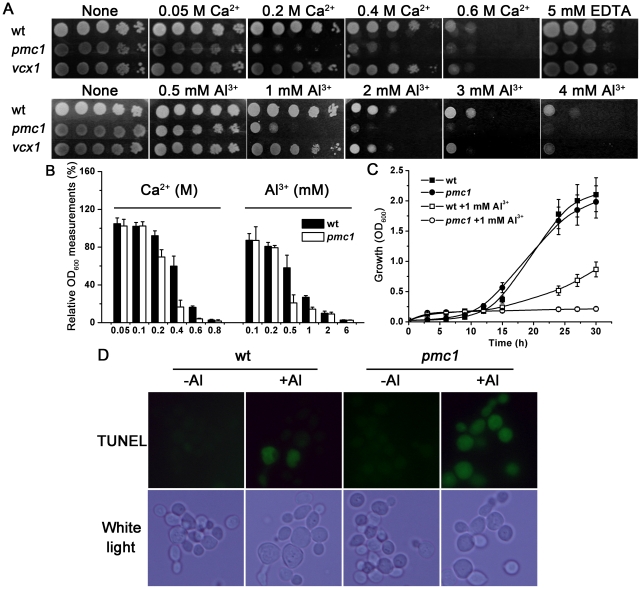
Figure 1. Al sensitivity in the pmc1 mutant.
(A) Growth properties of the wild-type (wt) strain and pmc1 and vcx1 mutants under Ca and Al stresses. (B) Comparison of growth (OD600) between wt and pmc1 mutant yeast strains incubated in liquid 1/2 SD/Gal-Raf/-Ura medium containing a series of doses of CaCl2 or AlCl3 for 24 h. Relative OD600 measurements were calculated as the OD600 of treated cells divided by the OD600 of untreated cells. The value of untreated strains was set at 100%. (C) Yeast wt and pmc1 strains were incubated in liquid 1/2 SD/Gal-Raf/-Ura medium with or without 1 mM AlCl3. The cell densities (OD600 values) were determined at 3-h intervals over a 30-h period. (D) Comparison of Al-induced PCD between wt and pmc1 mutant by TUNEL. No TUNEL signal was detected in the absence of Al treatment
As shown in Figure 1B, the pmc1 mutant grew as robustly as the wild-type strain in medium containing low concentrations of Ca2+ (0–100 mM) but grew at a much slower pace under conditions of high concentrations of Ca2+ (200–800 mM). Furthermore, compared to the wild-type control, the pmc1 mutant was more sensitive to moderate levels (0.5–1 mM) of Al3+ in liquid medium. We then performed a time-course assay to monitor yeast growth with 1 mM Al treatment. As shown in Figure 1C, when comparing the two growth curves generated from conditions of no Al exposure, the mutant cells exhibited a very similar rate to that of wild-type cells. However, the pmc1 mutant displayed more sensitivity to Al treatment. The difference in growth (OD600) appeared early, between the 10th–15th h, and became increasingly significant thereafter when mutant cells completely ceased to grow. In addition, both strains exhibited positive effects of Al toxicity within the first 10 h. This result indicated that the loss of vacuolar PMC1 in the mutant cells led to a failure in the regulative response to Al-elicited Ca2+ afflux.
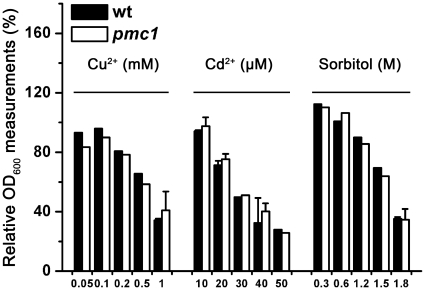
To ensure that the Al susceptibility of the pmc1 mutant was not an artifact, we tested the vulnerable specificity of the pmc1 mutant. As shown in Figure 2, the yeast cells were exposed to diverse stresses that could induce apoptosis in yeast, including different concentrations of sorbitol or other metal ions, such as copper ion (Cu2+) or cadmium ion (Cd2+). The pmc1 mutant displayed enhanced sensitivity to only Ca2+ and Al3+ but not to any of the other substances.
Figure 2. Stress specificity test on the pmc1 mutant.
Comparison of growth (OD600) between wt and pmc1 mutant yeast strains incubated in liquid 1/2 SD/Gal-Raf/-Ura medium containing a series of doses of sorbitol, CuCl2 or CdCl2 for 24 h. Relative OD600 measurements were calculated as the OD600 of treated cells divided by the OD600 of untreated cells. The value of untreated strains was set at 100%.
Taking into account all of these results along with those from our previous studies [13], Al toxicity in yeast appears to be mediated by interrupting the normal cytoplasmic Ca2+ pool and, consequently, by activating Ca signaling.
Al can induce both apoptotic-like cell death and an increase of Ca2+ level in yeast [13]. However, it remains to be determined whether Ca signals mediate Al-induced PCD. For this purpose, the TUNEL assay was used to detect apoptosis in wild-type yeast and the pmc1 mutant under Al stress. As shown in Figure 1D, TUNEL-positive cells were observed in both Al-treated strains. Moreover, the number of TUNEL-positive pmc1 mutant cells was much higher than that of the wild-type cells, indicating Al-elicited Ca signaling is an early mechanism of Al-induced PCD.
Overexpression of PMC1 reduced Al sensitivity in yeast
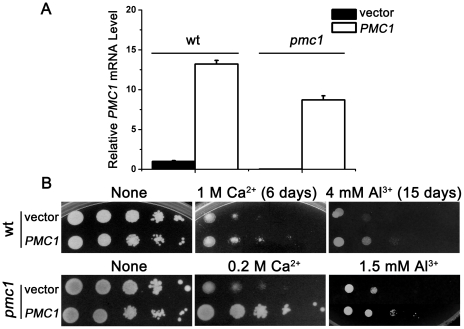
To investigate if PMC1 assumes an Al tolerance function in complementation tests, the exogenous PMC1 gene was transformed into wild-type yeast or back into the pmc1 mutant. Quantitative RT-PCR analysis showed that the levels of PMC1 mRNA in wild-type overexpressing exogenous PMC1 was 12 times higher than wild-type, and pmc1 mutant overexpressing PMC1 exhibited a level of PMC1 mRNA that was about 8 times higher than wild-type yeast (Figure 3A). Due to the enhanced susceptibility of the pmc1 mutant, lower concentrations of Ca2+ or Al3+ were used, compared with those concentrations used for the wild-type strain. As shown in Figure 3B, both wild-type and pmc1 strains exhibited increased Ca and Al tolerance in response to PMC1 overexpression.
Figure 3. Overexpressed Ca2+-ATPase PMC1 alleviates Al3+-induced cell death in yeast.
(A) Quantitative RT-PCR analysis of PMC1 expression in transformants. ACT1 was the internal standard. (B) Growth properties of transformed wt and pmc1 mutant strains stressed with Ca2+ or Al3+. Log-phase cells were diluted in a 10-fold series and spotted on 1/2 SD/Gal-Raf/-Ura plates containing the indicated stresses. The plates were then incubated at 30°C. Photos were taken after three or the indicated number of days of incubation.
To examine the causality between Al stress and Ca signals, we tested the alteration of cytosolic Ca2+ levels under Al treatment. Flow cytometry studies were performed to quantify Ca2+ levels using the Ca-specific probe Fluo-3/AM. As shown in Table 2, cytosolic Ca2+ levels were increased in wild-type cells when Al was present in the medium, and this result is consistent with previous studies [13]. Furthermore, the Ca2+ levels in wild-type cells overexpressing PMC1 were distinctly less than wild-type cells transformed with vector only in response to Al3+ at all concentrations tested. These data suggest that sequestration of Ca2+ into the vacuole for cytoplasmic Ca2+ homeostasis was achieved by genetic engineering with the overexpression Ca2+-ATPase PMC1, which represents a novel approach to the improvement of Al tolerance.
Table 2. Alteration of intracellular Ca2+ levels by PMC1.
| Ca2+ Responses in Yeast After Stimulation With Different Concentrations of Al3+ a | |||
| Ca2+ response (fluorescence ratio)b | |||
| Al3+(mM) | 0.5 | 1 | 4 |
| wt | 1.60±0.62 | 1.78±0.04* | 2.52±0.53** |
| wt/PMC1 | 1.28±0.46 | 1.40±0.50* | 1.91±0.86* |
Data are presented as mean±SD for at least three experiments. * p<0.05, and ** p<0.01 versus the untreated control values
Fluorescence ratio = value of mean FL1 in Al3+-stimulated yeast/mean FL1 in Al3+-unstimulated yeast. The value of untreated strains was set at 1.
Al toxicity is reduced by treatment with Ca2+ chelator BAPTA-AM
BAPTA-AM is a membrane permeable compound that is capable of mediating the redistribution of Ca2+ throughout diverse intracellular compartments; thus, BAPTA-AM can control cytoplasmic free Ca2+ at low levels [45], [46], [47]. To provide direct evidence that Ca2+ is an important mediator of Al toxicity, we tested yeast cells with the Ca2+ chelator BAPTA-AM to inhibit excessive cytosol free Ca2+ and monitored the effects.
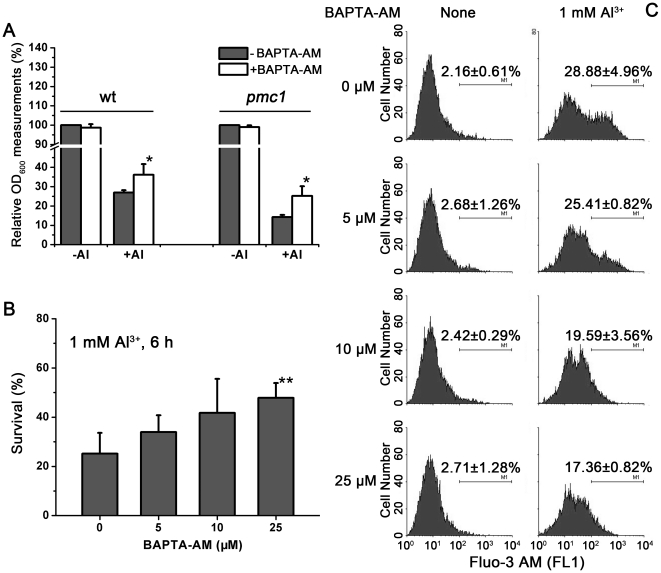
As shown in Figure 4A, yeast cells grew in the presence of different amounts of chelator BAPTA-AM. Treatment with 25 µM BAPTA-AM appeared to favor cell growth when Al stress was also present. During a 24-h incubation period, both wild-type and pmc1 mutant cells with BAPTA-AM treatment had higher densities than the cells without BAPTA-AM treatment in the presence of Al3+. BAPTA-AM treatment alleviated exogenous Ca2+ stress in wild-type cells after about 20 h, whereas in pmc1 this alleviation occurred at an earlier time point (data not shown). The chelator had little effect on unstressed cells. Furthermore, all the concentrations of BAPTA-AM used in this study effectively enhanced the viability of Al-exposed cells (Figure 4B). These data suggest that treatment with the cytosolic Ca2+ chelator BAPTA-AM can alleviate Al toxicity in yeast, directly supporting the idea that Al-induced cell death is mediated by cytosolic Ca signaling flux and Ca2+ homeostasis, which are both essential for cell viability.
Figure 4. Reduced Al toxicity with the Ca2+ chelator BAPTA-AM.
(A) Comparison of growth (OD600) with or without BAPTA-AM in wt and pmc1 mutants. Yeast strains were incubated in liquid 1/2 SD/Gal-Raf/-Ura medium containing 1 mM AlCl3 with or without BAPTA-AM for 24 h. Relative OD600 measurements were calculated as the OD600 of treated cells divided by the OD600 of untreated cells. The value of untreated strains was set at 100%. (B) Survival tests of yeast cells under Al stress with different concentrations of BAPTA-AM. The yeast cells were pretreated with the indicated concentrations of BAPTA-AM in liquid medium for 30 min, followed by treatment with 1 mM Al3+ for 6 h, and then, the cells were plated for survivors on YPD plates. The values were calculated as the percentage of the number of surviving cells without Al treatment. Viability without Al was set at 100%. The results are the means of at least three independent experiments. *p<0.05, and **p<0.01 versus the untreated control values. (C) Al stress-increased cytoplasmic Ca signals can be alleviated by BAPTA-AM. Flow cytometry analysis of 1 mM Al3+-challenged cytosolic Ca2+ levels in wt yeast.
To further explore the chelator BAPTA-AM function on Ca2+ levels in Al tolerance, the changes in cytosolic Ca2+ levels were monitored by flow cytometry. As shown in Figure 4C, BAPTA-AM caused a redistribution of cytosolic Ca2+ in the cells exposed to Al. The basal cytosolic Ca2+ concentrations in unstressed cells remained largely unaffected. When incubated with 1 mM Al3+, however, BAPTA-AM exposure led to a dose-dependent decrease in cytosolic Ca2+ in cells. Similar to the above results, BAPTA-AM exposure also resulted in a dose-dependent decrease in cytosolic Ca2+ under 0.5 mM Al3+ or 0.2 M Ca2+ treatment (Figure S2). These data are consistent with what we observed in the survival tests and strongly suggest that inhibiting the Al-elicited Ca2+ burst can reduce Al toxicity.
Heterogeneous anti-apoptotic proteins improved Al tolerance of the pmc1 mutant
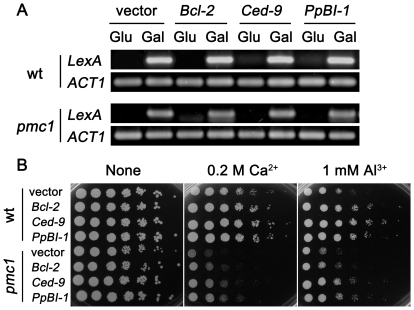
Previous studies showed that the Al-triggered Ca2+ level increase could be blocked by anti-apoptotic proteins [13]. This result led us to test whether the expression of anti-apoptotic members restore Al tolerance in the pmc1 mutant. As described before, all three transformants were morphologically indistinguishable, and their growth rates were similar. Semi-quantitative RT-PCR showed equally abundant expression of the three transgenes in induced medium (Gal) but almost no PCR signal in uninduced medium (Glu) (Figure 5A). As shown in Figure 5B, the strains expressing Bcl-2, Ced-9 or PpBI-1 exhibited robust growth under both Ca and Al stresses, suggesting that the negative regulation of PCD alleviated Al toxicity through the regulation of Ca homeostasis.
Figure 5. Heterogeneous anti-apoptotic members improve the Al tolerance of the pmc1 mutant.
(A) Semi-quantitative RT-PCR analysis of the expression of anti-apoptotic members in the wt and pmc1 mutant strains after incubation in induced (Gal) or uninduced (Glu) medium. ACT1 served as the internal standard. (B) Growth properties of yeast cells transformed with anti-apoptosis members under Ca and Al stresses. Log-phase cells were diluted in a 5-fold series with an initial OD600 of 1, and then, 5 µL of each dilution was spotted on 1/2 SD/Gal-Raf/-His plates containing different stresses. Photos were taken after three days of incubation at 30°C.
We examined the alteration of cytosolic Ca2+ levels in the wild-type, pmc1 mutant and transgenic cells expressing anti-apoptotic members under Al stress. Al3+ treatment (1 mM) resulted in increased Ca2+ levels in both wild-type and pmc1 mutant cells compared to the unstressed cells. Al-induced cytoplasmic Ca2+ levels in cells expressing anti-apoptotic members was distinctly decreased compared to the cells transformed with vector only (Table 3), suggesting that anti-apoptotic members can complement the function of Ca2+-ATPase PMC1 and act upstream of intracellular Ca2+ flux in the pathway mediating Al-induced cell death.
Table 3. Alteration of intracellular Ca2+ levels by anti-apoptotic members.
| Ca2+ Responses in Yeast After Stimulation With 1 mM Al3+ a | ||||
| Ca2+ response(fluorescence ratio) b | ||||
| vector | Bcl-2 | Ced-9 | PpBI-1 | |
| wt | 1.72±0.23** | 1.58±0.27 | 1.47±0.08* | 1.35±0.39 |
| pmc1 | 1.32±0.12* | 0.95±0.21 | 1.16±0.27 | 0.91±0.18 |
Data are presented as mean±SD for at least three experiments. * p<0.05, and ** p<0.01 versus the untreated control values
Fluorescence ratio = value of mean FL1 in Al3+-stimulated yeast/mean FL1 in Al3+-unstimulated yeast. The value of untreated strains was set at 1.
Ca pathway components participate in Al tolerance
As shown above, regulating cytosolic Ca2+ significantly improved Al tolerance in yeast cells. To investigate the molecular mechanisms underlying Ca signaling, we determined the expression levels of certain Ca pathway components using quantitative RT-PCR. Three strains were selected for this assay: wild-type cells, cells pretreated with BAPTA-AM, and cells transformed with Ced-9.
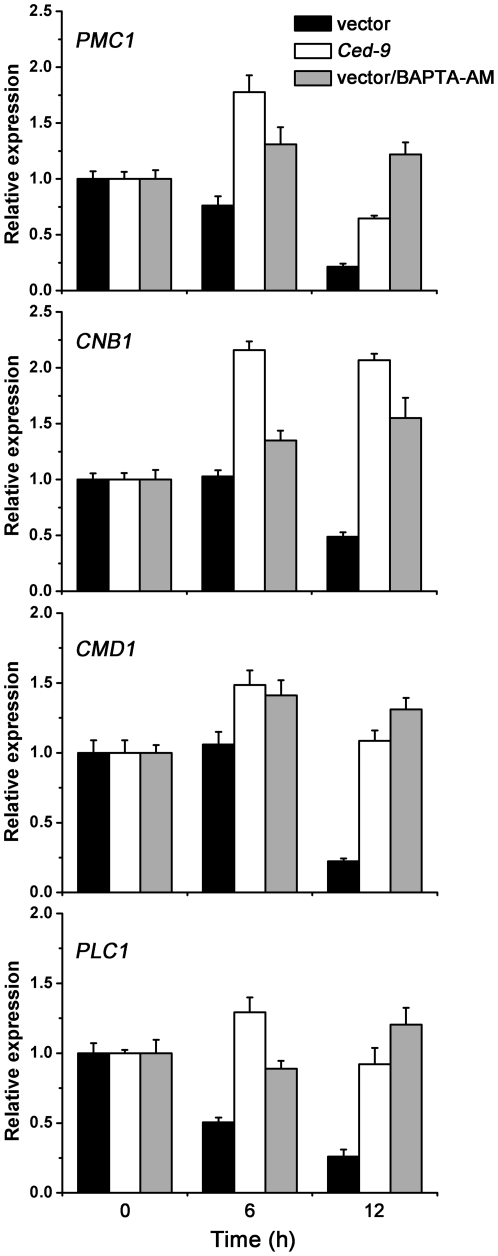
Figure 6 shows that accumulation of PMC1 mRNA is significantly reduced when cells are exposed to Al. Expression levels of PMC1 in Ced-9 transformants or BAPTA-AM pretreated cells increased by about 3- or 6-fold over the wild-type cells when exposed to Al3+ for 12 h. Consistent with Al sensitivity of the pmc1 mutant, these results suggest that cytosolic Ca2+ homeostasis and PMC1 activation are important for Al tolerance.
Figure 6. Ca pathway components participate in Al tolerance of yeast.
wt/pGilda, wt/pGilda-Ced-9 and wt/pGilda/BAPTA-AM were treated with or without Al3+. The relative expression levels of genes were determined by quantitative RT-PCR. ACT1 was used as an internal control. 0 h represents yeast with Al treatment at 0 h. Data are the mean ± SD (n = 6; two biological replicates and three PCR replicates).
Small variations in cytosolic Ca2+ that occur in response to a number of stimuli are sufficient to activate a variety of Ca-sensing proteins, such as CaM and calcineurin; this activation then leads to the induction of various downstream signal transduction pathways [24], [36]. The expression of CMD1 (which encodes CaM) and CNB1 (which encodes the regulatory subunit of calcineurin) decreased upon exposure to Al (Figure 6). It has been reported that PLC activity is inhibited by Al in plants [37]. Our data showed a similar result for PLC1, which encodes PLC and was down-regulated when cells were exposed to Al3+. However, the expression of CMD1, CNB1 and PLC1 were restored to normal or even higher levels in Ced-9 transgenic colonies and BAPTA-AM pretreated cells. These results indicate that these Ca2+-relevant genes in Ca signaling pathways participate in Al tolerance in yeast.
Discussion
In this study, we investigated the relevance of a vacuole-located Ca2+-ATPase PMC1 in modulating Al stress responses and the function of cytoplasmic Ca homeostasis on Al tolerance in yeast. When compared to wild-type, the pmc1 mutant exhibited more sensitivity to Al stress (Figure 1), as well as to high Ca2+ concentrations [39]. Al induces apoptosis in yeast via the elevation of cytosolic Ca2+, which is released from intracellular sources, and apoptotic suppressors enhance Al tolerance with decreased Ca signals [13]. PMR1, a Golgi Ca2+/Mn2+-ATPase, plays a role in Al tolerance [48]. In particular, PMR1 is important for intracellular Ca homeostasis, and PMC1 transcription increases in the pmr1 mutant to sequester cellular Ca2+ into intracellular stores [49], suggesting that Ca2+-mediated signaling plays a pivotal role in Al tolerance of yeast.
Since the first description of apoptosis in yeast [17], many factors, including sorbitol [50], Al [13], Cu [51], Cd [52] and others, have been found to induce yeast apoptosis. Sorbitol and Cu both trigger apoptosis via a mitochondrial pathway, with caspase and cytochrome c occasionally involved [50], [51]. Cd2+ induces ER and oxidative stresses in yeast. Cd toxicity is a direct consequence of Cd2+ accumulation in the ER [52], [53], [54]. In particular, PMR1 has a central role in the regulation of intracellular levels of Cd2+ and Cd2+ detoxification [55]. By contrast, pmc1 mutant and wild-type cells show the same tolerance to CdCl2 [55], which is consistent with our results. These results clearly indicate that PMC1 plays selective roles in Al tolerance as opposed to the other stresses we tested.
The addition of Al to cultures changes pH values. However, based on our experimental data, Ca2+ changed little in response to pH variations, which was different with Al3+ (Figure S3A). Particularly when the pH was 4.0 or 3.8, both 0.5 mM Al and 1 mM Al medium, which we often use, have a similar pH. Ca2+ level in the pmc1 mutant was elevated when the pH reached 3.4. The proton (H+) concentration may be too high and may affect both the H+ gradient and the pH of the vacuole or other organelles. The growth properties of wild-type yeast and the pmc1 mutant were similar when cells were exposed to different pH values (Figure S3B), whereas the pmc1 mutant was more sensitive to Al than wild-type yeast. These results suggest that the differences between the pmc1 mutant and wild-type yeast under Al stress are caused by Al3+ rather than pH change.
Al3+ exposure can elicit a striking and rapid increase in cytosolic Ca2+ in plants and yeast [2], [13]. Ca2+ transport across the plasma membrane and intracellular sequestration are tightly regulated events that maintain the cytosolic Ca2+ concentrations within a range of 50–200 nM [49]. As such, the regulation of intracellular Ca homeostasis in eukaryotic cells is a remarkably intricate and dynamic process, which led us to explore the relation between cytosolic Ca2+ fluctuation and Al tolerance. PMC1 overexpression enhanced Al and Ca tolerance in wild-type yeast and the pmc1 mutant (Figure 3). Endomembrane Ca2+-ATPases appear to be important for intracellular Ca2+ distribution [28], [40], [56]. Recent studies have shown that PMCA overexpression depletes intracellular Ca2+ stores and induces apoptosis through the mitochondrial pathway in clonal β-cells [57]. Because PMCA and PMC1 are localized to different regions, we hypothesized that the vacuole was the key Ca2+ store involved in regulating cytosolic Ca2+ in response to Al stress in yeast. In addition to Ca2+ and Al3+, we also found that the pmc1 mutant displayed sensitivity to H2O2 (Figure S4), which suggested that Ca signals are related reactive oxygen species (ROS) production [58]. A recent study also showed that lethal H2O2 shock predominantly mobilized the vacuolar Ca2+ in yeast [59]. The studies of Ca2+-ATPases AtACA4 and AtACA11 in plant also indicate that endomembrane Ca2+ pumps function as suppressors of a salicylic acid-dependent PCD pathway and that vacuoles can modulate Ca signals [60].
When subjected to Al stress, the cytosolic Ca2+ concentration in PMC1 transformants was lower than in wild-type cells (Table 2). Down-regulation of Al-elicited cytosolic Ca2+ enhanced Al tolerance. Buffering cytosolic Ca2+ with BAPTA-AM resulted in the inhibition of apoptosis, which would have otherwise been caused by oxidative stress [45], Cd [46], [47], Galectin-9 [61], and various other conditions [62], [63], [64]. Consistent with the BAPTA-AM-induced down-regulation of cytosolic Ca2+, the elevation of cell death stimulated by Al was abolished (Figure 4). These results indicated that Ca oscillation played a pivotal role in mediating Al toxicity. In particular, the decrease of cytoplasmic free Ca2+ may be one mechanism that reduces Al toxicity.
Bcl-2 family and BI-1 proteins regulate intracellular Ca2+ homeostasis [65], [66], [67]. Earlier studies have shown that Bcl-2 overexpression leads to increased Sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) expression [68], [69]. Bcl-2, Ced-9 and PpBI-1 significantly improve Al tolerance and block Al-elicited Ca signals [13]. Ced-9 inhibits Al-induced activity of caspase-like vacuolar processing enzyme (VPE) [12]. These apoptotic suppressors restored both wild-type and pmc1 mutant growth under Al and Ca stresses (Figure 5). We chose 1/2 SD instead of full SD because yeast growth exhibited more Al sensitivity and the Bcl-2 gene enhanced Al tolerance in this medium. Cytosolic Ca signal levels detected in wild-type and pmc1 mutant cells expressing apoptotic suppressors were distinctly less than the levels in the cells transformed with vector only after exposure to 1 mM Al3+ (Table 3), suggesting that anti-apoptotic members complemented the function of Ca2+-ATPase PMC1 and enhanced Al tolerance to some extent.
Our study demonstrates, for the first time, that Al toxicity is mediated by Ca signals and that the vacuolar Ca2+-ATPase Pmc1p plays a pivotal role in Al tolerance in yeast. These findings may make it possible to genetically improve Al tolerance in plant species by protecting intracellular Ca2+ homeostasis.
Supporting Information
Effect of Ca and Al stresses on the cell growth of pmr1 and pmc1 pmr1 cnb1 mutants. Growth properties of wt and mutant strains under Ca (A) and Al (B) stresses.
(TIF)
Al and Ca stress-increased cytoplasmic Ca signals can be alleviated by BAPTA-AM. Flow cytometry analysis of 0.5 mM Al3+- and 0.2 M Ca2+-challenged cytosolic Ca2+ levels in wt yeast.
(TIF)
Ca signals and growth properties of wt and pmc1 mutant strains in response to pH variations. A. Ca2+ changed little in response to pH variations. Flow cytometry analysis of pH-challenged cytosolic Ca2+ levels in wt and pmc1 mutant strains. B. Growth properties of yeast cells under different pH values.
(TIF)
H2O2 sensitivity in the pmc1 mutant. Growth properties of the wt strain and the pmc1 mutant under H2O2 stress.
(TIF)
Acknowledgments
The authors sincerely thank Dr. Kyle W. Cunningham (Johns Hopkins University, Baltimore, MD, USA), Dr. Michael G. Palmgren (University of Copenhagen, Denmark), Dr. John C. Reed, Dr. P.H. Ho and Dr. G.S. Feng (the Burnham Institute, La Jolla, CA, USA), and Prof. Lone Bækgaard (The Royal Veterinary and Agricultural University, Copenhagen, Denmark) for gifts of either plasmids or strains.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the National Science Foundation of China (Grant No.30771347, No.30870034), National High Technology Research and Development Program of China (863 Program) (No.2007AA10Z141), and the Key Program of Science and technology Department of Zhejiang Province (No.2009C12072, No.2009C32064). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Panda S, Baluska F, Matsumoto H. Aluminum stress signaling in plants. Plant Signal Behav. 2009;4:592–597. doi: 10.4161/psb.4.7.8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma J. Syndrome of aluminum toxicity and diversity of aluminum resistance in higher plants. Int Rev Cytol. 2007;264:225–252. doi: 10.1016/S0074-7696(07)64005-4. [DOI] [PubMed] [Google Scholar]
- 3.Ma JF. Physiological Mechanisms of Al Resistance in Higher Plants. Soil Science & Plant Nutrition. 2005a;51:609–612. [Google Scholar]
- 4.Sasaki T, Yamamoto Y, Ezaki B, Katsuhara M, Ahn S, et al. A wheat gene encoding an aluminum-activated malate transporter. Plant J. 2004;37:645–653. doi: 10.1111/j.1365-313x.2003.01991.x. [DOI] [PubMed] [Google Scholar]
- 5.Furukawa J, Yamaji N, Wang H, Mitani N, Murata Y, et al. An Aluminum-Activated Citrate Transporter in Barley. Plant Cell Physiol. 2007;48:1081–1091. doi: 10.1093/pcp/pcm091. [DOI] [PubMed] [Google Scholar]
- 6.Magalhaes J, Liu J, Guimaraes C, Lana U, Alves V, et al. A gene in the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nat Genet. 2007;39:1156–1161. doi: 10.1038/ng2074. [DOI] [PubMed] [Google Scholar]
- 7.Larsen P, Cancel J, Rounds M, Ochoa V. Arabidopsis ALS1 encodes a root tip and stele localized half type ABC transporter required for root growth in an aluminum toxic environment. Planta. 2007;225:1447–1458. doi: 10.1007/s00425-006-0452-4. [DOI] [PubMed] [Google Scholar]
- 8.Larsen P, Geisler M, Jones C, Williams K, Cancel J. ALS3 encodes a phloem-localized ABC transporter-like protein that is required for aluminum tolerance in Arabidopsis. Plant J. 2005;41:353–363. doi: 10.1111/j.1365-313X.2004.02306.x. [DOI] [PubMed] [Google Scholar]
- 9.Huang CF, Yamaji N, Mitani N, Yano M, Nagamura Y, et al. A Bacterial-Type ABC Transporter Is Involved in Aluminum Tolerance in Rice. Plant Cell. 2009;21:655–667. doi: 10.1105/tpc.108.064543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamaji N, Huang CF, Nagao S, Yano M, Sato Y, et al. A Zinc Finger Transcription Factor ART1 Regulates Multiple Genes Implicated in Aluminum Tolerance in Rice. Plant Cell. 2009;21:3339–3349. doi: 10.1105/tpc.109.070771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia J, Yamaji N, Kasai T, Ma JF. Plasma membrane-localized transporter for aluminum in rice. Proceedings of the National Academy of Sciences. 2010;107:18381–18385. doi: 10.1073/pnas.1004949107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W, Pan J, Zheng K, Chen H, Shao H, et al. Ced-9 inhibits Al-induced programmed cell death and promotes Al tolerance in tobacco. Biochem Biophys Res Commun. 2009;383:141–145. doi: 10.1016/j.bbrc.2009.03.125. [DOI] [PubMed] [Google Scholar]
- 13.Zheng K, Pan J-W, Ye L, Fu Y, Peng H-Z, et al. Programmed Cell Death-Involved Aluminum Toxicity in Yeast Alleviated by Antiapoptotic Members with Decreased Calcium Signals. Plant Physiol. 2007;143:38–49. doi: 10.1104/pp.106.082495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunawardena AHLAN. Programmed cell death and tissue remodelling in plants. Journal of Experimental Botany. 2008;59:445–451. doi: 10.1093/jxb/erm189. [DOI] [PubMed] [Google Scholar]
- 15.Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. The Journal of Experimental Medicine. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerr J, Wyllie A, Currie A. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madeo F, Fröhlich E, Fröhlich K-U. A Yeast Mutant Showing Diagnostic Markers of Early and Late Apoptosis. The Journal of Cell Biology. 1997;139:729–734. doi: 10.1083/jcb.139.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reed JC. Bcl-2-family proteins and hematologic malignancies: history and future prospects. Blood. 2008;111:3322–3330. doi: 10.1182/blood-2007-09-078162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reimers K, Choi C, Bucan V, Vogt P. The Bax Inhibitor-1 (BI-1) family in apoptosis and tumorigenesis. Curr Mol Med. 2008;8:148–156. doi: 10.2174/156652408783769562. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe N, Lam E. BAX Inhibitor-1 Modulates Endoplasmic Reticulum Stress-mediated Programmed Cell Death in Arabidopsis. Journal of Biological Chemistry. 2008;283:3200–3210. doi: 10.1074/jbc.M706659200. [DOI] [PubMed] [Google Scholar]
- 21.Ghribi O, Herman M, Spaulding N, Savory J. Lithium inhibits aluminum-induced apoptosis in rabbit hippocampus, by preventing cytochrome c translocation, Bcl-2 decrease, Bax elevation and caspase-3 activation. J Neurochem. 2002;82:137–145. doi: 10.1046/j.1471-4159.2002.00957.x. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Rao R. A spoke in the wheel: calcium spikes disrupt yeast cell cycle. Cell Cycle. 2008;7:870–873. doi: 10.4161/cc.7.7.5616. [DOI] [PubMed] [Google Scholar]
- 23.Demaurex N, Poburko D. A Revolving Door for Calcium. Science. 2009;326:57–58. doi: 10.1126/science.1180482. [DOI] [PubMed] [Google Scholar]
- 24.Kudla J, Batistic O, Hashimoto K. Calcium Signals: The Lead Currency of Plant Information Processing. Plant Cell. 2010;22:541–563. doi: 10.1105/tpc.109.072686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cunningham K, Fink G. Ca2+ transport in Saccharomyces cerevisiae. J Exp Biol. 1994;196:157–166. doi: 10.1242/jeb.196.1.157. [DOI] [PubMed] [Google Scholar]
- 26.Pottosin II, Schönknecht G. Vacuolar calcium channels. Journal of Experimental Botany. 2007;58:1559–1569. doi: 10.1093/jxb/erm035. [DOI] [PubMed] [Google Scholar]
- 27.Brini M, Carafoli E. Calcium Pumps in Health and Disease. Physiol Rev. 2009;89:1341–1378. doi: 10.1152/physrev.00032.2008. [DOI] [PubMed] [Google Scholar]
- 28.Anil VS, Rajkumar P, Kumar P, Mathew MK. A Plant Ca2+ Pump, ACA2, Relieves Salt Hypersensitivity in Yeast. Journal of Biological Chemistry. 2008;283:3497–3506. doi: 10.1074/jbc.M700766200. [DOI] [PubMed] [Google Scholar]
- 29.Ton V-K, Rao R. Functional expression of heterologous proteins in yeast: insights into Ca2+ signaling and Ca2+-transporting ATPases. Am J Physiol Cell Physiol. 2004;287:C580–589. doi: 10.1152/ajpcell.00135.2004. [DOI] [PubMed] [Google Scholar]
- 30.Cessna SG, Chandra S, Low PS. Hypo-osmotic Shock of Tobacco Cells Stimulates Ca2+ Fluxes Deriving First from External and then Internal Ca2+ Stores. Journal of Biological Chemistry. 1998;273:27286–27291. doi: 10.1074/jbc.273.42.27286. [DOI] [PubMed] [Google Scholar]
- 31.Pittman JK. Vacuolar Ca2+ uptake. Cell Calcium. 2011. In Press, Corrected Proof. [DOI] [PubMed]
- 32.Peiter E. The plant vacuole: Emitter and receiver of calcium signals. Cell Calcium. 2011. In Press, Corrected Proof. [DOI] [PubMed]
- 33.Cunningham KW. Acidic calcium stores of Saccharomyces cerevisiae. Cell Calcium. 2011. In Press, Corrected Proof. [DOI] [PMC free article] [PubMed]
- 34.Pozos T, Sekler I, Cyert M. The product of HUM1, a novel yeast gene, is required for vacuolar Ca2+/H+ exchange and is related to mammalian Na+/Ca2+ exchangers. Mol Cell Biol. 1996;16:3730–3741. doi: 10.1128/mcb.16.7.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cunningham K, Fink G. Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:2226–2237. doi: 10.1128/mcb.16.5.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim MC, Chung WS, Yun D-J, Cho MJ. Calcium and Calmodulin-Mediated Regulation of Gene Expression in Plants. Molecular Plant. 2009;2:13–21. doi: 10.1093/mp/ssn091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramos-Diaz A, Brito-Argaez L, Munnik T, Hernandez-Sotomay S. Aluminum inhibits phosphatidic acid formation by blocking the phospholipase C pathway. Planta. 2007;225:393–401. doi: 10.1007/s00425-006-0348-3. [DOI] [PubMed] [Google Scholar]
- 38.Yoko-o T, Matsui Y, Yagisawa H, Nojima H, Uno I, et al. The putative phosphoinositide-specific phospholipase C gene, PLC1, of the yeast Saccharomyces cerevisiae is important for cell growth. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:1804–1808. doi: 10.1073/pnas.90.5.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cunningham K, Fink G. Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+ ATPases. The Journal of Cell Biology. 1994;124:351–363. doi: 10.1083/jcb.124.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geisler M, Frangne N, Gomes E, Martinoia E, Palmgren MG. The ACA4 Gene of Arabidopsis Encodes a Vacuolar Membrane Calcium Pump That Improves Salt Tolerance in Yeast. Plant Physiol. 2000;124:1814–1827. doi: 10.1104/pp.124.4.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elble R. A simple and efficient procedure for transformation of yeasts. Biotechniques. 1992;13:18–20. [PubMed] [Google Scholar]
- 42.Teste M, Duquenne M, Francois J, Parrou J. Validation of reference genes for quantitative expression analysis by real-time RT-PCR in Saccharomyces cerevisiae. BMC Mol Biol. 2009;10:99. doi: 10.1186/1471-2199-10-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Madeo F, Fröhlich E, Ligr M, Grey M, Sigrist SJ, et al. Oxygen Stress: A Regulator of Apoptosis in Yeast. The Journal of Cell Biology. 1999;145:757–767. doi: 10.1083/jcb.145.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scoltock AB, Bortner CD, St JBirdG, Putney JW, Cidlowski JA. A Selective Requirement for Elevated Calcium in DNA Degradation, but Not Early Events in Anti-Fas-induced Apoptosis. Journal of Biological Chemistry. 2000;275:30586–30596. doi: 10.1074/jbc.M004058200. [DOI] [PubMed] [Google Scholar]
- 45.Zhang B, Ma J-X. Kallikrein-Binding Protein Protects Retinal Muller Cells Against H2O2-Induced Cell Death by Inhibiting Calcium Overload. Invest Ophthalmol Vis Sci. 2007;48:573. [Google Scholar]
- 46.Son Y-O, Lee J-C, Hitron JA, Pan J, Zhang Z, et al. Cadmium Induces Intracellular Ca2+- and H2O2-Dependent Apoptosis through JNK- and p53-Mediated Pathways in Skin Epidermal Cell line. Toxicological Sciences. 2010;113:127–137. doi: 10.1093/toxsci/kfp259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang S, Shih Y, Lee C, Chen W, Lin C, et al. The role of endoplasmic reticulum in cadmium-induced mesangial cell apoptosis. Chem Biol Interact. 2009;181:45–51. doi: 10.1016/j.cbi.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 48.Kakimoto M, Kobayashi A, Fukuda R, Ono Y, Ohta A, et al. Genome-wide screening of aluminum tolerance in Saccharomyces cerevisiae. Biometals. 2005;18:467–474. doi: 10.1007/s10534-005-4663-0. [DOI] [PubMed] [Google Scholar]
- 49.Kellermayer R, Aiello DP, Miseta A, Bedwell DM. Extracellular Ca2+ sensing contributes to excess Ca2+ accumulation and vacuolar fragmentation in a pmr1{Delta} mutant of S. cerevisiae. J Cell Sci. 2003;116:1637–1646. doi: 10.1242/jcs.00372. [DOI] [PubMed] [Google Scholar]
- 50.Silva RD, Sotoca R, Johansson B, Ludovico P, Sansonetty F, et al. Hyperosmotic stress induces metacaspase- and mitochondria-dependent apoptosis in Saccharomyces cerevisiae. Molecular Microbiology. 2005;58:824–834. doi: 10.1111/j.1365-2958.2005.04868.x. [DOI] [PubMed] [Google Scholar]
- 51.Liang Q, Zhou B. Copper and Manganese Induce Yeast Apoptosis via Different Pathways. Mol Biol Cell. 2007;18:4741–4749. doi: 10.1091/mbc.E07-05-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nargund A, Avery S, Houghton J. Cadmium induces a heterogeneous and caspase-dependent apoptotic response in Saccharomyces cerevisiae. Apoptosis. 2008;13:811–821. doi: 10.1007/s10495-008-0215-8. [DOI] [PubMed] [Google Scholar]
- 53.Muthukumar K, Nachiappan V. Cadmium-induced oxidative stress in Saccharomyces cerevisiae. Indian J Biochem Biophys. 2010;47:383–387. [PubMed] [Google Scholar]
- 54.Gardarin A, Chédin S, Lagniel G, Aude J-C, Godat E, et al. Endoplasmic reticulum is a major target of cadmium toxicity in yeast. Molecular Microbiology. 2010;76:1034–1048. doi: 10.1111/j.1365-2958.2010.07166.x. [DOI] [PubMed] [Google Scholar]
- 55.Lauer Júnior CM, Bonatto D, Mielniczki-Pereira AA, Zilles Schuch A, Dias JF, et al. The Pmr1 protein, the major yeast Ca2+-ATPase in the Golgi, regulates intracellular levels of the cadmium ion. FEMS Microbiology Letters. 2008;285:79–88. doi: 10.1111/j.1574-6968.2008.01214.x. [DOI] [PubMed] [Google Scholar]
- 56.Hirschi KD, Korenkov VD, Wilganowski NL, Wagner GJ. Expression of Arabidopsis CAX2 in Tobacco. Altered Metal Accumulation and Increased Manganese Tolerance. Plant Physiol. 2000;124:125–134. doi: 10.1104/pp.124.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang L, Allagnat F, Nguidjoe E, Kamagate A, Pachera N, et al. Plasma Membrane Ca2+-ATPase Overexpression Depletes Both Mitochondrial and Endoplasmic Reticulum Ca2+ Stores and Triggers Apoptosis in Insulin-secreting BRIN-BD11 Cells. Journal of Biological Chemistry. 2010;285:30634–30643. doi: 10.1074/jbc.M110.116681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu S-S. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 59.Popa C, Dumitru I, Ruta L, Danet A, Farcasanu I. Exogenous oxidative stress induces Ca2+ release in the yeast Saccharomyces cerevisiae. FEBS J. 2010;277:4027–4038. doi: 10.1111/j.1742-4658.2010.07794.x. [DOI] [PubMed] [Google Scholar]
- 60.Boursiac Y, Lee SM, Romanowsky S, Blank R, Sladek C, et al. Disruption of the Vacuolar Calcium-ATPases in Arabidopsis Results in the Activation of a Salicylic Acid-Dependent Programmed Cell Death Pathway. Plant Physiol. 2010;154:1158–1171. doi: 10.1104/pp.110.159038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kashio Y, Nakamura K, Abedin MJ, Seki M, Nishi N, et al. Galectin-9 Induces Apoptosis Through the Calcium-Calpain-Caspase-1 Pathway. J Immunol. 2003;170:3631–3636. doi: 10.4049/jimmunol.170.7.3631. [DOI] [PubMed] [Google Scholar]
- 62.Seo Y-W, Woo H-N, Piya S, Moon AR, Oh J-W, et al. The Cell Death–Inducing Activity of the Peptide Containing Noxa Mitochondrial-Targeting Domain Is Associated with Calcium Release. Cancer Research. 2009;69:8356–8365. doi: 10.1158/0008-5472.CAN-09-0349. [DOI] [PubMed] [Google Scholar]
- 63.Zhao D, Chu W, Wu L, Li J, Liu Q, et al. PAF exerts a direct apoptotic effect on the rat H9c2 cardiomyocytes in Ca2+-dependent manner. Int J Cardiol. 2010;143:86–93. doi: 10.1016/j.ijcard.2009.01.068. [DOI] [PubMed] [Google Scholar]
- 64.Bacus SS, Hill JE, Trusk P, Seger R, Spector NL. Activation of the AMPK regulated metabolic stress response by a small molecule HER2/EGFR tyrosine kinase inhibitor protects cardiac myocytes from apoptotic stimuli. J Clin Oncol (Meeting Abstracts) 2007;25:14000–. [Google Scholar]
- 65.Szegezdi E, MacDonald DC, Ni Chonghaile T, Gupta S, Samali A. Bcl-2 family on guard at the ER. Am J Physiol Cell Physiol. 2009;296:C941–953. doi: 10.1152/ajpcell.00612.2008. [DOI] [PubMed] [Google Scholar]
- 66.Xu C, Xu W, Palmer AE, Reed JC. BI-1 Regulates Endoplasmic Reticulum Ca2+ Homeostasis Downstream of Bcl-2 Family Proteins. Journal of Biological Chemistry. 2008;283:11477–11484. doi: 10.1074/jbc.M708385200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Westphalen B, Wessig J, Leypoldt F, Arnold S, Methner A. BI-1 protects cells from oxygen glucose deprivation by reducing the calcium content of the endoplasmic reticulum. Cell Death Differ. 2005;12:304–306. doi: 10.1038/sj.cdd.4401547. [DOI] [PubMed] [Google Scholar]
- 68.Kuo T, Kim H, Zhu L, Yu Y, Lin H, et al. Modulation of endoplasmic reticulum calcium pump by Bcl-2. Oncogene. 1998;17:1903–1910. doi: 10.1038/sj.onc.1202110. [DOI] [PubMed] [Google Scholar]
- 69.Vanden Abeele F, Skryma R, Shuba Y, Van Coppenolle F, Slomianny C, et al. Bcl-2-dependent modulation of Ca(2+) homeostasis and store-operated channels in prostate cancer cells. Cancer Cell. 2002;1:169–179. doi: 10.1016/s1535-6108(02)00034-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of Ca and Al stresses on the cell growth of pmr1 and pmc1 pmr1 cnb1 mutants. Growth properties of wt and mutant strains under Ca (A) and Al (B) stresses.
(TIF)
Al and Ca stress-increased cytoplasmic Ca signals can be alleviated by BAPTA-AM. Flow cytometry analysis of 0.5 mM Al3+- and 0.2 M Ca2+-challenged cytosolic Ca2+ levels in wt yeast.
(TIF)
Ca signals and growth properties of wt and pmc1 mutant strains in response to pH variations. A. Ca2+ changed little in response to pH variations. Flow cytometry analysis of pH-challenged cytosolic Ca2+ levels in wt and pmc1 mutant strains. B. Growth properties of yeast cells under different pH values.
(TIF)
H2O2 sensitivity in the pmc1 mutant. Growth properties of the wt strain and the pmc1 mutant under H2O2 stress.
(TIF)