Abstract
Changes in renal function are one of the most common manifestations of severe illness. There is a clinical need to intervene early with proven treatments in patients with potentially deleterious changes in renal function. Unfortunately progress has been hindered by poor definitions of renal dysfunction and a lack of early biomarkers of renal injury. In recent years, the definitional problem has been addressed with the establishment of a new well-defined diagnostic entity, acute kidney injury (AKI), which encompasses the wide spectrum of kidney dysfunction, together with clearer definition and sub-classification of the cardio-renal syndromes. From the laboratory have emerged new biomarkers which allow early detection of AKI, including neutrophil gelatinase-associated lipocalin (NGAL) and cystatin C. This review describes the new concepts of AKI and the cardio-renal syndromes as well as novel biomarkers which allow early detection of AKI. Panels of AKI biomarker tests are likely to revolutionise the diagnosis and management of critically ill patients in the coming years. Earlier diagnosis and intervention should significantly reduce the morbidity and mortality associated with acute kidney damage.
Keywords: Acute kidney injury, Cardio-renal syndrome, Acute renal failure, Neutrophil gelatinase-associated lipocalin, Cystatin C, Creatinine, Renal function, Heart failure
INTRODUCTION
Changes in renal function are one of the most common manifestations of severe illness. Their importance is reflected in the routine physiological and biochemical monitoring of kidney function via urine output measures and blood laboratory measurements in critically ill patients. Despite improvements in health outcomes in many areas in recent years, mortality and morbidity rates associated with acute renal dysfunction remain high. There is a clinical need to intervene early with proven treatments in patients with potentially deleterious changes in renal function. Unfortunately progress has been hindered by poor definitions of renal dysfunction and a lack of early biomarkers of renal injury. In recent years, the definitional problem has been addressed with the establishment of a new well-defined diagnostic entity, acute kidney injury (AKI), which encompasses the wide spectrum of kidney dysfunction, together with clearer definition and sub-classification of the cardio-renal syndromes. From the laboratory have emerged new biomarkers which allow early detection of AKI, including neutrophil gelatinase-associated lipocalin (NGAL) and cystatin C. These new approaches offer increased hope that early effective treatments for AKI can improve the clinical outcomes of seriously ill patients. This review will describe the new concepts of AKI and the cardio-renal syndromes as well as novel biomarkers which allow early detection of AKI.
NEW DISEASE CONCEPTS
1. Acute kidney injury
The term "acute renal failure" was first used in 1951 by Homer Smith with reference to acute renal failure related to traumatic injury [1]. It has since entered the mainstream medical lexicon with over 18,000 PubMed references by the end of 2010. Despite its popularity, the term has suffered from a lack of clear definition which limited discussion in the area and has complicated comparisons of epidemiological and therapeutic studies. A variety of different prevalence figures have been reported, reflecting more than 35 different definitions of acute renal failure found in the literature [2]. This has led to a wide range of mortality rates from 27% to 60% associated with acute renal failure [3-6]. Generally the definitions have been based on absolute or relative concentration or changes in concentration of serum creatinine. Some have used extremely complicated criteria based on different increases in serum creatinine concentration from different baseline values [7, 8].
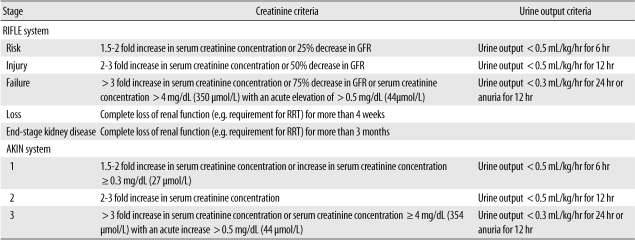
In an effort to improve consensus and allow the development of evidence-based guidelines for the management of acute kidney dysfunction, the Acute Dialysis Quality Initiative (ADQI) suggested a graded definition called the RIFLE criteria in 2004 [9, 10]. The RIFLE criteria uses three levels of injury (Risk, Injury and Failure) based on either serum creatinine concentration, glomerular filtration rate (GFR) or urine output and two levels of outcome (Loss and End-stage Renal Disease [ESRD]) based on the need for renal replacement therapy (RRT) and the time period. The different levels are shown in Table 1.
Table 1.
Acute kidney injury classification systems
RRT, renal replacement therapy.
The patient should be classified at the least favourable level resulting from assessment of the different variables (serum creatinine concentration, GFR and urine output). The diagnostic descriptor used should be "RIFLE-R", "RIFLE-I", etc. as appropriate. It has suggested that a subscript "o" should be added (e.g. RIFLE-Fo) if the classification results from urine output assessment and "c" to denote the presence of pre-existing renal disease [11].
The RIFLE criteria have been adopted by the nephrology community but some limitations have been highlighted in recent years. The choice of matching serum creatinine and urine output measures at each level was not evidence based and the predictive power of the creatinine and urine output criteria is not equivalent. One study has found the serum creatinine criteria of the classification seems to be a better predictor of mortality than urine output [12]. Use of the RIFLE criteria is limited in patients who present with AKI but without a baseline creatinine concentration. Back-calculation of a baseline creatinine concentration using the 4-parameter MDRD equation assuming a baseline GFR of 75 mL/min/1.73 m2 has been suggested to overcome this problem but further work to support this approach is needed [9].
In 2007 the Acute Kidney Injury Network (AKIN) suggested a modified set of criteria based on the RIFLE approach [13-15]. The term acute kidney "injury" as opposed to "failure" was used to reflect coverage of the full range of acute renal dysfunction and has been adopted as a Medical Subject Heading (MeSH) by the U.S. National Library of Medicine in 2011. AKIN proposed 3 separate stages for AKI which correspond to the RIFLE criteria; Stage 1 (Risk), Stage 2 (Injury) and Stage 3 (Failure). RIFLE levels Loss and Failure are considered outcomes rather than stages. The AKIN approach is shown in Table 1.
The diagnostic criteria should be used only following adequate resuscitation when applicable [15]. This is to exclude changes in creatinine concentration or urine output secondary to transient easily reversible fluid depletion. Exclusion of urinary tract obstruction is also suggested if oliguria is the only diagnostic criterion met. As a consequence, a patient with urinary tract obstruction and oliguria alone will not meet the AKI criteria while another with urinary tract obstruction and raised creatinine concentration will do so. Both the fluid replacement and urinary tract obstruction restrictions may exclude some of the traditional pre-renal and post-renal cases of acute renal failure from the AKI category. However, the main focus in developing the criteria was to allow for epidemiological and outcome comparisons between different sites and treatments, rather than for individual patient diagnosis and management. Hence these changes will result in arguably smaller but cleaner, more homogeneous groups that will simplify inter-study comparisons.
A cornerstone of both the RIFLE and AKIN criteria is the measurement of serum creatinine concentration as a marker of renal function. The shortcomings of serum creatinine concentration in reflecting GFR are well known. Serum creatinine is only useful in patients with stable renal function - in acute renal dysfunction, the GFR may be reduced but the serum creatinine concentration may be normal or low as there has been insufficient time for creatinine to accumulate [16]. Although creatinine freely filters across the glomerular membrane and is neither absorbed nor metabolised by the kidney, 10-40% of urinary creatinine is secreted by the proximal tubule into the urine [17]. As GFR falls in chronic kidney disease, the increase in serum creatinine can be blunted by increasing creatinine secretion [17-21]. Nephrotic syndrome can also lead to increased creatinine secretion and a reduction in serum creatinine concentration [22]. Creatinine concentrations may be lowered in advanced renal disease where intestinal bacterial overgrowth leads to increased bacterial creatininase activity and enhanced extrarenal creatinine elimination [23].
Creatinine production varies between individuals based on dietary intake and body habitus. There are sex and race differences in serum creatinine concentration reflecting differences in muscle mass and thus differences in the rates of creatinine production from muscle creatine. Men have higher serum creatinine concentrations than women and blacks have higher values than whites, who in turn have higher values than Malays, Indians and Chinese [24, 25]. Creatinine concentrations measured using the alkaline picrate method are prone to drug (e.g. cefoxitin, flucytosine) or metabolite (e.g. ketone) interference [26-30]. Drugs such as trimethoprim and cimetidine may decrease tubular creatinine secretion leading to elevation in serum creatinine concentration by up to 0.4-0.5 mg/dL (35-44 µmol/L) [31-33]. Creatinine is also removed by dialysis, limiting the usefulness of creatinine measurement once dialysis has begun.
In addition to serum creatinine concentration's limitations in reflecting changes in GFR, compensatory hypertrophy and hyperfiltration of unaffected glomeruli in cases of progressive glomerular loss may mean levels of both creatinine and GFR may not necessarily reflect the underlying renal injury [18, 19, 34]. Proteinuria, abnormalities of urine sediment, and systemic hypertension may be the only signs of such silent damage.
2. Cardio-renal syndrome
Another clinical concept whose definition has been refined in recent years is the cardio-renal syndrome (CRS). There is a close association between renal and cardiac function in both acute and chronic diseases. Cardiovascular disease causes over 50% of deaths in patients with renal failure while poor renal function increases mortality in patients with heart failure [35-37]. The term "cardio-renal syndrome" had been loosely used in the past to describe the relationship between renal and cardiac function but it was not until 2004 that the National Heart, Lung, and Blood Institute defined CRS as a condition in which therapy to relieve congestive symptoms of heart failure is limited by a decline in renal function as manifested by a reduction in glomerular filtration rate. Although this definition suggests a one-way effect of renal on cardiac function, further work has shown the interactions to work in both directions and in a variety of clinical conditions [38]. In 2008 the ADQI suggested the use of CRS to identify a disorder of the heart and kidneys whereby acute or chronic dysfunction in one organ may induce acute or chronic dysfunction in the other organ [39]. The ADQI report had similar goals to the RIFLE and AKIN initiatives in encouraging comparison between epidemiological and interventional studies as well as the development of diagnostic tools for the prevention and management of the different syndromes.
Five different subtypes of syndrome were identified:
Acute cardio-renal syndrome (type 1): acute worsening of heart function leading to kidney injury and/or dysfunction. Up to 40% of patients with acute decompensated heart failure develop AKI and fall into this category [40, 41]. AKI was defined as the secondary event using RIFLE-AKIN criteria.
Chronic cardio-renal syndrome (type 2): chronic abnormalities in heart function leading to kidney injury and/or dysfunction. Up to 63% of hospitalised patients with congestive heart failure fall into this category [42, 43]. Renal dysfunction was defined as the secondary event using KDOQI criteria [42].
Acute reno-cardiac syndrome (type 3): acute worsening of kidney function (AKI) leading to heart injury and/or dysfunction. AKI was defined as the primary event using RIFLE-AKIN criteria.
Chronic reno-cardiac syndrome (type 4): chronic kidney disease leading to heart injury, disease, and/or dysfunction. Excess cardiovascular deaths associated with increasing renal dysfunction have been estimated at over 50% [44]. Renal dysfunction was defined as the secondary event using KDOQI criteria [42].
Secondary CRS (type 5): systemic conditions (e.g. sepsis, systemic lupus erythematosus, diabetes mellitus, amyloidosis, or other chronic inflammatory conditions) leading to simultaneous injury and/or dysfunction of heart and kidney. AKI was defined as one of the possible secondary events using RIFLE-AKIN criteria.
As can be seen, identification of AKI is key to the definitions of types 1, 3 and 5 CRS. Adoption of the RIFLE-AKIN criteria enables common dialogue between the cardiology and nephrology communities but the reliance of the RIFLE and AKIN systems on serum creatinine measurement and its limitations discussed earlier remains. There is a need for new specific biomarkers that identify kidney injury early and that can replace serum creatinine in both the definition of AKI for epidemiological and study purposes as well as in guiding individual patient management.
BIOMARKERS OF ACUTE KIDNEY INJURY
In recent years, a number of novel biomarkers have been developed which promise earlier and more specific detection of acute kidney injury. Following their initial identification and preliminary work in animal and human AKI models, they are becoming available from a number of vendors for use in the clinical laboratory.
1. Neutrophil gelatinase-associated lipocalin
NGAL is arguably the most promising emerging bioma-rker for detection of AKI. It is composed of 8 β-strands that form a β-barrel enclosing a calyx which binds and transports low-molecular-weight substances [45]. In humans, NGAL is a 25 kDa 178 amino acid chain which is predominantly in a monomeric form. It is expressed by neutrophils and other epithelial cells including those in the proximal collecting tubule [46]. It appears to have a role in binding siderophores (small iron-carrying molecules) [47]. Studies in mouse models of renal ischemia reperfusion showed NGAL production to be highly up-regulated at an early stage and NGAL was detected in the urine within 2 hr following ischemia [48]. It has also been shown to be a marker of cisplatin nephrotoxicity in an animal model [49]. The physiological role of NGAL in this setting may be to decrease injury by reducing apoptosis and increasing the normal proliferation of kidney tubule cells. NGAL may also enhance delivery of iron and cause up-regulation of heme oxygenase-1, further protecting kidney tubule cells [48, 50-53]. There is the intriguing possibility of using NGAL as a renoprotective agent in acute ischemic renal injury. In a murine model of renal ischemia-reperfusion injury, intravenous administration of purified recombinant NGAL administered before, during, or after ischemia resulted in marked amelioration of the morphologic and functional consequences with a reduction in the number of apoptotic tubule cells and an increase in proliferating proximal tubule cells after ischemic injury [51].
Many clinical studies have demonstrated the ability of NGAL to allow early identification of AKI. A variety of clinical settings have been examined, including cardiac surgery [54-59], contrast procedures [60, 61], intensive care units [62-64] and the emergency department [65]. A representative study examined the performance of NGAL as an early biomarker for ischemic renal injury after cardiopulmonary bypass in 71 children undergoing cardiopulmonary bypass. Serial urine and blood samples were analysed by western blots and ELISA for NGAL expression. The primary outcome measure was acute renal injury, defined as a 50% increase in serum creatinine from baseline. 20 children (28%) developed acute renal injury, but diagnosis with serum creatinine was only possible 1-3 days after cardiopulmonary bypass. Urinary NGAL rose from a mean of 1.6 µg/L at baseline to 147 µg/L 2 hr after cardiopulmonary bypass, and the amount in the serum increased from a mean of 3.2 µg/L at baseline to 61 µg/L 2 hr after the procedure [59]. A recent systematic review and meta-analysis of the diagnostic and prognostic value of NGAL in AKI examined data from 19 studies and 8 countries involving 2,538 patients, of whom 19.2% developed AKI [66]. Overall, the area under the receiver operating curve (AUC) of NGAL to predict AKI was 0.815 (cardiac surgery patients: 0.775; critically ill patients: 0.728; after contrast infusion: 0.894). The diagnostic accuracy of blood NGAL (AUC 0.775) was similar to that of urine NGAL (AUC 0.837). NGAL had better predictive value in children (AUC 0.930) compared with adults (AUC 0.782). It was useful in predicting both renal replacement therapy (AUC 0.782) and in-hospital mortality (AUC 0.706).
NGAL measurement has now been commercialised and can be performed in both blood (Triage Meterpro System, Alere International Sàrl, Morges Switzerland) and urine samples (Abbott Architect, Abbott Laboratories, Abbott Park, Illinois, USA). Both urine and blood samples have their advantages and shortcomings. Blood NGAL measurements are invasive and may potentially reflect the effect of extra-renal disease on NGAL concentrations. However samples are readily available and the measurement can be performed rapidly on whole blood or plasma (15-20 min) on a point-of-care device. Urine sampling is non-invasive and there are less potentially interfering proteins present than in blood specimens. However disadvantages include the lack of available specimen in oliguric patients, the effect of over- or under-hydration and diuretic treatment on measured urinary NGAL concentrations and a longer analytical time on a laboratory-based analyser. This choice in sample type and platform allows the flexibility to offer the test in a variety of different clinical settings.
There are many scenarios where NGAL measurement could be useful, including critical illness, sepsis, oliguria, contrast procedures, cardiopulmonary bypass and polytrauma [56, 59-63, 67-69]. NGAL may be helpful in disease monitoring in other renal diseases, lupus nephritis, IgA nephropathy and polycystic kidney disease [70-73]. It could also be useful in non-renal conditions such as brain tumors, inflammatory bowel disease and pre-eclampsia [74-76]. NGAL appears to be an exciting marker of AKI but more work needs to be done to confirm its utility in routine clinical practice and to fine-tune the choice of appropriate cut-offs for different clinical settings and populations.
2. Cystatin C
Cystatin C is a 13.3 kDa low molecular weight member of the cystatin superfamily of cysteine protease inhibitors which is synthesised by all nucleated cells at a constant production rate. It is freely filtered at the glomerulus and is not reabsorbed, although it is metabolised by the renal tubules, which limits the utility of urinary measurement. Although it was purported to be unaffected by gender, age or muscle mass, more recent work has shown higher concentrations in men, those of greater height and weight, higher lean body mass and increasing age [77-79]. Studies over 20 yr have generally shown cystatin C to be a better predictor of GFR than creatinine. A meta-analysis of 49 studies covering 4,492 individuals showed cystatin C to have a greater AUC than creatinine (0.926 vs 0.837) in predicting GFR [80]. Cystatin C measurement allows earlier detection of AKI than serum creatinine measurement [81]. In 85 patients at high risk to develop AKI using RIFLE criteria, serum creatinine and cystatin C was measured daily. In the 44 patients who developed AKI, the increase of cystatin C (>50% over baseline) preceded that of creatinine by 1.5 days. A more recent study has shown cystatin C to rise significantly at 12 hr after pediatric cardiopulmonary bypass in patients who subsequently developed AKI [82]. Compared to NGAL, cystatin C rises later in AKI, with several studies in adults administered contrast showing an early rise in both urine (4 hr) and plasma (2 hr) NGAL compared to a later increase in cystatin C (8-24 hr) [83-85]. Cystatin C suffers from a lack of assay standardisation, which limits the generalisability of many study findings.
3. Other biomarkers
Kidney injury molecule-1 (KIM-1) is a transmembrane glycoprotein whose production is up-regulated in the proximal tubule in AKI [86-88]. It can be measured in urine and can help identify the etiology of AKI. Higher concentrations are seen in ischemic AKI compared to other forms of AKI and chronic kidney disease [89]. Urinary concentrations rise 6-12 hr following cardiopulmonary bypass in patients who subsequently develop AKI in a timeframe similar to that of NGAL [90].
Interleukin-18 (IL-18) is a mediator of inflammation which is induced in the proximal tubule and can be measured in urine as an early marker of AKI. Concentrations rise 6 hr after cardiopulmonary bypass in patients subsequently diagnosed with AKI [91]. It is more specific for ischemic insult and is unaffected by chronic kidney disease, urinary tract infections or nephrotoxins [92]. However the literature on its utility is mixed with a study of 100 patients undergoing cardiac surgery suggesting it was not valuable in identifying patients who develop AKI after cardiac surgery but was a nonspecific marker of cardiopulmonary bypass-associated systemic inflammation [93].
Other potential biomarkers include urinary tubular enzymes which are released from proximal tubular epithelial cells within 12 hr of AKI. They include N-acetyl-β-glucosa-minidase (NAG), proximal renal tubular epithelial antigen, α-glutathione S-transferase, π-glutathione S-transferase, γ-glutamyltranspeptidase, alanine aminopeptidase, lactate dehydrogenase, and alkaline phosphatase. Urinary low-molecular-weight proteins such as α1-microglobulin, β2-mic-roglobulin, retinol binding protein, adenosine deaminase binding protein are produced at different sites in the body, filtered at the glomerulus, and reabsorbed at the proximal tubule with no secretion. Although some of these analytes appear promising, clinical evaluations are limited and there is little published data on their specificity for AKI [94, 95].
4. Acute kidney injury panels
Of the many new biomarkers available, NGAL appears to be the most promising but it is likely to be most useful when combined with other markers such as cystatin C or KIM-1 to form an "AKI panel" [96-99]. Several studies have examined the use of such biomarkers in combination [88, 100, 101]. In a study of 100 adult cardiac surgical patients measuring NGAL and cystatin C as well as urea and creatinine, the new biomarkers were superior to the conventional measures in predicting AKI. Blood NGAL and cystatin C were independent predictors of AKI [100]. Another study measured KIM-1, NAG and NGAL in 90 adults undergoing cardiac surgery. The AUCs for KIM-1 to predict AKI immediately and 3 hr after operation were 0.68 and 0.65; 0.61 and 0.63 for NAG; and 0.59 and 0.65 for NGAL, respectively. Combining the three biomarkers enhanced the sensitivity of early detection of postoperative AKI compared with individual biomarkers: the AUCs for the three biomarkers combined were 0.75 and 0.78. The performance of combining biomarkers was even better among 16 early postoperative AKI patients with AUCs of 0.80 and 0.84, respectively [88].
CONCLUSION
The recent development of the RIFLE and AKIN criteria for AKI and the sub-classifications of CRS have prompted increased research and clinical interest in earlier and more specific markers of AKI. These tests are now becoming available in the clinical sphere and promise to change the paradigm for AKI diagnosis and management. Identification of AKI will no longer rely on functional markers such as serum creatinine concentration with its temporal and analytical shortcomings. The availability of panels of tests such as NGAL, cystatin C and KIM-1 are likely to revolutionise the diagnosis and management of critically ill patients in the coming years. Earlier diagnosis and intervention should significantly reduce the morbidity and mortality associated with acute kidney damage.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Smith HW. The kidney: structure and function in health and disease. New York: Oxford University Press; 1951. p. 764. [Google Scholar]
- 2.Kellum JA, Mehta RL, Angus DC, Palevsky P, Ronco C. The first international consensus conference on continuous renal replacement therapy. Kidney Int. 2002;62:1855–1863. doi: 10.1046/j.1523-1755.2002.00613.x. [DOI] [PubMed] [Google Scholar]
- 3.Liaño F, Pascual J Madrid Acute Renal Failure Study Group. Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Kidney Int. 1996;50:811–818. doi: 10.1038/ki.1996.380. [DOI] [PubMed] [Google Scholar]
- 4.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 5.Brivet FG, Kleinknecht DJ, Loirat P, Landais PJ French Study Group on Acute Renal Failure. Acute renal failure in intensive care units--causes, outcome, and prognostic factors of hospital mortality: a prospective, multicenter study. Crit Care Med. 1996;24:192–198. doi: 10.1097/00003246-199602000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Mehta RL. Outcomes research in acute renal failure. Semin Nephrol. 2003;23:283–294. doi: 10.1016/s0270-9295(03)00064-0. [DOI] [PubMed] [Google Scholar]
- 7.Hou SH, Bushinsky DA, Wish JB, Cohen JJ, Harrington JT. Hospital-acquired renal insufficiency: a prospective study. Am J Med. 1983;74:243–248. doi: 10.1016/0002-9343(83)90618-6. [DOI] [PubMed] [Google Scholar]
- 8.Mehta RL, Chertow GM. Acute renal failure definitions and classification: time for change? J Am Soc Nephrol. 2003;14:2178–2187. doi: 10.1097/01.asn.0000079042.13465.1a. [DOI] [PubMed] [Google Scholar]
- 9.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ronco C, Kellum JA, Mehta R. Acute dialysis quality initiative (ADQI) Nephrol Dial Transplant. 2001;16:1555–1558. doi: 10.1093/ndt/16.8.1555. [DOI] [PubMed] [Google Scholar]
- 11.Brenner BM. Brenner and Rector's the kidney. 8th ed. Philadelphia: Saunders; 2008. p. 943. [Google Scholar]
- 12.Cruz DN, Bolgan I, Perazella MA, Bonello M, de Cal M, Corradi V, et al. North east Italian prospective hospital renal outcome survey on Acute Kidney Injury (NEiPHROS-AKI): targeting the pro-blem with the RIFLE Criteria. Clin J Am Soc Nephrol. 2007;2:418–425. doi: 10.2215/CJN.03361006. [DOI] [PubMed] [Google Scholar]
- 13.Levin A, Warnock DG, Mehta RL, Kellum JA, Shah SV, Molitoris BA, et al. Improving outcomes from acute kidney injury: report of an initiative. Am J Kidney Dis. 2007;50:1–4. doi: 10.1053/j.ajkd.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Molitoris BA, Levin A, Warnock DG, Joannidis M, Mehta RL, Kellum JA, et al. Improving outcomes of acute kidney injury: report of an initiative. Nat Clin Pract Nephrol. 2007;3:439–442. doi: 10.1038/ncpneph0551. [DOI] [PubMed] [Google Scholar]
- 15.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rule AD, Bergstralh EJ, Slezak JM, Bergert J, Larson TS. Glomerular filtration rate estimated by cystatin C among different clinical presentations. Kidney Int. 2006;69:399–405. doi: 10.1038/sj.ki.5000073. [DOI] [PubMed] [Google Scholar]
- 17.Doolan PD, Alpen EL, Theil GB. A clinical appraisal of the plasma concentration and endogenous clearance of creatinine. Am J Med. 1962;32:65–79. doi: 10.1016/0002-9343(62)90183-3. [DOI] [PubMed] [Google Scholar]
- 18.Petri M, Bockenstedt L, Colman J, Whiting-O'Keefe Q, Fitz G, Sebastian A, et al. Serial assessment of glomerular filtration rate in lupus nephropathy. Kidney Int. 1988;34:832–839. doi: 10.1038/ki.1988.257. [DOI] [PubMed] [Google Scholar]
- 19.van Acker BA, Koomen GC, Koopman MG, de Waart DR, Arisz L. Creatinine clearance during cimetidine administration for measurement of glomerular filtration rate. Lancet. 1992;340:1326–1329. doi: 10.1016/0140-6736(92)92502-7. [DOI] [PubMed] [Google Scholar]
- 20.Kim KE, Onesti G, Ramirez O, Brest AN, Swartz C. Creatinine clearance in renal disease. A reappraisal. Br Med J. 1969;4:11–14. doi: 10.1136/bmj.4.5674.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levey AS. Measurement of renal function in chronic renal disease. Kidney Int. 1990;38:167–184. doi: 10.1038/ki.1990.182. [DOI] [PubMed] [Google Scholar]
- 22.Branten AJ, Vervoort G, Wetzels JF. Serum creatinine is a poor marker of GFR in nephrotic syndrome. Nephrol Dial Transplant. 2005;20:707–711. doi: 10.1093/ndt/gfh719. [DOI] [PubMed] [Google Scholar]
- 23.Dunn SR, Gabuzda GM, Superdock KR, Kolecki RS, Schaedler RW, Simenhoff ML. Induction of creatininase activity in chronic renal failure: timing of creatinine degradation and effect of antibiotics. Am J Kidney Dis. 1997;29:72–77. doi: 10.1016/s0272-6386(97)90010-x. [DOI] [PubMed] [Google Scholar]
- 24.Hawkins RC. Differences in serum creatinine concentration be tween Caucasians, Chinese, Indians and Malays. Clin Chim Acta. 2010;411:1393. doi: 10.1016/j.cca.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 25.Jones CA, McQuillan GM, Kusek JW, Eberhardt MS, Herman WH, Coresh J, et al. Serum creatinine levels in the US population: third National Health and Nutrition Examination Survey. Am J Kidney Dis. 1998;32:992–999. doi: 10.1016/s0272-6386(98)70074-5. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell EK. Flucytosine and false elevation of serum creatinine level. Ann Intern Med. 1984;101:278. doi: 10.7326/0003-4819-101-2-278_1. [DOI] [PubMed] [Google Scholar]
- 27.Saah AJ, Koch TR, Drusano GL. Cefoxitin falsely elevates creatinine levels. JAMA. 1982;247:205–206. [PubMed] [Google Scholar]
- 28.Molitch ME, Rodman E, Hirsch CA, Dubinsky E. Spurious serum creatinine elevations in ketoacidosis. Ann Intern Med. 1980;93:280–281. doi: 10.7326/0003-4819-93-2-280. [DOI] [PubMed] [Google Scholar]
- 29.Miller WG, Myers GL, Ashwood ER, Killeen AA, Wang E, Thienpont LM, et al. Creatinine measurement: state of the art in accuracy and interlaboratory harmonization. Arch Pathol Lab Med. 2005;129:297–304. doi: 10.5858/2005-129-297-CMSOTA. [DOI] [PubMed] [Google Scholar]
- 30.Soldin SJ, Henderson L, Hill JG. The effect of bilirubin and ketones on reaction rate methods for the measurement of creatinine. Clin Biochem. 1978;11:82–86. doi: 10.1016/s0009-9120(78)90028-0. [DOI] [PubMed] [Google Scholar]
- 31.Rocci ML, Jr, Vlasses PH, Ferguson RK. Creatinine serum concentrations and H2-receptor antagonists. Clin Nephrol. 1984;22:214–215. [PubMed] [Google Scholar]
- 32.Berg KJ, Gjellestad A, Nordby G, Rootwelt K, Djøseland O, Fauchald P, et al. Renal effects of trimethoprim in ciclosporin- and azathioprine-treated kidney-allografted patients. Nephron. 1989;53:218–222. doi: 10.1159/000185747. [DOI] [PubMed] [Google Scholar]
- 33.Hilbrands LB, Artz MA, Wetzels JF, Koene RA. Cimetidine improves the reliability of creatinine as a marker of glomerular filtration. Kidney Int. 1991;40:1171–1176. doi: 10.1038/ki.1991.331. [DOI] [PubMed] [Google Scholar]
- 34.Chagnac A, Kiberd BA, Fariñas MC, Strober S, Sibley RK, Hoppe R, et al. Outcome of the acute glomerular injury in proliferative lupus nephritis. J Clin Invest. 1989;84:922–930. doi: 10.1172/JCI114254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hillege HL, Nitsch D, Pfeffer MA, Swedberg K, McMurray JJ, Yusuf S, et al. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006;113:671–678. doi: 10.1161/CIRCULATIONAHA.105.580506. [DOI] [PubMed] [Google Scholar]
- 36.Smith GL, Lichtman JH, Bracken MB, Shlipak MG, Phillips CO, DiCapua P, et al. Renal impairment and outcomes in heart failure: systematic review and meta-analysis. J Am Coll Cardiol. 2006;47:1987–1996. doi: 10.1016/j.jacc.2005.11.084. [DOI] [PubMed] [Google Scholar]
- 37.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 38.Bock JS, Gottlieb SS. Cardiorenal syndrome: new perspectives. Circulation. 2010;121:2592–2600. doi: 10.1161/CIRCULATIONAHA.109.886473. [DOI] [PubMed] [Google Scholar]
- 39.Ronco C, McCullough P, Anker SD, Anand I, Aspromonte N, Bagshaw SM, et al. Cardio-renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur Heart J. 2010;31:703–711. doi: 10.1093/eurheartj/ehp507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gottlieb SS, Abraham W, Butler J, Forman DE, Loh E, Massie BM, et al. The prognostic importance of different definitions of worsening renal function in congestive heart failure. J Card Fail. 2002;8:136–141. doi: 10.1054/jcaf.2002.125289. [DOI] [PubMed] [Google Scholar]
- 41.Forman DE, Butler J, Wang Y, Abraham WT, O'Connor CM, Gottlieb SS, et al. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol. 2004;43:61–67. doi: 10.1016/j.jacc.2003.07.031. [DOI] [PubMed] [Google Scholar]
- 42.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 43.Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail. 2007;13:422–430. doi: 10.1016/j.cardfail.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 44.Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, et al. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17:2034–2047. doi: 10.1681/ASN.2005101085. [DOI] [PubMed] [Google Scholar]
- 45.Flower DR, North AC, Sansom CE. The lipocalin protein family: structural and sequence overview. Biochim Biophys Acta. 2000;1482:9–24. doi: 10.1016/s0167-4838(00)00148-5. [DOI] [PubMed] [Google Scholar]
- 46.Uttenthal LO. NGAL: a marker molecule for the distressed kidney? Clin Lab Int. 2005;29:39–41. [Google Scholar]
- 47.Schmidt-Ott KM, Mori K, Kalandadze A, Li JY, Paragas N, Nicholas T, et al. Neutrophil gelatinase-associated lipocalin-mediated iron traffic in kidney epithelia. Curr Opin Nephrol Hypertens. 2006;15:442–449. doi: 10.1097/01.mnh.0000232886.81142.58. [DOI] [PubMed] [Google Scholar]
- 48.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 49.Mishra J, Mori K, Ma Q, Kelly C, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin: a novel early urinary biomarker for cisplatin nephrotoxicity. Am J Nephrol. 2004;24:307–315. doi: 10.1159/000078452. [DOI] [PubMed] [Google Scholar]
- 50.Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest. 2005;115:610–621. doi: 10.1172/JCI23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mishra J, Mori K, Ma Q, Kelly C, Yang J, Mitsnefes M, et al. Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2004;15:3073–3082. doi: 10.1097/01.ASN.0000145013.44578.45. [DOI] [PubMed] [Google Scholar]
- 52.Kjeldsen L, Johnsen AH, Sengeløv H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993;268:10425–10432. [PubMed] [Google Scholar]
- 53.Herget-Rosenthal S. One step forward in the early detection of acute renal failure. Lancet. 2005;365:1205–1206. doi: 10.1016/S0140-6736(05)74787-5. [DOI] [PubMed] [Google Scholar]
- 54.Tuladhar SM, Puntmann VO, Soni M, Punjabi PP, Bogle RG. Rapid detection of acute kidney injury by plasma and urinary neutrophil gelatinase-associated lipocalin after cardiopulmonary bypass. J Cardiovasc Pharmacol. 2009;53:261–266. doi: 10.1097/FJC.0b013e31819d6139. [DOI] [PubMed] [Google Scholar]
- 55.Xin C, Yulong X, Yu C, Changchun C, Feng Z, Xinwei M. Urine neutrophil gelatinase-associated lipocalin and interleukin-18 predict acute kidney injury after cardiac surgery. Ren Fail. 2008;30:904–913. doi: 10.1080/08860220802359089. [DOI] [PubMed] [Google Scholar]
- 56.Wagener G, Jan M, Kim M, Mori K, Barasch JM, Sladen RN, et al. Association between increases in urinary neutrophil gelatinase-associated lipocalin and acute renal dysfunction after adult cardiac surgery. Anesthesiology. 2006;105:485–491. doi: 10.1097/00000542-200609000-00011. [DOI] [PubMed] [Google Scholar]
- 57.Bennett M, Dent CL, Ma Q, Dastrala S, Grenier F, Workman R, et al. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol. 2008;3:665–673. doi: 10.2215/CJN.04010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dent CL, Ma Q, Dastrala S, Bennett M, Mitsnefes MM, Barasch J, et al. Plasma neutrophil gelatinase-associated lipocalin predicts acute kidney injury, morbidity and mortality after pediatric cardiac surgery: a prospective uncontrolled cohort study. Crit Care. 2007;11:R127. doi: 10.1186/cc6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 60.Bachorzewska-Gajewska H, Malyszko J, Sitniewska E, Malyszko JS, Pawlak K, Mysliwiec M, et al. Could neutrophil-gelatinase-associated lipocalin and cystatin C predict the development of contrast-induced nephropathy after percutaneous coronary interventions in patients with stable angina and normal serum creatinine values? Kidney Blood Press Res. 2007;30:408–415. doi: 10.1159/000109102. [DOI] [PubMed] [Google Scholar]
- 61.Hirsch R, Dent C, Pfriem H, Allen J, Beekman RH, 3rd, Ma Q, et al. NGAL is an early predictive biomarker of contrast-induced nephropathy in children. Pediatr Nephrol. 2007;22:2089–2095. doi: 10.1007/s00467-007-0601-4. [DOI] [PubMed] [Google Scholar]
- 62.Makris K, Markou N, Evodia E, Dimopoulou E, Drakopoulos I, Ntetsika K, et al. Urinary neutrophil gelatinase-associated lipocalin (NGAL) as an early marker of acute kidney injury in critically ill multiple trauma patients. Clin Chem Lab Med. 2009;47:79–82. doi: 10.1515/CCLM.2009.004. [DOI] [PubMed] [Google Scholar]
- 63.Wheeler DS, Devarajan P, Ma Q, Harmon K, Monaco M, Cvijanovich N, et al. Serum neutrophil gelatinase-associated lipocalin (NGAL) as a marker of acute kidney injury in critically ill children with septic shock. Crit Care Med. 2008;36:1297–1303. doi: 10.1097/CCM.0b013e318169245a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zappitelli M, Washburn KK, Arikan AA, Loftis L, Ma Q, Devarajan P, et al. Urine neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in critically ill children: a prospective cohort study. Crit Care. 2007;11:R84. doi: 10.1186/cc6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nickolas TL, O'Rourke MJ, Yang J, Sise ME, Canetta PA, Barasch N, et al. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med. 2008;148:810–819. doi: 10.7326/0003-4819-148-11-200806030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54:1012–1024. doi: 10.1053/j.ajkd.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 67.Arnaoutakis GJ, Bihorac A, Martin TD, Hess PJ, Jr, Klodell CT, Ejaz AA, et al. RIFLE criteria for acute kidney injury in aortic arch surgery. J Thorac Cardiovasc Surg. 2007;134:1554–1560. doi: 10.1016/j.jtcvs.2007.08.039. discussion 60-1. [DOI] [PubMed] [Google Scholar]
- 68.Bagshaw SM, Bellomo R, Kellum JA. Oliguria, volume overload, and loop diuretics. Crit Care Med. 2008;36:S172–S178. doi: 10.1097/CCM.0b013e318168c92f. [DOI] [PubMed] [Google Scholar]
- 69.Wagener G, Gubitosa G, Wang S, Borregaard N, Kim M, Lee HT. Urinary neutrophil gelatinase-associated lipocalin and acute kidney injury after cardiac surgery. Am J Kidney Dis. 2008;52:425–433. doi: 10.1053/j.ajkd.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 70.Bolignano D, Coppolino G, Campo S, Aloisi C, Nicocia G, Frisina N, et al. Neutrophil gelatinase-associated lipocalin in patients with autosomal-dominant polycystic kidney disease. Am J Nephrol. 2007;27:373–378. doi: 10.1159/000103912. [DOI] [PubMed] [Google Scholar]
- 71.Suzuki M, Wiers KM, Klein-Gitelman MS, Haines KA, Olson J, Onel KB, et al. Neutrophil gelatinase-associated lipocalin as a biomarker of disease activity in pediatric lupus nephritis. Pediatr Nephrol. 2008;23:403–412. doi: 10.1007/s00467-007-0685-x. [DOI] [PubMed] [Google Scholar]
- 72.Parikh CR, Jani A, Mishra J, Ma Q, Kelly C, Barasch J, et al. Urine NGAL and IL-18 are predictive biomarkers for delayed graft function following kidney transplantation. Am J Transplant. 2006;6:1639–1645. doi: 10.1111/j.1600-6143.2006.01352.x. [DOI] [PubMed] [Google Scholar]
- 73.Brunner HI, Mueller M, Rutherford C, Passo MH, Witte D, Grom A, et al. Urinary neutrophil gelatinase-associated lipocalin as a biomarker of nephritis in childhood-onset systemic lupus erythematosus. Arthritis Rheum. 2006;54:2577–2584. doi: 10.1002/art.22008. [DOI] [PubMed] [Google Scholar]
- 74.D'Anna R, Baviera G, Giordano D, Todarello G, Corrado F, Buemi M. Second trimester neutrophil gelatinase-associated lipocalin as a potential prediagnostic marker of preeclampsia. Acta Obstet Gynecol Scand. 2008;87:1370–1373. doi: 10.1080/00016340802464463. [DOI] [PubMed] [Google Scholar]
- 75.Manfredi MA, Zurakowski D, Rufo PA, Walker TR, Fox VL, Moses MA. Increased incidence of urinary matrix metalloproteinases as predictors of disease in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:1091–1096. doi: 10.1002/ibd.20419. [DOI] [PubMed] [Google Scholar]
- 76.Smith ER, Zurakowski D, Saad A, Scott RM, Moses MA. Urinary biomarkers predict brain tumor presence and response to therapy. Clin Cancer Res. 2008;14:2378–2386. doi: 10.1158/1078-0432.CCR-07-1253. [DOI] [PubMed] [Google Scholar]
- 77.Macdonald J, Marcora S, Jibani M, Roberts G, Kumwenda M, Glover R, et al. GFR estimation using cystatin C is not independent of body composition. Am J Kidney Dis. 2006;48:712–719. doi: 10.1053/j.ajkd.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 78.Groesbeck D, Köttgen A, Parekh R, Selvin E, Schwartz GJ, Coresh J, et al. Age, gender, and race effects on cystatin C levels in US adolescents. Clin J Am Soc Nephrol. 2008;3:1777–1785. doi: 10.2215/CJN.00840208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Knight EL, Verhave JC, Spiegelman D, Hillege HL, de Zeeuw D, Curhan GC, et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004;65:1416–1421. doi: 10.1111/j.1523-1755.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 80.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40:221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 81.Herget-Rosenthal S, Marggraf G, Hüsing J, Göring F, Pietruck F, Janssen O, et al. Early detection of acute renal failure by serum cystatin C. Kidney Int. 2004;66:1115–1122. doi: 10.1111/j.1523-1755.2004.00861.x. [DOI] [PubMed] [Google Scholar]
- 82.Krawczeski CD, Vandevoorde RG, Kathman T, Bennett MR, Woo JG, Wang Y, et al. Serum cystatin C is an early predictive biomarker of acute kidney injury after pediatric cardiopulmonary bypass. Clin J Am Soc Nephrol. 2010;5:1552–1557. doi: 10.2215/CJN.02040310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ling W, Zhaohui N, Ben H, Leyi G, Jianping L, Huili D, et al. Urinary IL-18 and NGAL as early predictive biomarkers in contrast-induced nephropathy after coronary angiography. Nephron Clin Pract. 2008;108:c176–c181. doi: 10.1159/000117814. [DOI] [PubMed] [Google Scholar]
- 84.Bachorzewska-Gajewska H, Malyszko J, Sitniewska E, Malyszko JS, Dobrzycki S. Neutrophil-gelatinase-associated lipocalin and renal function after percutaneous coronary interventions. Am J Nephrol. 2006;26:287–292. doi: 10.1159/000093961. [DOI] [PubMed] [Google Scholar]
- 85.Bachorzewska-Gajewska H, Malyszko J, Sitniewska E, Malyszko JS, Dobrzycki S. Neutrophil gelatinase-associated lipocalin (NGAL) correlations with cystatin C, serum creatinine and eGFR in patients with normal serum creatinine undergoing coronary angiography. Nephrol Dial Transplant. 2007;22:295–296. doi: 10.1093/ndt/gfl408. [DOI] [PubMed] [Google Scholar]
- 86.Steiner RW. Interpreting the fractional excretion of sodium. Am J Med. 1984;77:699–702. doi: 10.1016/0002-9343(84)90368-1. [DOI] [PubMed] [Google Scholar]
- 87.Vaidya VS, Ramirez V, Ichimura T, Bobadilla NA, Bonventre JV. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol. 2006;290:F517–F529. doi: 10.1152/ajprenal.00291.2005. [DOI] [PubMed] [Google Scholar]
- 88.Han WK, Wagener G, Zhu Y, Wang S, Lee HT. Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol. 2009;4:873–882. doi: 10.2215/CJN.04810908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 90.Han WK, Waikar SS, Johnson A, Betensky RA, Dent CL, Devarajan P, et al. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int. 2008;73:863–869. doi: 10.1038/sj.ki.5002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Parikh CR, Mishra J, Thiessen-Philbrook H, Dursun B, Ma Q, Kelly C, et al. Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2006;70:199–203. doi: 10.1038/sj.ki.5001527. [DOI] [PubMed] [Google Scholar]
- 92.Parikh CR, Devarajan P. New biomarkers of acute kidney injury. Crit Care Med. 2008;36:S159–S165. doi: 10.1097/CCM.0b013e318168c652. [DOI] [PubMed] [Google Scholar]
- 93.Haase M, Bellomo R, Story D, Davenport P, Haase-Fielitz A. Urinary interleukin-18 does not predict acute kidney injury after adult cardiac surgery: a prospective observational cohort study. Crit Care. 2008;12:R96. doi: 10.1186/cc6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Herget-Rosenthal S, Poppen D, Hüsing J, Marggraf G, Pietruck F, Jakob HG, et al. Prognostic value of tubular proteinuria and enzymuria in nonoliguric acute tubular necrosis. Clin Chem. 2004;50:552–558. doi: 10.1373/clinchem.2003.027763. [DOI] [PubMed] [Google Scholar]
- 95.Trof RJ, Di Maggio F, Leemreis J, Groeneveld AB. Biomarkers of acute renal injury and renal failure. Shock. 2006;26:245–253. doi: 10.1097/01.shk.0000225415.5969694.ce. [DOI] [PubMed] [Google Scholar]
- 96.Devarajan P. Emerging biomarkers of acute kidney injury. Contrib Nephrol. 2007;156:203–212. doi: 10.1159/000102085. [DOI] [PubMed] [Google Scholar]
- 97.Ho E, Fard A, Maisel A. Evolving use of biomarkers for kidney injury in acute care settings. Curr Opin Crit Care. 2010;16:399–407. doi: 10.1097/MCC.0b013e32833e10bc. [DOI] [PubMed] [Google Scholar]
- 98.Soni SS, Ronco C, Katz N, Cruz DN. Early diagnosis of acute kidney injury: the promise of novel biomarkers. Blood Purif. 2009;28:165–174. doi: 10.1159/000227785. [DOI] [PubMed] [Google Scholar]
- 99.Nguyen MT, Devarajan P. Biomarkers for the early detection of acute kidney injury. Pediatr Nephrol. 2008;23:2151–2157. doi: 10.1007/s00467-007-0470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Haase-Fielitz A, Bellomo R, Devarajan P, Story D, Matalanis G, Dragun D, et al. Novel and conventional serum biomarkers predicting acute kidney injury in adult cardiac surgery--a prospective cohort study. Crit Care Med. 2009;37:553–560. doi: 10.1097/CCM.0b013e318195846e. [DOI] [PubMed] [Google Scholar]
- 101.Liangos O, Tighiouart H, Perianayagam MC, Kolyada A, Han WK, Wald R, et al. Comparative analysis of urinary biomarkers for early detection of acute kidney injury following cardiopulmonary bypass. Biomarkers. 2009;14:423–431. doi: 10.1080/13547500903067744. [DOI] [PMC free article] [PubMed] [Google Scholar]