Abstract
Rhabdomyolysis-induced renal failure represents up to 15% of all cases of acute renal failure. Many studies over the past four decades have demonstrated that accumulation of myoglobin in the kidney is central in the mechanism leading to kidney injury. However, some discussion exists regarding the mechanism mediating this oxidant injury. Although free iron-catalyzed fenton reaction has been proposed to explain the tissue injury, more recent evidence strongly suggests that the main cause of oxidant injury is myoglobin redox cycling and generation of oxidized lipids. These molecules can propagate tissue injury and cause renal vasoconstriction, two of the three main conditions associated with acute renal failure. This review presents the evidence supporting the two mechanisms of oxidative injury, describes the central role of myoglobin redox cycling in the pathology of renal failure associated with rhabdomyolysis, and discuss the value of therapeutic interventions aiming at inhibiting myoglobin redox cycling for the treatment of rhabdomyolysis-induced renal failure.
Introduction
Rhabdomyolysis affects about 1 in 10,000 persons in the USA and accounts for an estimated 8 to 15 % of all cases of acute renal failure (www.rhabdomyolysis.org). About 5% of rhabdomyolysis cases result in death (about 1,500 for the USA). Some data suggests that the number of patients with rhabdomyolysis following trauma may be underestimated and that the current treatments may not improve the outcome in a large proportion of patients with renal failure following rhabdomyolysis [1]. In this review we will describe the current treatments and discuss possible new therapeutic venues derived from recent new advances in the understanding of the pathophysiology of rhabdomyolysis–induced renal failure.
Rhabdomyolysis
Rhabdomyolysis, the breakdown of striated muscle, results in the release of potentially toxic compounds in the circulation that may affect kidney function. The syndrome has been recognized for a few thousands years (Old Testament, Book of Numbers, Ch. 11, verses 31–35). However, the link between renal failure and rhabdomyolysis was clearly established for the first time by the classic description of the crush syndrome following London bombardment during the Second World War [2]. The authors later determined the presence of abnormal levels of myoglobin (Mb) in the urine of these patients [3].
Etiology
The causes of rhabdomyolysis are diverse ranging from crush injury to genetic disorders or drug induced (Table 1). These disorders can by due to mechanical or electrical trauma [4–8], muscle injury following intense exercise, heatstroke, malignant hyperthermia, seizure or long term immobilization [8–16]. Lysis of myocytes can also occur following ischemia [17–20] or be caused by metabolic disorders leading to hypokalemia, hypernatremia or hypophosphatemia [21–26]. Many drugs administered in overdose but also in chronic normal dose administration have been shown to cause rhabdomyolysis [27–45]. Finally, genetic disorders leading to deregulations of enzymes involved in metabolic pathways have also been linked to rhabdomyolysis [46–50].
Table 1. Causes of rhabdomyolysis.
(www.nlm.nih.gov/medlineplus, for review Khan, 2009, NJM, 272).
| Crush injury | |
| Muscle injuries | Severe exertion, muscle ischemia, heat stroke, prolonged immobilization. |
| Drugs | Cocaine, heroin, alcohol, statins. |
| Electrolyte imbalance | Hyponatremia, hypokalemia and hypophosphatemia. |
| Genetic disorders | Deficiencies of glycolytic enzymes, abnormal lipid metabolism and other. |
Pathophysiology
Although the causes are numerous, the etiology of the disease results from the lysis of the myocytes and the release of their content in the circulation (Table 1, for review see [51, 52]). Following lysis of myocytes, large amounts of salts, enzymes (aldolase, Creatine kinase (CK), lactate dehydrogenase) and Mb, an 18,800 Da oxygen carrier [53–55] are released in the circulation. Circulating Mb then becomes deposited in the kidney causing renal tubular obstruction and necrosis, which is accompanied by intense renal vasoconstriction [51, 56–59].
Renal failure
Although it is well accepted that renal failure is caused by Mb deposition in the kidney, the mechanism by which it occurs is still debated. Over time, number of mechanisms have been proposed to explain the pathophysiology of rhabdomyolysis-induced renal failure, including decreased delivery of blood to the glomerulus [56, 60], reduced glomerular filtration rate [61], leakage of filtrate across a damaged tubular epithelium [62] or tubular obstruction by Mb casts [62]. The current consensus is that renal failure is due to the combined effects of hypovolemia, aciduria, and direct cytotoxicity due to accumulation of renal tubular Mb (for review, see [63, 64]).
Oxidant injury and rhabdomyolysis-induced renal failure
There is accumulating evidence for a causative role of Mb-mediated oxidative injury to the kidney in the development of rhabdomyolysis-induced renal failure. Two hypotheses have been proposed to explain the mechanism by which Mb can cause oxidative injury to the kidney: 1) release of free iron from the Mb, catalyzing Fenton reactions, and 2) Mb redox cycling induced lipid peroxidation. In this section we will analyze the merit of both theories.
Free iron-mediated Fenton reaction
Most data suggested that free iron released from Mb degradation in the kidney is involved in the generation of oxidative species through catalysis of Fenton reaction. The strongest evidence for this mechanism came from data showing that desferioxamine, an iron chelator, decreased rhabdomyolysis-induced renal injury in the rat [59] and prevented cell toxicity induced by direct exposure to Mb [65, 66]. Although the role of free iron in renal injury can not be totally excluded, there are reasons to think that this is unlikely to be a major contributor. First, several mechanisms are in place in the body to bind free iron and prevent its toxic effect in vivo. Experiments designed to measure formation of hydroxyl radical (•OH), the reactive oxygen species produced by the Fenton reaction, in proximal tubular segments of the rat model of glycerol-induced rhabdomyolysis demonstrated that levels of OH• are decreased after glycerol injection compared with control animals [67]. Using cultured kidney cells subjected to Mb, Zager et al. demonstrated that compounds that scavenge •OH were unable to protect the cells from the Mb-induced injury [66].
Cytochrome P-450s present in the kidney were proposed as an other source for free iron [68, 69]. This hypothesis was based on observations that kidney content of P-450 was decreased following rhabdomyolysis in rats and that P-450 specific inhibitors decreased tissue injury [68]. Although several lines of evidence suggest that P-450s are a source of free iron in chronic kidney disease such as minimal change nephrotic syndrome [70–73], their roles in rhabdomyolysis-induced renal failure is more controversial [68, 69, 74].
The effects of deferoxamine observed both in vivo and in cells in culture [59, 65, 66] can be explained in view of the evidence that deferoxamine is known to be a reductant of ferryl Mb [75, 76], the species responsible for the pseudo-peroxidase activity of Mb which mediates lipid peroxidation (see section below). Finally, degradation of hemoproteins by induction of heme oxygenase is known to prevent renal failure following induction of rhabdomyolysis in the rat [77, 78] and following injection of hemoglobin in mouse [79], processes that release the heme from the proteins. Not only does this evidence support a role for Mb in rhabdomyolysis-induced renal failure, it also argues against the role of free iron in the oxidative damage, as degradation of the heme by heme oxygenase generates free iron [80].
Myoglobin redox cycling and lipid peroxidation
The normal function of Mb is to transport oxygen in muscles. It does so because of its ability, in its ferrous form (FeII), to bind reversibly molecular oxygen. In pathologic conditions in which this hemoprotein is released from the reducing environment of cells, the ferrous heme undergoes oxidation to the ferric state (FeIII). In the presence of peroxides, such as hydrogen peroxide or lipid hydroperoxides, hemoproteins are oxidized to the ferryl form (FeIV=O), which often accompanied by the formation of a globin-based free radical [81–84]. The ferryl form of these hemoproteins can generate lipid-based radical species through abstraction of a lipid hydrogen atom and the globin radical can propagate to exogenous substrates [85]. In the absence of antioxidants, these reactions can initiate a cascade in which free and membrane-bound lipids become oxidized (Figure 1).
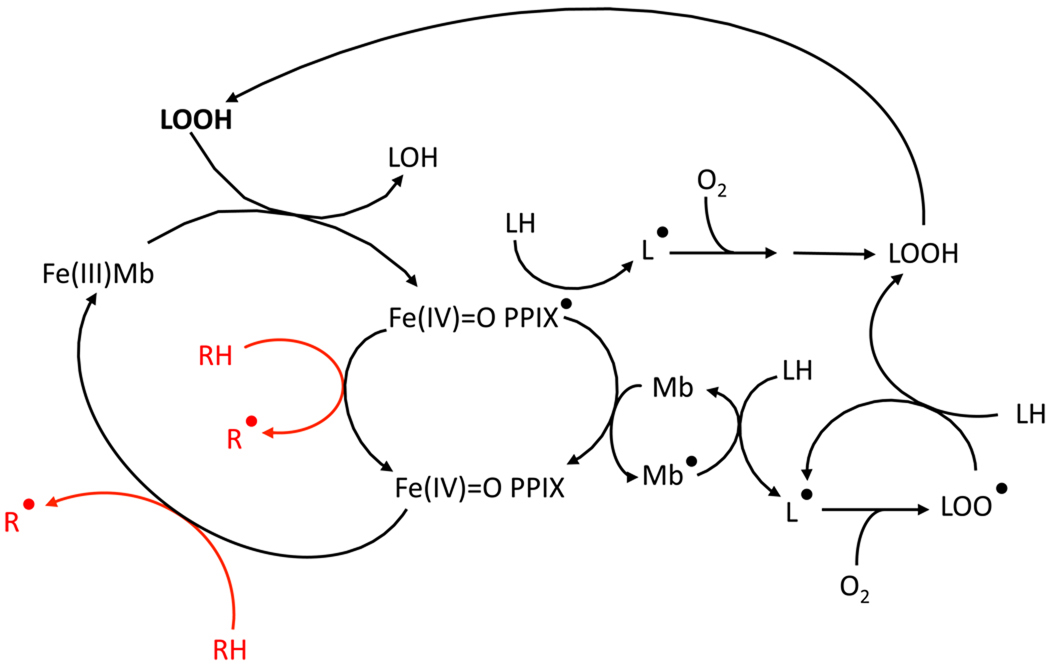
Figure 1. Mechanism for Mb-induced lipid peroxidation and its inhibition by acetaminophen.
Oxidation of ferric Mb (Fe(III)Mb) by a lipid hydroperoxide (LOOH) yields the ferryl Mb protoporphyrin radical (Fe(IV)=O PPIX •). This radical can delocalize to the protein to form a globin radical (Mb •), which can in turn catalyze lipid peroxidation by abstracting an electron from a lipid (LH). The lipid radical (L •) can then react to molecular oxygen to form the peroxyl radical (LOO •), which can propagate lipid peroxidation. Reducing co-substrates such as ApAP (RH) reduce the ferryl Mb back to its ferric state, thus inhibiting Mb-catalyzed lipid peroxidation.
Several lines of evidence support the role of Mb redox cycling in the development of rhabdomyolysis-induced renal failure. Mb by itself can catalyze peroxidation of arachidonic acid [81]. The high levels of Mb accumulating in the kidney have been shown to cause lipid peroxidation in that target organ as reflected by markedly increased F2-isoprostanes (F2-IsoPs) excretion in the urine and increased levels of F2-IsoPs in the kidney following rhabdomolysis in the rat [74]. Moreover, the urine of humans with rhabdomyolysis contains increased levels of F2-IsoPs and hemeprotein crosslinks of Mb [86]. These data unambiguously demonstrate that Mb redox cycling occurs in the kidney of patients with rhabdmyolysis, as the heme-protein crosslink only forms by the reaction of the ferryl heme and the globin radical [87].
Supporting evidence for the role of Mb redox cycling in renal failure is also shown by the attenuation of renal failure by alkalinization [74] a process that is known to inhibit Mb redox cycling [74, 88]. Classically the beneficial effects of alkalinization were thought to be due to enhanced solubilization of Mb, which increased the excretion of Mb from the kidney. Moreover, arachidonic acid oxidation catalyzed by Mb has been shown to be increased at acidic pH [89]. Inhibitors of lipid peroxidation also has been shown to protect rhabdomyolysis-induced injury to the kidney [90] and deferoxamine can inhibit lipid peroxidation by reducing ferryl Mb to its ferric state [75, 91, 92] without chelating free iron [91]. All these data support the mechanism by which ferric Mb deposited in the kidney undergoes redox cycling by reacting with peroxides present in the milieu, which in turn induces an oxidant injury to the kidney as a result of enhanced lipid peroxidation.
Further support for this hypothesis that Mb redox cycling is responsible for renal injury associated with rhabdomyolysis comes from the recent evidence that acetaminophen (ApAP) inhibits lipid peroxidation and significantly improves renal function in a rat model of rhabdomyolysis, with a reduction in the amount of structural renal damage [93]. The basis for considering that ApAP might inhibit the pseudoperoxidase-initiated formation of hemeprotein radicals derived from our work that addressed the cellular selectivity of inhibition of the prostaglandin H synthases (PGHSs) by ApAP. The PGHSs are bifunctional enzymes that contain both cyclooxygenase and peroxidase catalytic sites [94]. The catalytic activity is initiated by reduction of a hydroperoxide in the peroxidase site leading to formation of a protoporphyrin radical cation similar to what happens with Mb. Abundant evidence indicates that ApAP is a reducing cosubstrate of the PGHS peroxidase [95–98]. ApAP also has been found to inhibit myeloperoxidase-induced lipid peroxidation [99]. The analogy of the PGHS catalytic cycle and that of ferryl Mb-mediated lipid peroxidation led us to hypothesize that ApAP may be able to reduce ferryl Mb and prevent its redox cycling, similarly to what happens in PGHS (Figure 1). In this manuscript [93], we demonstrated that ApAP inhibits the oxidation of free arachidonic acid catalyzed by Mb in vitro, via reduction of ferryl Mb to its ferric state. All the in vitro experiments were done in presence of EDTA, a free-iron chelator, ruling out the participation of free-iron in this process. We also have shown that ApAP prevents formation of the protein radicals, which result from incubation of Mb with hydrogen peroxide as well as the formation of heme-protein crosslinks. We then showed that treatment of rats with ApAP before or after induction of rhabdomyolysis by glycerol injection in skeletal muscle, led to a decrease urinary F2-isoprostanes levels as well as inhibition of heme-protein crosslinks in the urine of the animals when compared to controls. Notably, heme-protein crosslinking can only occur if Mb has passed through the ferryl state [87]. Taken together, our results clearly demonstrate that compounds that can reduce the ferryl form of Mb can be used in vivo to protect the kidney from injury following rhabdomyolysis, establishing a basis for new therapeutic hypotheses.
Current treatments
The standard of care of rhabdomyolysis is aggressive IV rehydration with or without addition of mannitol and bicarbonate. Mannitol is thought to protect the kidney by decreasing the intratubular accumulation of Mb while bicarbonate increases the urine pH above 6.5, which is thought to help solubilize Mb thus enhancing its excretion from the kidney. This treatment was first described in 1984 by Ron et al. in patients suffering from crush injury [100]. However this study did not include a control group, thus undermining its interpretation. Since then, several studies, both prospective and retrospective, suggested that large volume infusion of fluid with balanced salt solution is sufficient to protect the kidney from injury and that bicarbonate seems to be unnecessary [65, 101, 102]. In 2004 in a retrospective study evaluating over 2,000 cases, Brown et al. found that patients with CK levels over 5,000 U/l do not benefit from administration of bicarbonate and mannitol. The authors concluded that “the standard of administering bicarbonate and mannitol to patients with post-traumatic rhabdomyolysis should be reevaluated” [1]. The conclusion of that study stresses the necessity to explore new therapeutic venues for the prevention of rhabdomyolysis-induced renal failure.
New hypothetical pharmacologic interventions
The three major mechanisms by which Mb induces acute renal failure include renal vasoconstriction, tubular obstruction by Mb casts and lipid peroxidation-mediated tubular injury. Tubular obstruction seems to be a result of the kidney injury [65, 101, 102] and, as such, may not necessitate specific interventions. On the other hand, targeting either of the other two mechanisms or both together may have profound effects against acute renal failure.
Inhibition of glomerular vasoconstriction
One of the other two mechanisms participating in the development of acute renal failure is the decrease in blood flow in the glomerulus (Figure 2). The current treatment with large volume infusion of fluid with balanced salts supports the idea that restoring glomerular filtration and flushing the intra-tubular Mb helps protecting the kidney.
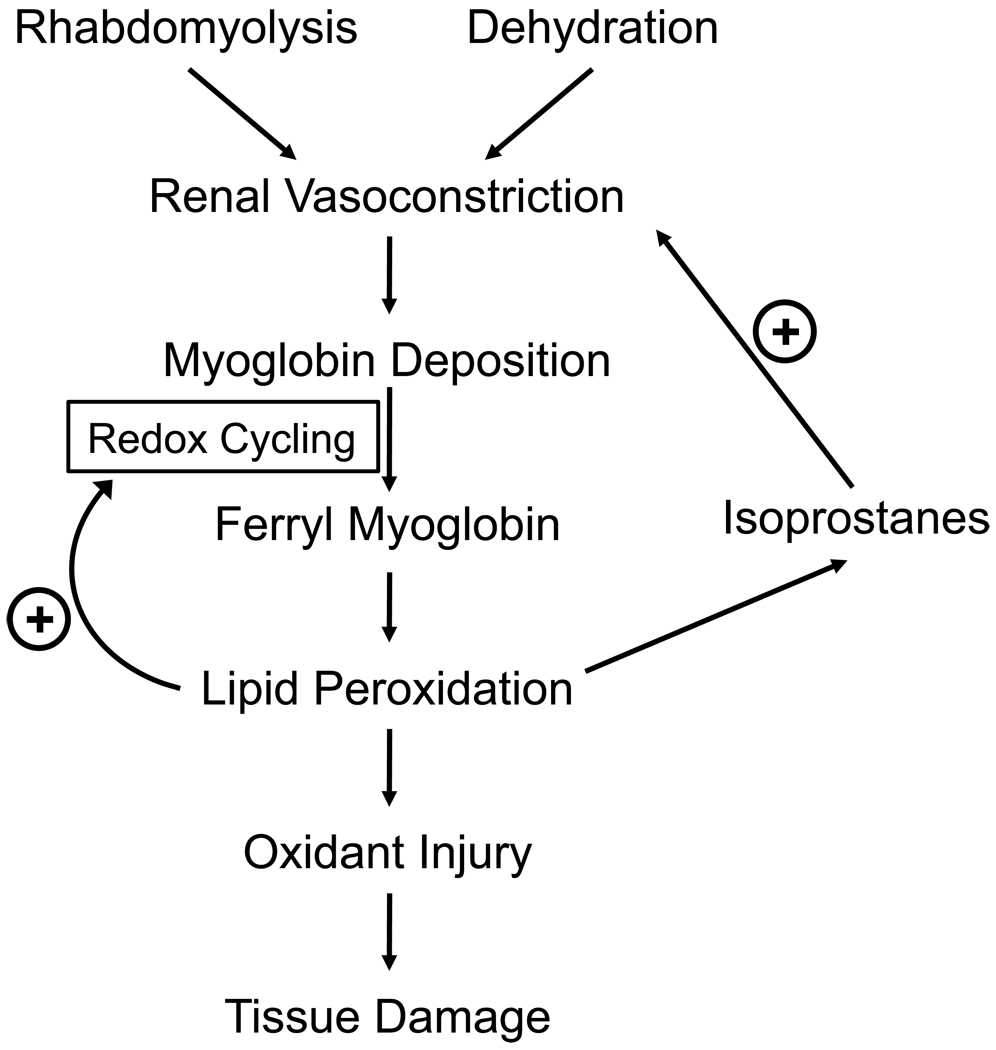
Figure 2. Pathophysiologic mechanism leading to kidney injury following rhabdomyolysis.
Release of Mb following rhabdomyolysis associated with hypovolemia induces renal vasoconstriction and Mb deposition in the kidney. Mb gets oxidized to its ferryl state inducing lipid peroxidation. Products of lipid peroxidation play a central role in the pathology via three different mechanisms: 1) the exacerbate lipid peroxidation by increasing the rate of formation of ferryl Mb; 2) they contribute to the oxidative injury of the kidney; and 3) they cause vasoconstriction preventing normal function of the kidney.
Several vasoactive factors seem to play an important role in modifying renal blood flow. Nitric oxide (NO) production is decreased during rhabdomyolydid-induced acute renal failure as measured by levels of urinary nitrite and kidney injury is increased by treatment of the animals with L-NAME (an inhibitor of NO synthesis) [103]. This can potentially be explained by scavenging of NO by Mb in the kidney. However, the kidneys are protected from injury by treatment with L-arginine (a precursor of NO) or by molsidomine (a NO donor) [103]. These results suggest that increasing renal production of NO may help protect the kidney against oxidant injury following rhabdomyolysis.
Overproduction of F2-isoprostanes in the kidney in rhabdomyolysis induced renal failure may also be an important contributor to the renal vasoconstriction. F2-isoprostanes [104] have been shown to be remarkably potent renal vasoconstrictors [105–107], which is mediated by activation of vascular thromboxane receptors [108]. The notion that F2-isoprostanes are an important of the renal vasoconstriction is supported by the findings that they are generated in the kidney following rhabdomyolysis both in an animal model [74] and in patients [109], and that thromboxane receptor antagonists have been shown to reduce renal vasoconstriction following rhabdomyolysis in the rat [110]. This evidence that Mb-induced isoprostanes can cause renal vasoconstriction, which can be prevented by antagonists of the thromboxane receptor, provides a mechanistic basis for developing additional therapeutic strategies to improve renal function in rhabdomyolysis induced renal failure.
Inhibition of myoglobin redox-cycling
Because the products of lipid peroxidation can cause both oxidant injury and vasoconstriction (figure 2), inhibiting Mb redox cycling might possibly represent the most efficient therapeutic intervention.
Lipid soluble antioxidants, if distributed to the target lipids, would be expected to break the chain reaction triggered by interaction of the hemoprotein with a lipid interface and could thereby attenuate the propagation of radicals that escape inhibition by a hemoprotein reductant. Although antioxidants would inhibit propagation of the free radical oxidation reaction, it would not eliminate the formation of fatty acid hydroperoxides that can serve as substrates for the pseudoperoxidase activity of Mb, sustaining the initiation phase of lipid-based radicals that would decrease the efficiency of the antioxidant. Indeed, the importance of hydroperoxides in the mechanism of renal failure caused by hemoproteins can be inferred from experiments demonstrating that scavenging hydrogen peroxide protects the kidney from injury following rhabdomyolysis induction in rats [111]. Similarly, a therapeutic intervention that increases the level in the aqueous phase of the glutathione peroxidase co-substrate, glutathione, could accelerate disposition of the lipid hydroperoxides and attenuate this additional positive feedback on radical generation. Thus, a therapy combining chain breaking antioxidants and glutathione precursor, such as N-acetyl cysteine, or hydrogen peroxyde scavengers may provide a synergistic effect on acute kidney failure.
Optimal therapy to inhibit lipid-peroxidation catalyzed by hemoproteins clinically may well be based on a drug with the properties to block radical initiation, with the likelihood that interruption of the more distal steps in the chain reaction by lipid soluble anti-oxidants could be additive or synergistic in inhibiting the feed-forward process of hemoprotein-catalyzed lipid peroxidation. The peroxidation of lipids by Mb is a multistep process, initiated by oxidation of hemoproteins in an aqueous environment that is accessible poorly if at all by highly lipid soluble antioxidants such as vitamin E. Thus, aqueous soluble inhibitors of formation of hemoprotein radicals may be optimal for blocking the initiation of the radical cascade.
Our findings that ApAP can inhibit oxidative injury of the kidney and protect rats from renal failure following rhabdolyolysis provides proof of the concept that the inhibition of Mb-induced lipid peroxidation can alter a pathophysiologic process in vivo. Importantly, this effect was seen when ApAP was given not only before induction of rhabdomyolysis but also when administered after rhabdomyolysis was induced [93]. Although ApAP is the only compound presently available for human use with a potency within its range of safety, many compounds that are peroxidase substrates [98, 112] may potentially exert similar effects as ApAP.
Conclusions
In conclusion, this review describes the central role of Mb redox cycling in the pathology of renal failure associated with rhabdomyolysis. The standard of care for rhabdomyolysis-induced renal failure consists in aggressive rehydration and may be sufficiently effective for patients with a mild form of the disease. However, the evidence suggests that pharmacologic agents that inhibit Mb redox cycling may represent the best additive therapeutic intervention for patients with a more severe form of this disease. The report that ApAP prevents renal failure in an animal model of rhabdomyolysis-induced renal failure at plasma concentrations that are within the therapeutic concentrations in humans, provides the basis for future studies aiming at evaluating the therapeutic value of ApAP in treating patients with rhabdomyolysis. The data also supports the development of novel drugs that would be better reducing agents and/or have less hepatotoxicity than ApAP. Finally, although this review focuses specifically on rhabdomyolysis-induced renal failure, the approach described above could be extended to other conditions caused by hemoprotein-mediated oxidant injury such as myocardial infarction, subarachnoid hemorrhage, Plasmodium falciparum malaria, and sickle cell disease.
Acknowledgement
This work was funded in part by grants GM15431 and GM42056 from the National Institutes of Health.
List of Abbreviations
- ApAP
acetaminophen
- CK
Creatine kinase
- EDTA
Ethylenediaminetetraacetic acid
- F2-IsoPs
F2-isoprostanes
- L-NAME
N (G)-nitro-L- arginine methyl ester
- NO
Nitric oxide
- OH•
hydroxyl radical
- PGHSs
prostaglandin H synthases
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brown CV, Rhee P, Chan L, Evans K, Demetriades D, Velmahos GC. Preventing renal failure in patients with rhabdomyolysis: do bicarbonate and mannitol make a difference? J. Trauma. 2004;56:1191–1196. doi: 10.1097/01.ta.0000130761.78627.10. [DOI] [PubMed] [Google Scholar]
- 2.Bywaters EG, Beall D. Crush injuries with impairment of renal function. Br. Med. J. 1941;1:427–432. doi: 10.1136/bmj.1.4185.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bywaters EG, Delory GE, Rimington C, Smiles J. Myohaemoglobin in the urine of air raid casualties with crushing injury. Biochem. J. 1941;35:1164–1168. doi: 10.1042/bj0351164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malinoski DJ, Slater MS, Mullins RJ. Crush injury and rhabdomyolysis. Crit. Care Clin. 2004;20:171–192. doi: 10.1016/s0749-0704(03)00091-5. [DOI] [PubMed] [Google Scholar]
- 5.Brumback RA, Feeback DL, Leech RW. Rhabdomyolysis following electrical injury. Semin. Neurol. 1995;15:329–334. doi: 10.1055/s-2008-1041040. [DOI] [PubMed] [Google Scholar]
- 6.Brown CV, Rhee P, Evans K, Demetriades D, Velmahos G. Rhabdomyolysis after penetrating trauma. Am. Surg. 2004;70:890–892. [PubMed] [Google Scholar]
- 7.Storey PA, Arrowsmith J, Burke FD. Re: Rhabdomyolysis following hand crush injury. J. Hand Surg. Eur. Vol. 2009;34:134–135. doi: 10.1177/17531934097488. [DOI] [PubMed] [Google Scholar]
- 8.Patel DR, Gyamfi R, Torres A. Exertional rhabdomyolysis and acute kidney injury. Phys. Sportsmed. 2009;37:71–79. doi: 10.3810/psm.2009.04.1685. [DOI] [PubMed] [Google Scholar]
- 9.Demos MA, Gitin EL, Kagen LJ. Exercise myoglobinemia and acute exertional rhabdomyolysis. Arch. Intern. Med. 1974;134:669–673. [PubMed] [Google Scholar]
- 10.Singh KV. Exercise induced rhabdomyolysis with acute renal failure. J. Indian Med. Assoc. 1983;81:97. [PubMed] [Google Scholar]
- 11.Gagliano M, Corona D, Giuffrida G, Giaquinta A, Tallarita T, Zerbo D, Sorbello M, Paratore A, Virgilio C, Cappellani A, Veroux P, Veroux M. Low-intensity body building exercise induced rhabdomyolysis: a case report. Cases J. 2009;2:7. doi: 10.1186/1757-1626-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fidler S, Fagan E, Williams R, Dewhurst I, Cory CE. Heatstroke and rhabdomyolysis presenting as fulminant hepatic failure. Postgrad. Med. J. 1988;64:157–159. doi: 10.1136/pgmj.64.748.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Messing ML, Feinzimer ET, Brosnan JJ, Rochester D. CT of rhabdomyolysis associated with malignant hyperthermia and seizures. Clin. Imaging. 1993;17:258–259. doi: 10.1016/0899-7071(93)90064-t. [DOI] [PubMed] [Google Scholar]
- 14.Capacchione JF, Muldoon SM. The relationship between exertional heat illness, exertional rhabdomyolysis, and malignant hyperthermia. Anesth. Analg. 2009;109:1065–1069. doi: 10.1213/ane.0b013e3181a9d8d9. [DOI] [PubMed] [Google Scholar]
- 15.Penn AS, Rowland LP, Fraser DW. Drugs, coma, and myoglobinuria. Arch. Neurol. 1972;26:336–343. doi: 10.1001/archneur.1972.00490100066006. [DOI] [PubMed] [Google Scholar]
- 16.Szewczyk D, Ovadia P, Abdullah F, Rabinovici R. Pressure-induced rhabdomyolysis and acute renal failure. J. Trauma. 1998;44:384–388. doi: 10.1097/00005373-199802000-00028. [DOI] [PubMed] [Google Scholar]
- 17.Sapin SO, Rosengart RM, Salem MM. Chest pain during stenting of a native aortic coarctation: a case for acute intercostal muscle ischemia and rhabdomyolysis. Catheter Cardiovasc. Interv. 2002;57:217–220. doi: 10.1002/ccd.10293. [DOI] [PubMed] [Google Scholar]
- 18.Williams JE, Jr, Tucker DB, Read JM., 3rd Rhabdomyolysis-myoglobinurea: consequences of prolonged tourniquet. J. Foot Surg. 1983;22:52–56. [PubMed] [Google Scholar]
- 19.Taylor DC, Salvian AJ, Shackleton CR. Crush syndrome complicating pneumatic antishock garment (PASG) use. Injury. 1988;19:43–44. doi: 10.1016/0020-1383(88)90178-7. [DOI] [PubMed] [Google Scholar]
- 20.Adiseshiah M, Round JM, Jones DA. Reperfusion injury in skeletal muscle: a prospective study in patients with acute limb ischaemia and claudicants treated by revascularization. Br. J. Surg. 1992;79:1026–1029. doi: 10.1002/bjs.1800791013. [DOI] [PubMed] [Google Scholar]
- 21.Singhal PC, Abramovici M, Venkatesan J, Mattana J. Hypokalemia and rhabdomyolysis. Miner. Electrolyte Metab. 1991;17:335–339. [PubMed] [Google Scholar]
- 22.Lucatello A, Sturani A, Di Nardo A, Fusaroli M. Acute renal failure in rhabdomyolysis associated with hypokalemia. Nephron. 1994;67:115–116. doi: 10.1159/000187899. [DOI] [PubMed] [Google Scholar]
- 23.Acquarone N, Garibotto G, Pontremoli R, Gurreri G. Hypernatremia associated with severe rhabdomyolysis. Nephron. 1989;51:441–442. doi: 10.1159/000185349. [DOI] [PubMed] [Google Scholar]
- 24.Vikrant S, Pandey D, Raina R, Sharma A. Deep vein thrombosis complicating severe hypernatremia, rhabdomyolysis, and acute renal failure in a patient with untreated seizure disorder. Clin. Exp. Nephrol. 2007;11:88–91. doi: 10.1007/s10157-006-0449-0. [DOI] [PubMed] [Google Scholar]
- 25.Knochel JP, Barcenas C, Cotton JR, Fuller TJ, Haller R, Carter NW. Hypophosphatemia and rhabdomyolysis. J. Clin. Invest. 1978;62:1240–1246. doi: 10.1172/JCI109244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar D, McAlister FA. Rhabdomyolysis complicating unrecognized hypophosphatemia in an alcoholic patient. Can. J. Gastroenterol. 1999;13:165–167. doi: 10.1155/1999/376034. [DOI] [PubMed] [Google Scholar]
- 27.Klock JC, Sexton MJ. Rhabdomyolysis and acute myoglobinuric renal failure following heroin use. Calif. Med. 1973;119:5–8. [PMC free article] [PubMed] [Google Scholar]
- 28.Deighan CJ, Wong KM, McLaughlin KJ, Harden P. Rhabdomyolysis and acute renal failure resulting from alcohol and drug abuse. QLM. 2000;93:29–33. doi: 10.1093/qjmed/93.1.29. [DOI] [PubMed] [Google Scholar]
- 29.Bessa O., Jr Alcoholic rhabdomyolysis: a review. Conn. Med. 1995;59:519–521. [PubMed] [Google Scholar]
- 30.Hojs R, Sinkovic A. Rhabdomyolysis and acute renal failure following methadone abuse. Nephron. 1992;62:362. doi: 10.1159/000187076. [DOI] [PubMed] [Google Scholar]
- 31.Crowe AV, Howse M, Bell GM, Henry JA. Substance abuse and the kidney. QLM. 2000;93:147–152. doi: 10.1093/qjmed/93.3.147. [DOI] [PubMed] [Google Scholar]
- 32.Horowitz BZ, Panacek EA, Jouriles NJ. Severe rhabdomyolysis with renal failure after intranasal cocaine use. J. Emerg. Med. 1997;15:833–837. doi: 10.1016/s0736-4679(97)00193-5. [DOI] [PubMed] [Google Scholar]
- 33.Wrenn KD, Oschner I. Rhabdomyolysis induced by a caffeine overdose. Ann. Emerg. Med. 1989;18:94–97. doi: 10.1016/s0196-0644(89)80323-3. [DOI] [PubMed] [Google Scholar]
- 34.Terada Y, Shinohara S, Matui N, Ida T. Amphetamine-induced myoglobinuric acute renal failure. Jpn. J. Med. 1988;27:305–308. doi: 10.2169/internalmedicine1962.27.305. [DOI] [PubMed] [Google Scholar]
- 35.Coco TJ, Klasner AE. Drug-induced rhabdomyolysis. Curr. Opin. Pediatr. 2004;16:206–210. doi: 10.1097/00008480-200404000-00017. [DOI] [PubMed] [Google Scholar]
- 36.Prendergast BD, George CF. Drug-induced rhabdomyolysis--mechanisms and management. Postgrad. Med. J. 1993;69:333–336. doi: 10.1136/pgmj.69.811.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khosla U, Ruel KS, Hunt DP. Antihistamine-induced rhabdomyolysis. South. Med. J. 2003;96:1023–1026. doi: 10.1097/01.SMJ.0000076461.67623.E4. [DOI] [PubMed] [Google Scholar]
- 38.Montgomery H, Porter JC, Bradley RD. Salicylate intoxication causing a severe systemic inflammatory response and rhabdomyolysis. Am. J. Emerg. Med. 1994;12:531–532. doi: 10.1016/0735-6757(94)90271-2. [DOI] [PubMed] [Google Scholar]
- 39.Terrovitou CT, Milionis HJ, Elisaf MS. Acute rhabdomyolysis after bezafibrate re-exposure. Nephron. 1998;78:336–337. doi: 10.1159/000044947. [DOI] [PubMed] [Google Scholar]
- 40.Smals AG, Beex LV, Kloppenborg PW. Clofibrate-induced muscle damage with myoglobinuria and cardiomyopathy. N. Engl. J. Med. 1977;296:942. doi: 10.1056/nejm197704212961617. [DOI] [PubMed] [Google Scholar]
- 41.Lim AK, Ho L, Levidiotis V. Quinine-induced renal failure as a result of rhabdomyolysis, haemolytic uraemic syndrome and disseminated intravascular coagulation. Intern. Med. J. 2006;36:465–467. doi: 10.1111/j.1445-5994.2006.01104.x. [DOI] [PubMed] [Google Scholar]
- 42.Jose J, Saravu K, Shastry BA. Atorvastatin-induced early-onset rhabdomyolysis in a patient with nephrotic syndrome. Am. J. Health. Syst. Pharm. 2007;64:726–729. doi: 10.2146/ajhp060241. [DOI] [PubMed] [Google Scholar]
- 43.Khan FY, Ibrahim W. Rosuvastatin induced rhabdomyolysis in a low risk patient: a case report and review of the literature. Curr. Clin. Pharmacol. 2009;4:1–3. doi: 10.2174/157488409787236056. [DOI] [PubMed] [Google Scholar]
- 44.Bakri R, Wang J, Wierzbicki AS, Goldsmith D. Cerivastatin monotherapy-induced muscle weakness, rhabdomyolysis and acute renal failure. Int. J. Cardiol. 2003;91:107–109. doi: 10.1016/s0167-5273(02)00581-8. [DOI] [PubMed] [Google Scholar]
- 45.Richards S, Umbreit JN, Fanucchi MP, Giblin J, Khuri F. Selective serotonin reuptake inhibitor-induced rhabdomyolysis associated with irinotecan. South. Med. J. 2003;96:1031–1033. doi: 10.1097/01.SMJ.0000084311.35864.D6. [DOI] [PubMed] [Google Scholar]
- 46.Lofberg M, Jankala H, Paetau A, Harkonen M, Somer H. Metabolic causes of recurrent rhabdomyolysis. Acta Neurol. Scand. 1998;98:268–275. doi: 10.1111/j.1600-0404.1998.tb07307.x. [DOI] [PubMed] [Google Scholar]
- 47.Poels PJ, Dolmans WM, Gabreels FJ. Rhabdomyolysis associated with malaria tertiana in a patient with myoadenylate deaminase deficiency. Trop. Geogr. Med. 1993;45:83–86. [PubMed] [Google Scholar]
- 48.Poels PJ, Gabreels FJ. Rhabdomyolysis: a review of the literature. Clin. Neurol. Neurosurg. 1993;95:175–192. doi: 10.1016/0303-8467(93)90122-w. [DOI] [PubMed] [Google Scholar]
- 49.Poels PJ, Wevers RA, Braakhekke JP, Benders AA, Veerkamp JH, Joosten EM. Exertional rhabdomyolysis in a patient with calcium adenosine triphosphatase deficiency. J. Neurol. Neurosurg. Psychiatry. 1993;56:823–826. doi: 10.1136/jnnp.56.7.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brumback RA, Feeback DL, Leech RW. Rhabdomyolysis in childhood. A primer on normal muscle function and selected metabolic myopathies characterized by disordered energy production. Pediatr. Clin. North Am. 1992;39:821–858. doi: 10.1016/s0031-3955(16)38377-8. [DOI] [PubMed] [Google Scholar]
- 51.Vanholder R, Sever MS, Erek E, Lameire N. Rhabdomyolysis. J Am Soc Nephrol. 2000;11:1553–1561. doi: 10.1681/ASN.V1181553. [DOI] [PubMed] [Google Scholar]
- 52.Zager RA. Rhabdomyolysis and myohemoglobinuric acute renal failure. Kidney Int. 1996;49:314–326. doi: 10.1038/ki.1996.48. [DOI] [PubMed] [Google Scholar]
- 53.Huerta-Alardin AL, Varon J, Marik PE. Bench-to-bedside review: Rhabdomyolysis -- an overview for clinicians. Crit. Care. 2005;9:158–169. doi: 10.1186/cc2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Knochel JP. Mechanisms of rhabdomyolysis. Curr. Opin. Rheumatol. 1993;5:725–731. doi: 10.1097/00002281-199305060-00006. [DOI] [PubMed] [Google Scholar]
- 55.Luck RP, Verbin S. Rhabdomyolysis: a review of clinical presentation, etiology, diagnosis, and management. Pediatr. Emerg. Care. 2008;24:262–268. doi: 10.1097/PEC.0b013e31816bc7b7. [DOI] [PubMed] [Google Scholar]
- 56.Ayer G, Grandchamp A, Wyler T, Truniger B. Intrarenal hemodynamics in glycerol-induced myohemoglobinuric acute renal failure in the rat. Circ. Res. 1971;29:128–135. doi: 10.1161/01.res.29.2.128. [DOI] [PubMed] [Google Scholar]
- 57.Kurtz TW, Maletz RM, Hsu CH. Renal cortical blood flow in glycerol-induced acute renal failure in the rat. Circ. Res. 1976;38:30–35. doi: 10.1161/01.res.38.1.30. [DOI] [PubMed] [Google Scholar]
- 58.Venkatachalam MA, Rennke HG, Sandstrom DJ. The vascular basis for acute renal failure in the rat. Preglomerular and postglomerular vasoconstriction. Circ. Res. 1976;38:267–279. doi: 10.1161/01.res.38.4.267. [DOI] [PubMed] [Google Scholar]
- 59.Paller MS. Hemoglobin- and myoglobin-induced acute renal failure in rats: role of iron in nephrotoxicity. Am. J. Physiol. 1988;255:F539–F544. doi: 10.1152/ajprenal.1988.255.3.F539. [DOI] [PubMed] [Google Scholar]
- 60.Chedru MF, Baethke R, Oken DE. Renal cortical blood flow and glomerular filtration in myohemoglobinuric acute renal failure. Kidney Int. 1972;1:232–239. doi: 10.1038/ki.1972.33. [DOI] [PubMed] [Google Scholar]
- 61.Cox JW, Baehler RW, Sharma H, O'Dorisio T, Osgood RW, Stein JH, Ferris TF. Studies of the mechanism of oliguria in a model of unilateral acute renal failure. J. Clin. Invest. 1974;53:1546–1558. doi: 10.1172/JCI107705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Flamenbaum W. Pathophysiology of acute renal failure. Arch. Intern. Med. 1973;131:911–928. [PubMed] [Google Scholar]
- 63.Khan FY. Rhabdomyolysis: a review of the literature. Neth. J. Med. 2009;67:272–283. [PubMed] [Google Scholar]
- 64.Zager RA. Acute renal failure in the setting of bone marrow transplantation. Kidney Int. 1994;46:1443–1458. doi: 10.1038/ki.1994.417. [DOI] [PubMed] [Google Scholar]
- 65.Zager RA. Combined mannitol and deferoxamine therapy for myohemoglobinuric renal injury and oxidant tubular stress. Mechanistic and therapeutic implications. J. Clin. Invest. 1992;90:711–719. doi: 10.1172/JCI115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zager RA, Burkhart K. Myoglobin toxicity in proximal human kidney cells: roles of Fe, Ca2+, H2O2, and terminal mitochondrial electron transport. Kidney Int. 1997;51:728–738. doi: 10.1038/ki.1997.104. [DOI] [PubMed] [Google Scholar]
- 67.Zager RA, Burkhart KM, Conrad DS, Gmur DJ. Iron, heme oxygenase, and glutathione: effects on myohemoglobinuric proximal tubular injury. Kidney Int. 1995;48:1624–1634. doi: 10.1038/ki.1995.457. [DOI] [PubMed] [Google Scholar]
- 68.Baliga R, Zhang Z, Baliga M, Shah SV. Evidence for cytochrome P-450 as a source of catalytic iron in myoglobinuric acute renal failure. Kidney Int. 1996;49:362–369. doi: 10.1038/ki.1996.53. [DOI] [PubMed] [Google Scholar]
- 69.Baliga R, Zhang Z, Shah SV. Role of cytochrome P-450 in hydrogen peroxide-induced cytotoxicity to LLC-PK1 cells. Kidney Int. 1996;50:1118–1124. doi: 10.1038/ki.1996.418. [DOI] [PubMed] [Google Scholar]
- 70.Ueda N, Baliga R, Shah SV. Role of 'catalytic' iron in an animal model of minimal change nephrotic syndrome. Kidney Int. 1996;49:370–373. doi: 10.1038/ki.1996.54. [DOI] [PubMed] [Google Scholar]
- 71.Liu H, Bigler SA, Henegar JR, Baliga R. Cytochrome P450 2B1 mediates oxidant injury in puromycin-induced nephrotic syndrome. Kidney Int. 2002;62:868–876. doi: 10.1046/j.1523-1755.2002.00515.x. [DOI] [PubMed] [Google Scholar]
- 72.Liu H, Shah SV, Baliga R. Cytochrome P-450 as a source of catalytic iron in minimal change nephrotic syndrome in rats. Am. J. Physiol. Renal Physiol. 2001;280:F88–F94. doi: 10.1152/ajprenal.2001.280.1.F88. [DOI] [PubMed] [Google Scholar]
- 73.Liu H, Baliga M, Bigler SA, Baliga R. Role of cytochrome P450 2B1 in puromycin aminonucleoside-induced cytotoxicity to glomerular epithelial cells. Nephron Exp. Nephrol. 2003;94:e17–e24. doi: 10.1159/000070815. [DOI] [PubMed] [Google Scholar]
- 74.Moore KP, Holt SG, Patel RP, Svistunenko DA, Zackert W, Goodier D, Reeder BJ, Clozel M, Anand R, Cooper CE, Morrow JD, Wilson MT, Darley-Usmar V, Roberts LJ., 2nd. A causative role for redox cycling of myoglobin and its inhibition by alkalinization in the pathogenesis and treatment of rhabdomyolysis-induced renal failure. J. Biol. Chem. 1998;273:31731–31737. doi: 10.1074/jbc.273.48.31731. [DOI] [PubMed] [Google Scholar]
- 75.Rice-Evans C, Okunade G, Khan R. The suppression of iron release from activated myoglobin by physiological electron donors and by desferrioxamine. Free Radic. Res. Commun. 1989;7:45–54. doi: 10.3109/10715768909088161. [DOI] [PubMed] [Google Scholar]
- 76.Reeder BJ, Hider RC, Wilson MT. Iron chelators can protect against oxidative stress through ferryl heme reduction. Free Radic. Biol. Med. 2008;44:264–273. doi: 10.1016/j.freeradbiomed.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 77.Nath KA, Balla G, Vercellotti GM, Balla J, Jacob HS, Levitt MD, Rosenberg ME. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J. Clin. Invest. 1992;90:267–270. doi: 10.1172/JCI115847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nath KA. Heme oxygenase-1: a provenance for cytoprotective pathways in the kidney and other tissues. Kidney Int. 2006;70:432–443. doi: 10.1038/sj.ki.5001565. [DOI] [PubMed] [Google Scholar]
- 79.Nath KA, Haggard JJ, Croatt AJ, Grande JP, Poss KD, Alam J. The indispensability of heme oxygenase-1 in protecting against acute heme protein-induced toxicity in vivo. Am. J. Pathol. 2000;156:1527–1535. doi: 10.1016/S0002-9440(10)65024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Abraham NG, Cao J, Sacerdoti D, Li X, Drummond G. Heme oxygenase: the key to renal function regulation. Am. J. Physiol. Renal Physiol. 2009;297:F1137–F1152. doi: 10.1152/ajprenal.90449.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grisham MB. Myoglobin-catalyzed hydrogen peroxide dependent arachidonic acid peroxidation. J. Free Radic. Biol. Med. 1985;1:227–232. doi: 10.1016/0748-5514(85)90122-9. [DOI] [PubMed] [Google Scholar]
- 82.Harel S, Kanner J. The generation of ferryl or hydroxyl radicals during interaction of haemproteins with hydrogen peroxide. Free Radic. Res. Commun. 1988;5:21–33. doi: 10.3109/10715768809068555. [DOI] [PubMed] [Google Scholar]
- 83.Hogg N, Rice-Evans C, Darley-Usmar V, Wilson MT, Paganga G, Bourne L. The role of lipid hydroperoxides in the myoglobin-dependent oxidation of LDL. Arch. Biochem. Biophys. 1994;314:39–44. doi: 10.1006/abbi.1994.1409. [DOI] [PubMed] [Google Scholar]
- 84.Patel RP, Svistunenko DA, Darley-Usmar VM, Symons MC, Wilson MT. Redox cycling of human methaemoglobin by H2O2 yields persistent ferryl iron and protein based radicals. Free Radic. Res. 1996;25:117–123. doi: 10.3109/10715769609149916. [DOI] [PubMed] [Google Scholar]
- 85.Ortiz de Montellano PR, Catalano CE. Epoxidation of styrene by hemoglobin and myoglobin. Transfer of oxidizing equivalents to the protein surface. J. Biol. Chem. 1985;260:9265–9271. [PubMed] [Google Scholar]
- 86.Reeder BJ, Sharpe MA, Kay AD, Kerr M, Moore K, Wilson MT. Toxicity of myoglobin and haemoglobin: oxidative stress in patients with rhabdomyolysis and subarachnoid haemorrhage. Biochem. Soc. Trans. 2002;30:745–748. doi: 10.1042/bst0300745. [DOI] [PubMed] [Google Scholar]
- 87.Reeder BJ, Svistunenko DA, Sharpe MA, Wilson MT. Characteristics and mechanism of formation of peroxide-induced heme to protein cross-linking in myoglobin. Biochemistry. 2002;41:367–375. doi: 10.1021/bi011335b. [DOI] [PubMed] [Google Scholar]
- 88.Rodriguez-Malaver AJ, Leake DS, Rice-Evans CA. The effects of pH on the oxidation of low-density lipoprotein by copper and metmyoglobin are different. FEBS Lett. 1997;406:37–41. doi: 10.1016/s0014-5793(97)00233-0. [DOI] [PubMed] [Google Scholar]
- 89.Fantone J, Jester S, Loomis T. Metmyoglobin promotes arachidonic acid peroxidation at acid pH. J. Biol. Chem. 1989;264:9408–9411. [PubMed] [Google Scholar]
- 90.Nath KA, Balla J, Croatt AJ, Vercellotti GM. Heme protein-mediated renal injury: a protective role for 21-aminosteroids in vitro and in vivo. Kidney Int. 1995;47:592–602. doi: 10.1038/ki.1995.75. [DOI] [PubMed] [Google Scholar]
- 91.Kanner J, Harel S. Desferrioxamine as an electron donor. Inhibition of membranal lipid peroxidation initiated by H2O2-activated metmyoglobin and other peroxidizing systems. Free Radic. Res. Commun. 1987;3:309–317. doi: 10.3109/10715768709069798. [DOI] [PubMed] [Google Scholar]
- 92.Reeder BJ, Wilson MT. Desferrioxamine inhibits production of cytotoxic heme to protein cross-linked myoglobin: a mechanism to protect against oxidative stress without iron chelation. Chem. Res.Toxicol. 2005;18:1004–1011. doi: 10.1021/tx049660y. [DOI] [PubMed] [Google Scholar]
- 93.Boutaud O, Moore KP, Reeder BJ, Harry D, Howie AJ, Wang S, Carney CK, Masterson TS, Amin T, Wright DW, Wilson MT, Oates JA, Roberts LJ., 2nd Acetaminophen inhibits hemoprotein-catalyzed lipid peroxidation and attenuates rhabdomyolysis-induced renal failure. Proc. Natl. Acad. Sci. U S A. 2010;107:2699–2704. doi: 10.1073/pnas.0910174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ohki S, Ogino N, Yamamoto S, Hayaishi O. Prostaglandin hydroperoxidase, an integral part of prostaglandin endoperoxide synthetase from bovine vesicular gland microsomes. J. Biol. Chem. 1979;254:829–836. [PubMed] [Google Scholar]
- 95.Boyd JA, Eling TE. Prostaglandin endoperoxide synthetase-dependent cooxidation of acetaminophen to intermediates which covalently bind in vitro to rabbit renal medullary microsomes. J. Pharmacol. Exp. Ther. 1981;219:659–664. [PubMed] [Google Scholar]
- 96.Harvison PJ, Egan RW, Gale PH, Christian GD, Hill BS, Nelson SS. Acetaminophen and analogs as cosubstrates and inhibitors of prostaglandin H synthase. Chem.-Biol Interactions. 1988;64:251–266. doi: 10.1016/0009-2797(88)90101-9. [DOI] [PubMed] [Google Scholar]
- 97.Harvison PJ, Egan RW, Gale PH, Nelson SD. Acetaminophen as a cosubstrate and inhibitor of prostaglandin H synthase. Adv. Exp. Med. Biol. 1986;197:739–747. doi: 10.1007/978-1-4684-5134-4_68. [DOI] [PubMed] [Google Scholar]
- 98.Markey CM, Alward A, Weller PE, Marnett LJ. Quantitative studies of hydroperoxide reduction by prostaglandin H synthase. Reducing substrate specificity and the relationship of peroxidase to cyclooxygenase activities. J. Biol. Chem. 1987;262:6266–6279. [PubMed] [Google Scholar]
- 99.Chou T, Greenspan P. Effect of acetaminophen on the myeloperoxidase-hydrogen peroxide-nitrite mediated oxidation of LDL. Biochim. Biophys. Acta. 2002;1581:57–63. doi: 10.1016/s1388-1981(02)00119-1. [DOI] [PubMed] [Google Scholar]
- 100.Ron D, Taitelman U, Michaelson M, Bar-Joseph G, Bursztein S, Better OS. Prevention of acute renal failure in traumatic rhabdomyolysis. Arch. Intern. Med. 1984;144:277–280. [PubMed] [Google Scholar]
- 101.Homsi E, Barreiro MF, Orlando JM, Higa EM. Prophylaxis of acute renal failure in patients with rhabdomyolysis. Ren. Fail. 1997;19:283–288. doi: 10.3109/08860229709026290. [DOI] [PubMed] [Google Scholar]
- 102.Ozguc H, Kahveci N, Akkose S, Serdar Z, Balci V, Ocak O. Effects of different resuscitation fluids on tissue blood flow and oxidant injury in experimental rhabdomyolysis. Crit. Care Med. 2005;33:2579–2586. doi: 10.1097/01.ccm.0000186767.67870.8c. [DOI] [PubMed] [Google Scholar]
- 103.Chander V, Chopra K. Molsidomine, a nitric oxide donor and L-arginine protects against rhabdomyolysis-induced myoglobinuric acute renal failure. Biochim. Biophys. Acta. 2005;1723:208–214. doi: 10.1016/j.bbagen.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 104.Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ., 2nd A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc. Natl. Acad. Sci. U S A. 1990;87:9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wright DH, Abran D, Bhattacharya M, Hou X, Bernier SG, Bouayad A, Fouron JC, Vazquez-Tello A, Beauchamp MH, Clyman RI, Peri K, Varma DR, Chemtob S. Prostanoid receptors: ontogeny and implications in vascular physiology. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;281:R1343–R1360. doi: 10.1152/ajpregu.2001.281.5.R1343. [DOI] [PubMed] [Google Scholar]
- 106.Hou X, Gobeil F, Jr, Peri K, Speranza G, Marrache AM, Lachapelle P, Roberts J, 2nd, Varma DR, Chemtob S, Ellis EF. Augmented vasoconstriction and thromboxane formation by 15-F(2t)-isoprostane (8-iso-prostaglandin F(2alpha)) in immature pig periventricular brain microvessels. Stroke. 2000;31:516–524. doi: 10.1161/01.str.31.2.516. discussion 525. [DOI] [PubMed] [Google Scholar]
- 107.Lahaie I, Hardy P, Hou X, Hassessian H, Asselin P, Lachapelle P, Almazan G, Varma DR, Morrow JD, Roberts LJ, 2nd, Chemtob S. A novel mechanism for vasoconstrictor action of 8-isoprostaglandin F2 alpha on retinal vessels. Am. J. Physiol. 1998;274:R1406–R1416. doi: 10.1152/ajpregu.1998.274.5.R1406. [DOI] [PubMed] [Google Scholar]
- 108.Hou X, Roberts LJ, 2nd, Gobeil F, Jr, Taber D, Kanai K, Abran D, Brault S, Checchin D, Sennlaub F, Lachapelle P, Varma D, Chemtob S. Isomer-specific contractile effects of a series of synthetic f2-isoprostanes on retinal and cerebral microvasculature. Free Radic. Biol. Med. 2004;36:163–172. doi: 10.1016/j.freeradbiomed.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 109.Holt S, Reeder B, Wilson M, Harvey S, Morrow JD, Roberts LJ, 2nd, Moore K. Increased lipid peroxidation in patients with rhabdomyolysis. Lancet. 1999;353:1241. doi: 10.1016/S0140-6736(98)05768-7. [DOI] [PubMed] [Google Scholar]
- 110.Newaz MA, Oyekan AO. Vascular responses to endothelin-1, angiotensin-II, and U46619 in glycerol-induced acute renal failure. J. Cardiovasc. Pharmacol. 2001;38:569–577. doi: 10.1097/00005344-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 111.Salahudeen AK, Clark EC, Nath KA. Hydrogen peroxide-induced renal injury. A protective role for pyruvate in vitro and in vivo. J. Clin. Invest. 1991;88:1886–1893. doi: 10.1172/JCI115511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Koshkin V, Dunford HB. Reactions of prostaglandin endoperoxide synthase with hydroperoxide and reducing substrates under single turnover conditions. Biochim. Biophys. Acta. 1999;1431:47–52. doi: 10.1016/s0167-4838(99)00041-2. [DOI] [PubMed] [Google Scholar]