Abstract
Introduction
Therapeutic hypothermia is being employed, clinically based, on its neuro-protective benefits. Both critical illness and therapeutic hypothermia significantly affect drug disposition, potentially contributing to drug-therapy and drug-disease interaction. Currently, there is limited written information of the known alterations in drug concentration and response during mild hypothermia treatment and there is a limited understanding of the specific mechanisms that underlie alterations in drug concentrations and the potential clinical importance of these changes.
Areas covered
A systemic review of the effect of therapeutic hypothermia on drug metabolism, disposition, and response is provided. Specifically, the clinical and preclinical evidence of the effects of therapeutic hypothermia on blood flow, specific hepatic metabolism pathways, transporter, renal excretion, pharmacodynamics and rewarming effect are reviewed.
Expert Opinion
Available evidence demonstrates that mild hypothermia decreases the clearance of a variety of drugs with apparently little change in drug protein binding. Recent evidence suggests that the magnitude of the change is elimination route specific. Further research is needed to determine the impact of these alterations on both drug concentration and response in order to optimize the hypothermia therapy in this vulnerable patient population.
Keywords: critical care, drug metabolism, drug response, pharmacokinetics, therapeutic hypothermia
1. Introduction
Hypothermia was first utilized over four decades ago to decrease the effects of organ ischemia during cardiac surgery by reducing metabolic rate and subsequent oxygen consumption [1]. From the early 1990’s, hypothermia was proven useful for treating focal and global ischemic brain injury in animal models [2–4]. Currently, the use of hypothermia has been increased clinically based on the results from randomized controlled clinical trials that have demonstrated the decreased mortality and improved neurological outcomes in post cardiac arrest adult patients and in neonates with hypoxic-ischemic encephalopathy (HIE) [5–8]. Therapeutic hypothermia is also being evaluated in several other diseases including, traumatic brain injury, focal brain ischemia, myocardial ischemia, spinal cord injury, hemorrhagic shock, pediatric cardiac arrest, and encephalopathy due to acute liver failure. Currently trials are going to determine the safety and efficacy in a number of these disease states [9–13].
Therapeutic hypothermia is induced by reducing core body temperature via a wide array of surface cooling and intravenous cooling methods. Randomized control trials demonstrating neuroprotection have employed therapeutic hypothermia at 32–34°C for 12–24 hours in adults and up to 48–72 hours for pediatric patients. Clinical implementation of therapeutic hypothermia can be divided into induction, maintenance and rewarming phases. Rapidly reducing core body temperature, stable and controlled maintenance in the range of 32–35°C, and slow passive rewarming are the key variables to yield beneficial effects [14–15]. The success of therapeutic hypothermia in improving neurological outcomes has been attributed to the fact that reduced body temperature alters multiple pathogenic mechanisms simultaneously. Hypothermia inhibits multiple steps in the biochemical cascade that contribute to brain injury after insult. Hypothermia, even mild cases, is associated with a wide range of systemic side effects. Although therapeutic hypothermia has demonstrated beneficial effects in neurological outcome, reduction of body temperature has the potential to alter other physiological processes which should be considered for optimizing this therapy. For example, many of the enzymes and transporters involved in drug absorption, distribution and metabolism are temperature dependent. Hypothermia has been utilized clinically without a full understanding of the effects on dug metabolism and drug disposition. The extensive pharmaco-therapeutic regimen employed in the critically ill patients, coupled with the difficulty identifying adverse drug reactions in these patients, creates a high likelihood of unrecognized therapy-drug interactions. For clinicians and health professionals to better understand the best method of applying hypothermia during pharmacological management, an understanding of the mechanism of hypothermia on drug disposition and response is necessary. In this review, the preclinical and clinical evidence and potential implications of altered drug disposition during mild and moderate hypothermia (30–35°C) have been detailed.
2. Pharmacological interventions involved in therapeutic hypothermia
A wide variety of medications are employed in critically ill patients who receiving therapeutic hypothermia. These medications may include anti-shivering and analgesics, cardiovascular drugs, anti-convulsant, anti-platelets, antimicrobial, and anti-inflammatory drugs [16–17]. Many of these drugs have narrow therapeutic windows and have not been thoroughly studied under hypothermia conditions. Adverse drug effects in critically ill patients are known to cause hypotension, increased ICU stay, prolonged cardiovascular support, prolonged respiratory depression, and hematologic side effect [18–20]. These adverse effects have the potential to diminish the overall efficacy of therapeutic hypothermia. We summarized the list of these medications in Table 1 with their pharmacological effects, related specific metabolic and elimination pathways, liver extraction ratios, protein binding and half-lives [21–24].
Table 1.
Pharmacological interventions involved in critical ill patients and their pharmacokinetic characteristics
| ANALGESICS/SEDATIVE | Half life | Protein binding | Hepatic extraction ratio to blood flow | CYP3A | CYP2C9/19 | CYP2D6 | Renal elimination | Transporter/OTHERS |
|---|---|---|---|---|---|---|---|---|
| Fentanyl | 2.5–6.5mins | 80–85% | High | √ | √ | |||
| Propofol | 30–60mins | 95–99% | High | √ | CYP2B/UGT | |||
| Alfentanil | 1.5–2 hrs | 92% | Low | √ | √ | |||
| Midazolam | 1.8–6.4hrs | 95% | Low/Intermediate | √ | √ | |||
| Morphine | 2–3hrs | 30–40% | High | √ | UGT | |||
| MUSCLE RELAXANT | ||||||||
| Vecuronium | 51–80mins | 30% | Low | √ | √ | PGP | ||
| Pancuronium | 1.5–2.7hrs | 77–91% | Low | √ +Bile | ||||
| ANTI-ARRYTHMICS | √ | |||||||
| Lidocaine | 1.5–2hrs | 61–80% | High | √ | √ | CYP1A2 | ||
| Amiodarone | 15–142 days | 33–65% | Low | √ | Bile | |||
| Digoxin | 38–48hrs | 25% | Low | √ | PGP | |||
| Procainamide | 2.5–4.5hrs | 15–20% | Intermediate | √ | √ | |||
| Bretylium | 7–8hrs | N/A | N/A | √ only | ||||
| ANTI-HYPERTENSIVE | ||||||||
| Verapamil | 2.8–7.4hrs | 90% | Intermediate | √ | √ | PGP | ||
| Enalapril | 11hrs | N/A | Intermediate | √ | OATP/MRP2 | |||
| Metoprolol | 3–7hrs | 15% | Intermediate/high | √ | √ | |||
| Propranolol | 4–5hrs | 88% | High | √ | ||||
| Atenolol | 6–7hrs | 6–16% | Low | √ | ||||
| Valsartan | 6hrs | 95% | N/A | √+Bile | OATP/MRP2 | |||
| Pravastatin | 77hrs | 50% | Intermediate/high | √ | OATP1B1/OATP2B1 | |||
| RESUSCITATION/INCREASE HEART RATE | ||||||||
| Epinephrine | 2mins | N/A | N/A | √ | MAO/COMT | |||
| Dopamine | 9mins | N/A | N/A | MAO/COMT | ||||
| ANTI-CONVULSANT | ||||||||
| Phenytoin | 6–24hrs | 90% | Intermediate | √ | √+Bile | UGT | ||
| Phenobarbital | 2–7 days | 20–45% | N/A | √ | √ | CYP2C19/UGT | ||
| Carbamazepine | 25–65hrs | 76% | Low | √ | PGP/UGT | |||
| MISCELLANEOUS | ||||||||
| Warfarin | 30–60hrs | 99.5% | Low | √ (S−,R−) | √ | CYP1A2(R−) | ||
| Mannitol | 100mins | N/A | N/A | √ only | ||||
| Gentamicin | 2 hrs | 0–10% | N/A | √ | ||||
| Rifampin | 1.5–5hrs | 90–95% | N/A | √ | Esterases | |||
| Ranitidine | 2–3hrs | 15% | N/A | √ | √ | PGP | ||
| Famotidine | 8–12hrs | 10–28% | N/A | √ | ||||
| Corticosteriods | Varies | varies | N/A | √ | √ | |||
| Magnesium Sulfate | N/A | NA | N/A | √ only |
Abbreviations: N/A: Not available; mins: Minutes; hrs: Hours; PGP: P-glycoprotein; UGT: UDP-galactose transporter; MAO: monoamine oxydase; COMT: catechol-O-methyltransferase; OATP: organic anion transporter; MRP2: Multidrug resistance protein 2.
3. Metabolism and hepatic clearance alterations during therapeutic hypothermia
In this section, we reviewed the current literature on the alterations in either drug concentrations or metabolism during hypothermia within the temperature range 30–35°C. It has been shown that hypothermia increases drug concentration and prolongs drug response in multiple clinical and animal studies. Based on the classic Pang et al paper describing the well-stirred model [25], factors that affect the hepatic clearance of a drug include: 1) blood flow to the liver; 2) intrinsic clearance; and 3) the fraction of drug bound to protein. Drugs can be classified into three categories according to the well-stirred model equation CLh=Q* [Fu*CL’int/(Q+Fu*CL’int)]: 1) Flow limited: in the case when intrinsic clearance (CL’int) is very large in comparison to blood flow (Q), hepatic clearance (CLh) proximately equals blood flow, CLh≈Q; 2) Capacity limited binding sensitive: for drug highly bound to plasma protein, a displacement from binding sites will significantly increase the concentration of drug at the hepatic enzyme site and the rate of metabolism will increase, CLh≈Fu*CL’int; 3) Capacity limited binding insensitive: When CL’int is very small comparison to hepatic blood flow, assigning drug protein binding did not change during the therapeutic range, CL≈CL’int. Therefore, in this section, we divided all the drugs into three categories based on the hepatic extraction ratio, protein binding and their specific metabolic pathway (Table 2).
Table 2.
Drug concentration and pharmacokinetic alterations in therapeutic hypothermia with their specific metabolism and elimination pathway
| Metabolic pathway | Temperature/subject population | Investigated Drug | concentration/PK parameters estimates |
|---|---|---|---|
| Flow/CYP2B/UGT | 34°C/healthy volunteers | Propofol [26] | Css↑ 28%, Inter-compartment CL↓ with temperature |
| Flow/CYP3A | 31.6°C/piglets | Fentayl [27] | Plasma conc↑25±11%, temperature and CYP3A4 dependency |
| Flow/bile/OATP | 32.5°C/male Wistar rats | ICG [28] | CL↓80%, MRT↑ 2-fold |
| Flow/bile/OATP | 32°C/rats | ICG [29] | Total CL↓ to 47%, AUC↑2-fold, CLb↓ |
| CYP3A | Moderate 32–34°C/TBI patients | Midazolam [35] | Plasma concentration ↑, Vd↑83%, CL↓, Ke↓ |
| CYP3A | 35.5–36.5°C/healthy volunteers | Midazolam [36] | CL↓ 11% per degree |
| CYP3A/PGP | <35, 35–35.9,36–36.9°C/volunteers | Vecuronium[37] | CL↓11.3% per degree |
| CYP3A | 32°C/In vitro microsome | Ethylmorphine [27] | Temperature dependent reaction, CYP3A4 activity ↓ to 69% |
| CYP2C9/CYP2C19 | 34 °C/brain damage patients | Phenytoin [30] | AUC↑180%, MRT↑180%, CL↓67% and Ke↓50% |
| CYP2C9/3A | 34.5°C/healthy volunteers | Neostigmine [39] | V1↓38%, no change in CL |
| CYP2C9/Renal | 32.5°C/male Wistar rats | S-acenocoumarol [28] | CL↓26% |
| CYP2C19/UGT | 30–31°C/TBI children | Phenobarbital [40] | Ke↓, renal metabolite excretion↓, Vd↑25% |
| CYP2D6/2B | Server <32°C/9 out of 11 children | Pentobarbital [41] | CL↓, Vd↓20%, no change in T1/2 |
| CYP2D6/2B | 30°C/perfused rat liver | Pentobarbital-2-c14 [42] | CLh↓50%, CLb↓ 71% |
| CYP2D6/Renal | 30.4°C/neurosurgical patients | Rocuronium [43] | CL↓ to 51%, MRT↑1.9 fold |
| CYP2E1 | 30°C/cardiac arrest rats | Chlorzoxazone [31] | Km↑116%, Clint↓44%,CL↓ 54%, Ke↓66% |
| UGT | 33–34°C/HIE infants | Morphine [46] | CL↓, potential toxic concentration in hypothermia |
| UGT | 30°C/dog | Morphine [47] | Conc in plasma and CSF↑, CL↓ 70%,T1/2β↑2-fold, MRT↑, Vd↓ |
| Transporter PGP | 32°C/in vitro kidney epithelial cell | Digoxin [48] | Direction from B to A ↓50% |
| PGP/OATP | 32°C/in vitro kidney epithelial cell | Quindine [48] | No change in 32°C |
| OATP/flow | 32.5°C/rats | ICG [28] | Bile excretion via OATP was extended |
| Renal filtration | 33.5°C/HIE infants | Gentamicin [50] | No change in CL, high concentration related to renal impairment |
| Renal filtration | 35°C/hypoxia newborn pig | Gentamicin [51] | No change in CL |
| Renal filtration/CYPs | 32°C/cats | Pancuronioum [53] | No change in CL, Vss↓ |
| Renal filtration/GFR | 32°C/rats | FD-4 [29] | No change in pharmacokinetics |
| Renal tubular secretion | 32°C/rats | PSP [29] | Total CL↓ 42%, plasma AUC↑2-fold, MRT↑, renal secretion ↓ |
Abbreviations: ICG: Indocyanine green; FD-4: Fluorescein isothiocyanate (FITC)-dextran; OATP: Organic anion transporter; CL: Systemic clearance; CLb: Bile clearance; Conc: Concentration; AUC: Area under curve; Ke: Elimination rate; Vd: Volume of distribution; V1: Volume of distribution in central compartment; Vss: Volume of distribution at steady state; MRT: Mean resident time; T1/2: Half- life; Km: Michaelis-Menten constant; Clint: Intrinsic clearance.
3.1 Flow limited drugs in therapeutic hypothermia
Flow limited drugs have a high hepatic extraction to blood flow ratio. Upon intravenous administration, the systemic clearance is highly dependent on the liver blood flow. Propofol and fentanyl are two examples of flow limited drugs. During hypothermia at 34°C, propofol steady state concentrations during constant infusion increased 28% compared to normothermia at 37°C in human volunteers (p<0.05) [26]. In addition, the effect of hypothermia on propofol concentration was more significant in the beginning (3.34 ± 1.62 vs 1.99 ± 1.30 pg/mL at 2 minutes, then the difference became smaller later). Three compartment model was used to estimate the pharmacokinetic parameters of propofol. The clearance of propofol at 34°C decreased 25% compared to normothermic condition (0.59 (95% CI: 0.24–1.38) vs 0.79 (0.58–1.08) L/min). The significant association was found between hypothermia and the inter-compartment clearances (1.73 (95% CI: 1.40–2.15) vs 3.51 (3.40–3.63) L/h for shallow, p < 0.005, and 2.13 (95% CI: 1.83–2.47) vs 2.63 (2.15–3.22) L/h for deep, p < 0.05). Hepatic blood flow decreased in both groups (33±11% in hypothermic volunteers and 23±11% in normothermic group) but they were not significant different from each other.
Similarly, in an animal study, Fritz et al showed that fentanyl plasma concentrations have been significantly elevated 25±11% in 31.6°C as compared with normothermic group in pig model [27]. Fentanyl was given infusion at a constant rate during a 6-hour period of hypothermia at 31.6±0.3°C. Fentanyl plasma concentration at the end of the cooling was 7.0±0.6 ng/mL, which was significantly elevated as compared with normothermic group (less than 6.0ng/mL). The authors suggested that the porcine model has similarities between swine and humans in the basic cardiovascular variables, as well as regional distribution of blood flows. Significant reduction in cardiac output and reduced flow to multiple organs were found during hypothermia. However, there was no significant alteration in hepatic artery flow during hypothermia.
Meanwhile, in two animal studies, the metabolism and elimination of indocyanine green (ICG), a commonly used marker for liver function, especially hepatic blood flow and biliary excretion has been tested in hypothermia. Daemen et al has shown that the plasma disappearance ICG was delayed in hypothermia 32.5°C and the clearance of ICG was significantly reduced from normothermia in male Wistar rats [28]. Pentobarbitone anesthesia was used in the study, ICG clearance decreased in the normothermia group as well (4.3±0.6 vs 8.0±1.7 ml/min in conscious rats). Hypothermia further reduced the clearance of ICG to 0.9±0.1ml/min, which were almost 80% decreases from normothermic group. Mean retention time (MRT) increased two-fold in hypothermia. In addition, Nishida et al has shown that during hypothermia at 32°C, significantly elevated ICG plasma concentration, increased area under the curve (AUC), and the decreased total clearance of ICG were found [29]. ICG was given IV at 1mg, plasma AUC at hypothermic group increased approximately two-fold compared to the AUC from control group (550±63.3 vs 271.4±53.4 μg·min/mL). Total clearance of ICG in hypothermic group has decreased to 47% compared to those in normothermia (2.28±0.26 vs 4.83±0.70 ml/min).
Based on these results, it is evident that the clearance of the high extraction ratio drugs, propofol and fentanyl are significantly reduced during hypothermia. Although this alteration is likely due to a reduction in hepatic blood flow, inconsistent results have been published demonstrating either no change or a decrease in hepatic blood flow during cooling. These inconsistent changes in blood flow are likely due to the assessment methods. Definitive studies employing more precise experimental methods of assessing hepatic blood flow are needed for fully elucidation of the mechanism that underlie changes in fentanyl or propofol clearance.
3.2 Capacity limited binding sensitive drugs in therapeutic hypothermia
Phenytoin is an example of capacity limited binding sensitive drugs. It is metabolized through CYP2C9 and CYP2C19 isoforms. Phenytoin metabolism and protein binding have been tested in mild hypothermia in the study by Iida et al in patients [30]. Fourteen traumatic brain injury patients were cooled to 34°C for 72 hours. Phenytoin AUC was increased by 180% (46.3±30.2 vs 16.6±7.3 min·mg/mL, p<0.02) and mean residence time was prolonged by 180% (194±130 vs 69±28 minutes, p<0.01) during hypothermia compared with the corresponding values after rewarming. The clearance of phenytoin was decreased by 67% (1.11±0.86 vs 3.42±2.67 L/h, p<0.02) and elimination constant (Ke) was decreased by 50% (0.10±0.06 vs 0.20±0.15 h−1, p<0.05) during hypothermia. In addition, plasma concentration of metabolite 5-pHPPH was found to be significantly lower during hypothermia in comparison with after hypothermia (p<0.05). Phenytoin protein binding did not change during hypothermia. Therefore, these results demonstrated that phenytoin metabolism was inhibited by mild therapeutic hypothermia in the study, whereas protein binding was not altered, thereby implying that hypothermia decreased the specific activities of CYP2C9 and CYP2C19.
In a cardiac arrest animal model, our lab has shown that protein binding for chlorzoxazone at 30°C did not change from normothermia values [31]. A confounder in assessment of protein binding was discussed by van den Broek et al who has pointed that the in vitro protein binding test might not be accurate because hypothermic blood has been warmed up towards room temperature. Therefore, the drug protein binding formed a new equilibrium before the analysis was carried out, which would introduces errors in predicting temperature effect [32]. However, in our observation with chlorzoxazone, careful temperature control was conducted throughout experimentation to decrease the likelihood of this confounder. Lin et al has suggested that use of the ratio of cerebrospinal fluid (CSF) drug concentration to plasma drug concentration to determine the drug binding in vivo, since CSF is a very low-protein fluid, drug in CSF is considered to be almost unbound [33]. Future studies providing available data from both blood and CSF concentrations during hypothermia will be helpful in further evaluating the protein binding change in vivo.
Collectively, the current evidence suggests that mild to moderate hypothermia does not significantly alter drug protein binding. However this conclusion is based on studies in a limited number of drugs, therefore future research is needed to determine the magnitude of hypothermia effect on protein binding across substrates.
3.3 Capacity limited binding insensitive drugs in therapeutic hypothermia
The clearance of capacity limited drugs is highly dependent on the hepatic intrinsic clearance, including, hepatic drug metabolism by CYP enzymes. Many drugs used in critical care medicine are metabolized by various isoforms of the CYP450 enzyme (Table 1). In this section, we reviewed the published evidence on evaluating drug concentration and pharmacokinetic alterations. Individual drugs have been divided by their specific metabolic CYP pathway (Table 2).
3.3.1 CYP3A
CYP3A is one of the most important CYP isoforms. CYP3A has broad substrate specificity, metabolizing a wide array of compounds such as the small molecules acetaminophen through the large molecule such as cyclosporine. A typical substrate structure includes lipophilic, 1–2 H-bond donor/acceptors at 5.5–7.5Å and 8–10Å from the binding site [34]. Commonly used drugs in critical care metabolized by CYP3A include midazolam, fentanyl, alfentanil, cardiovascular drugs such as lidocaine, calcium channel blockers vecuronium and the majority of corticosteriods. Since CYP3A is present in both liver and intestine, the changes in activity of CYP3A in the intestine could also affect both bioavailability and systemic clearance.
Midazolam is a well-known CYP3A4/5 substrate. A previous study by Fukuoka et al has reported a five-fold increase in plasma concentration of midazolam in hypothermic patients compared with the normothermic patients at steady state [35]. The highest concentration was found at 96 hours which was close to 10,000ng/mL in hypothermic group, while the concentration at steady state in normothermic group was less than 2000ng/mL. The hypothermic patients were maintained for 48 hours and slowly rewarmed. The complete sample collection time was 216 hours after ICU administration. There was greater than 100-fold decreases in clearance of midazolam in these brain injured patients when subjects core temperature were lower than 35°C. In addition, the calculated volume of distribution in hypothermia was 84% higher than normothermia. In another human study, our lab has evaluated midazolam clearance in normal healthy volunteers during mild hypothermia. We found that the midazolam clearance and inter-compartment clearance were associated with temperature reduction. Systemic clearance of midazolam has been estimated to decrease 11.1% per °C reduction in body temperature [36]. Therefore, the significant increases in midazolam concentration and decreased clearance observed in the studies are likely due to the depressed CYP3A intrinsic clearance. Similarly, neuromuscular blocker vecuronium is metabolized by CYP3A and the clearance of vecuronium was estimated to decrease 11.3% per °C in healthy human volunteers by Caldwell et al [37]. The percentage alteration in plasma clearance per °C reduction in body temperature can be very helpful in developing dosing guidelines in patients receiving therapeutic hypothermia.
The decreased CYP3A activity in vitro has been reported by Fritz et al, in which ethylmorphine-N-demethylation experiment showed strong temperature dependence of CYP3A activity (p<0.01) [27]. Hepatic CYP3A4 activity decreased to 69±1% at 32°C when compared with the CYP3A4 activity in normothermic control (p<0.001). Future in vitro study testing different concentrations of substrate as well as different temperatures will be helpful.
Collectively, these studies suggest that the metabolism of the drugs that depend on CYP3A activity would be inhibited due to the acute effect of hypothermia on CYP3A functional activity. The magnitude of the alteration is still open for debate given the data suggesting from 100-fold to as little as 20% changes during mild to moderate hypothermia. Future studies are warranted to determine the magnitude and clinical implications of these changes in CYP3A function.
3.3.2 CYP2C9 and CYP2C19
The CYP2C9 and CYP2C19 isoforms are involved in the metabolism of many commonly used medications in critically ill patients. Typical CYP2C9 substrate characteristics are weak acid, lipophilic, 1 or 2 H-bond donor/acceptors at 5–8Å from the site of metabolism. CYP2C9 also displays polymorphic in humans [34, 38]. CYP2C9 metabolizes drugs including phenytoin, carbamazepine, warfarin, tolbutamide, neostigmine and losartan. Typical substrates of CYP2C19 are neutral or weakly basic, 2–3 H-bond done/acceptors at 4.5Å apart and 5–8Å from the site. CYP2C19 is also involved in metabolizing phenytoin, phenobarbital and carbamazepine. In addition, it metabolizes omeprazole, diazepam and propranolol.
The metabolism of phenytoin has been discussed in the previous section, in which significant metabolism inhibition during hypothermia has been reported [30]. Neostigmine is metabolized by CYP2C9 and CYP3A. Heier et al demonstrated that at normothermia condition, the clearance and central volume of distribution of neostigmine were 696 ± (SE 175) ml/min and 5590 ± (SE 926) ml in healthy human volunteers. During mild hypothermia at 34.5°C, there was a 38% reduction in the central volume of distribution of neostigmine, and no significant alteration of total clearance was found [39]. In this case, the reduction of volume of distribution can describe the plasma concentration-time profile better than the reduction of clearance. Acenocoumarol is also metabolized by CYP2C9. Acenocoumarol is an anticoagulant that functions as a vitamin K antagonist. In the animal study, clearance of S-acenocoumarol decreased significantly during hypothermia at 32.5°C in male Wistar rats [30]. At normothermia 37.5 °C, pentobarbitone anesthesia decreased clearance of acenocoumarol from those in conscious rat (4.9 ± (SE 0.4) vs 3.4 ± (SE 0.3) ml/min). Hypothermia further reduced the clearance to 2.5 ± 0.2 ml/min, which was around 49% decrease from normothermia conscious state rats and 26.5% decrease from normothermia anesthesia group.
Phenobarbitone is metabolized by CYP2C19. Kadar et al performed the study in critically injured children and found that the reduction in body temperature influenced the rate of metabolism, urinary excretion and the volume of distribution of phenobarbitone [40]. Urine excretion for metabolite hydroxyphenobarbital at hypothermia 30–31°C was significantly decreased to 48% (1.87±0.71 vs 4.50±.90 μmol/h, p<0.005) while the urinary excretion rate of the parent drug was 52% higher (12.01±3.38 vs 6.25±1.25 μmol/h, p<0.05) during hypothermia. Volume of distribution increased in hypothermia (approximately 1.25 vs 0.79–1.01 L/kg). Relatively large inter-individual variability was observed in the study, however the change in the relative concentration of phenobarbitone and metabolites in urine was influenced primarily by body temperature. Collectively, these data suggest that hypothermia likely decreases the activity of CYP2C19 and rate of metabolism of its substrates.
Collectively, the metabolism and distribution for CYP2C9 and CYP2C19 substrates phenytoin, S-acenocoumaril and phenobarbital were significantly altered during hypothermia. Clinically, based on the narrow therapeutic window for anticoagulants and anticonvulsant, carefully clinical drug monitoring is warranted for these drugs during hypothermia.
3.3.3 CYP2D6
While CYP2D6 constitutes only about 2% of the total CYP content, it is an important isoform due to its role in the metabolism of a large number of pharmaceutical agents such as antiarrhythmics, CNS agents, codine and anticancer drugs. CYP2D6 also exhibits genetic polymorphisms in humans. While CYP3A4 can accommodate molecules that are structurally very diverse, CYP2D6 has a very strict structure requirement. Substrate normally have at least a basic nitrogen, have a flat hydrophobic area near the site of oxidation, be relatively hydrophilic, and have two potential hydrogen bonding groups in order to bind to the CYP2D6 active site [34].
Biotransformation of pentobarbital involves CYP2D6 and CYP2B6. The pharmacokinetics of pentobarbital during hypothermia (<32°C) was examined in 11 children with Reye syndrome, hypoxic encephalopathy, or acute head injury. The significant decreases in the systemic clearance and 20% decreases in volume of distribution at steady state of pentobarbital were observed in hypothermic patients when compared to previous data in normothermic adult volunteers [41]. The diminished systemic clearance of pentobarbital may result from the decrease in intrinsic enzyme activity that accompany hypothermia, as well as hepatic dysfunction in patients with Reye syndrome, hypoxic encephalopathy, or acute head injury. In the animal model, the metabolism of pentobarbital-2-C14 during hypothermia at 30°C was studied earlier by Kalser et al [42]. Since liver is the only site of metabolism of pentobarbital, the disappearance of the unchanged pentobarbital from the blood, after initial uptake by the liver, is due to its metabolism by the liver. The study has shown that the hepatic clearance decreased to 50% and the metabolite concentration in blood decreased 32% in hypothermia. In addition, bile clearance of pentobarbital decreased 71% and less metabolite excreted in bile as well. Therefore, hypothermia markedly decreased the amount of metabolites appearing in blood and bile as well as liver itself. There is a definitely slowing of the metabolic reaction rate under hypothermia.
Rocuronium is partially metabolized through CYP2D6 and CYP2C19 and large amount of rocuronium is excreted unchanged in bile and urine. Beaufort et al study showed that hypothermia at 30.4°C reduced the plasma clearance of rocuronium around 50% (from 4.26±0.50 to 2.17±0.62 mL/kg/min), and prolonged the mean residence time approximately 1.9-fold (from 56 ±19 to 108 ± 39 min) in nineteen neurosurgical patients [43]. The significant reduction in the clearance of pentobarbital and rocuronium are likely due to the reduction in the metabolic rate. Studies designed to determine the effect of hypothermia on the metabolism and pharmacokinetics of other CYP2D6 substrate drugs are needed to support these results for further conclusion on the effect of hypothermia on the activity of CYP2D6 isoform.
3.3.4 CYP2E1
CYP2E1 consists around 7% of total P450 content. It is implicated in the metabolism of volatile anesthetics, acetaminophen, ethanol, and a small number of therapeutic agents. CYP2E1 active site is relatively restricted compared to the large and open bonding pocket of CYP3A4. The active site contains two phenylalanines at alignment position 78 and 88 which appears to be able to adopt a coplanar arrangement of the phenyl rings, possibility represent an access channel for planar aromatic substrates [44]. In addition, humans exhibit wide inter-individual variability in concentrations of CYP2E1 mRNA and protein [44]. The CYP2E1 activity ranges four to fivefold differences from different individuals.
Chlorzoxazone is a well-known CYP2E1 probe. Rate of chlorzoxazone-6-hydroxylation was significantly correlated with concentrations of immunochemically measured CYP2E1 level [44]. Our laboratory demonstrated that hypothermia at 30°C decreased the systemic clearance of chlorzoxazone in cardiac arrest rats (1.26±0.34 vs 0.580±0.37 mL/min). Significant decreases in 6-hydroxychlorazoxone formation clearance and increases in plasma AUC of chlorzoxazone were found in temperature at 30°C compared to normothermia at 37°C [31]. In vitro analysis demonstrated that Michaelis-Menten constant (Km) was significantly increased at 30°C compared to 37°C (551±150 vs 255±52 μM), and CYP2E1 enzyme capacity (Vmax) was not altered. These alterations were due to the reduced intrinsic metabolic clearance produced by a decrease in enzyme substrate affinity and were not due to the alteration in drug protein binding.
Despite the limited number of studies, the results have consistently demonstrated the reduced intrinsic clearance and depressed enzyme activity, including multiple CYP isoforms during mild and moderate hypothermia.
4. Phase II enzymes function in therapeutic hypothermia
The activities of Phase II enzymes are important for drug biotransformation and elimination. UDP-glucuronosyltransferase (UGT) is one of the most studied phase II enzymes. Endogenous substrates of UGT enzymes include bilirubin, thyroxin, and steroid hormones. Commonly known UGT substrate drugs include benzodiazepines, morphine, propofol, phenobarbital, propranolol, aspirin, acetaminophen and valproic acid [34].
Morphine is primarily metabolized by UGT2B7, with little to no contribution by Phase I enzyme in the morphine elimination. Two major metabolites are morphine-3-glucuronide (M3G) and morphine-6-glucuronide (M6G). M6G binds to μ-receptors and is a more potent analgesic than morphine [45]. The effect of mild hypothermia at 33–34°C on morphine concentration in infants with hypoxic-ischemic encephalopathy was studied by Roka et al [46]. The study found that the mean morphine concentration in hypothermia was significant higher than normothermic group at 72 hours after birth. More notably, 6 of 7 infants in the hypothermia group vs 1 of 6 infant in the normothermia group demonstrated a concentration above the 300ng/mL. The clearance of morphine in hypothermia was significantly decreased from 0.89 (0.65–1.33) to 0.69 (0.58–1.12) mL/min per kg. The results showed that plasma morphine concentration were more likely to be elevated to potentially toxic concentrations in HIE patients receiving therapeutic hypothermia.
In the animal model, previous study by Bansinath et al has shown significant higher concentrations of morphine in both plasma and in cerebrospinal fluid (CSF) during hypothermia (30°C) in dogs [47]. Morphine sulfate was given IV bolus at dose 1mg/kg. Total clearance and volume of distribution of morphine were significantly decreased in hypothermia (25±4 vs 84±11 ml/kg/min, and 5.7±0.8 vs 8.5±0.7 L/kg, respectively) compared to those in normothermia at 37°C. Meanwhile, the elimination half-life and mean residence time were significantly increased during hypothermia (184±21 vs 81±8 minutes, and 3.6±0.8 vs 1.5±0.17 hours, respectively).
Collectively, these results demonstrated that morphine metabolism is inhibited during hypothermia, and careful monitoring the drug concentration and response is necessary. The reduced activity of UGT during hypothermia is a likely mechanism underlying the inhibition of metabolism. However, study on alternative UGT substrates and probe drugs during hypothermia will be necessary for further confirmation.
5. Transporter function in therapeutic hypothermia
A growing body of evidence has demonstrated a significant role of various transporters as effectors of both drug distribution and excretion. The major drug transporters, the ATP binding cassette (ABC) family are the major family involved in drug distribution, which include transporter ABCB1 or MDR1 P-glycoprotein (PGP). PGP has considerable influence on the systemic absorption and pharmacokinetics of drugs, especially for many calcium blockers and antiarrhythmic drugs used in critical care medicine. Previous study by Jin et al demonstrated that MDR1-mediated transport of digoxin was decreased in vitro at temperatures of 32°C [48]. The net B to A direction (basal side to the apical side) transporter ratio for digoxin decreased approximately 50% in lower temperature (7.5 vs 14), suggesting that MDR1 transport activity was reduced at 32°C or lower. No significant difference in passive diffusion process was observed at 32°C. In addition, the transport of inulin, as a marker of paracellular pathway, showed no effect of temperature at 25°C, suggesting that the tight junction was not affected during therapeutic hypothermia. Therefore, this study indicated that therapeutic hypothermia caused the suppression of drug transport via MDR1 rather than via passive diffusion.
In the same study, the decrease in the net transport ratio for quinidine, another MDR1 substrate, was not observed at 32°C. The authors mentioned that quinidine is also a substrate for organic anion transporter (OATP). Therefore, the OATP transporter might have affected the findings of temperature dependency [48]. OATP is an influx transporter. Previous discussed liver blood flow marker ICG is an OATP substrate. ICG is exclusively excreted into bile through OATP transporter. Biliary clearance of ICG decreased significantly to 46% in hypothermia at 32°C (1.94±0.26 vs 4.18±0.63 ml/min) [28].
Collectively, these studies suggested that the activity of MDR1 transporter reduces during hypothermia, while passive diffusion is not altered by cooling. ABC transporters are active transporters, and require energy in the form of adenosine triphosphate (ATP) to translocate substrates across cell membranes. Thereby, suggesting a reduction in energy dependent processes during hypothermia. More studies needed for determining the effect of hypothermia on transporters MDR1, OATP and other transporters in the future.
6. Renal elimination in therapeutic hypothermia
Renal elimination function can be divided into filtration, active secretion and re-absorption. The balance between renal clearance and metabolism is readily predicted by physio-chemical properties of the drug [49]. Gentamicin is a commonly used IV antibiotic that is eliminated largely by passive filtration with a minor contribution by tubular secretion. Previous study by Liu et al evaluated gentamicin in infant with HIE [50]. This study showed that the mean trough serum gentamicin concentration of hypothermia and normothermia were similar (2.19±1.7 vs 2.30±2.0 mg/L). The clearance of gentamicin was not altered by hypothermia at 33.5°C. A significant correlation was found between high serum gentamicin concentration and impaired renal function. Plasma creatinine concentrations were closely correlated (r2=0.36) with trough serum concentration in both hypothermia and normothermia groups. In an animal study, gentamicin pharmacokinetics was studied during hypothermia at 35°C in hypoxia piglets. Pharmacokinetics was evaluated after three gentamicin doses: before hypoxia-ischemia, after hypoxia-ischemia during mild hypothermia or normothermia, and during normothermia 48 h after the first dose. The study found that hypoxia-ischemia altered the renal function and the gentamicin clearance correlated with the creatinine plasma concentration (r=0.89) as well as kidney pathology score (r=0.55). There was no significant change in gentamicin pharmacokinetic parameters at 35°C compared to 39°C. Therefore, mild hypothermia after hypoxia-ischemia does not affect gentamicin pharmacokinetics over clinically employed therapeutic hypothermia temperatures [51]. Although studies evaluated temperatures below 30°C have shown reduced gentamicin clearance due to a reduced cardiac output and decreased glomerular filtration rate (GFR) [52], these reductions are not expected over the 32–35°C temperature range.
Pancuronium is another drug that is primary excreted through kidney filtration. Sixty to eighty percent of pancuronium is excreted through the kidneys and the remainder is metabolized in the liver and excreted in the bile. In an animal model, pancuronium was studied by Miller et al in cat at 39°C, 34°C and 29°C [53]. Central compartmente and total volume of distribution of pancuronium at steady state were both decreased at 34°C compared to 39°C. Total plasma clearance was found significantly reduced at 29°C compared to 39°C, while there was no difference of pancuronium clearance at 34°C vs 39°C (10.7±0.9 vs 10.9±1.5 ml/kg/min). There was a markedly reduced urinary and biliary excretion of pancuronium only when temperature was below 30°C. Fluorescein isothiocyanate (FITC)-dextran (FD-4), an index of GFR has been evaluated evaluated in the rat model during therapeutic hypothermia at 32°C [29]. FITC-dextran is eliminated by passive diffusion. Plasma concentrations were almost equal at all time points between 37°C and 32°C. The total clearance and renal clearance at 32°C and 37°C were also comparable (2.10±0.14 vs 1.88±0.13 ml/min for total, and 1.57±0.09 vs 1.45±0.05 ml/min for renal), while both of them significantly decreased only at a temperature of 28°C [29]. Collectively, these studies suggested that the kidney is able to maintain GFR during mild and moderate therapeutic hypothermia (30–35°C) in spite of possible variations in the systemic circulation. Therefore, renal passive filtration is not likely to be altered by mild hypothermia.
With respect to renal tubular secretion, PSP, a hydrophilic dye, has been used as a tool to assess renal function. PSP is excreted into the bile and urine as a free form or conjugative metabolite. Previous study by Nishida et al has shown that the plasma concentrations of PSP were increased significantly in the hypothermic groups (at both 32°C and 28°C) compared to the normothermic group at 37°C [29]. The plasma AUC of PSP in hypothermic group at 32°C was significantly larger than those in the normothermic group (1487.9±227 vs 788.6±0.9 μg·min/mL). The total clearance of PSP decreased about 42% at temperature 32°C (0.87±0.1 vs 1.51±0.15ml/min). In addition, both plasma MRT and bile MRT of PSP in the hypothermic group were significantly longer than those in normothermic group (95.1±15.7 vs 49.9±4.7 min for plasma MRT and 88.2±12.1 vs 49.5±.9mins for bile MRT). Furthermore, biliary and urinary recovery rates of PSP metabolite excretion were calculated as 37°C (66%, 34%) vs 32°C (75%, 25%), showing a tendency of decrease in urinary contrition by low body temperature. Therefore, PSP clearances (bile, urine and metabolites) in the hypothermic group were decreased, suggesting an effect of hypothermia on the active tubular secretion [29]. In addition, this study also suggested that active renal transporter would be attenuated at 32°C due to a failure in energy supply.
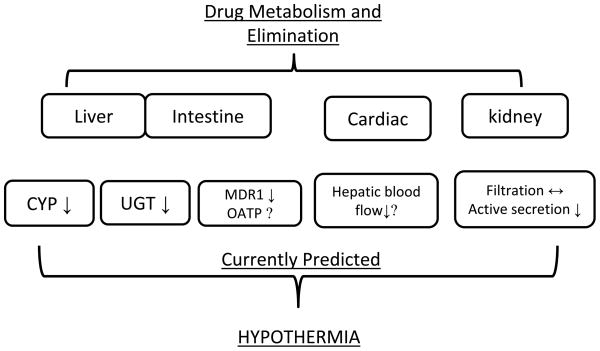
In summary, these studies suggest that active, energy requiring process such as tubular secretion is reduced during therapeutic hypothermia, whereas passive filtration is not significantly altered in mild and moderate hypothermia at 30°C–35°C. The effect of hypothermia on hepatic pathway, phase II enzyme, transporter and renal elimination based on the current evidence has been summarized in Figure 1.
Figure 1.
The effect of hypothermia on drug metabolism and elimination pathway
7. Current evidence on drug response changes during therapeutic hypothermia
Besides the effect of hypothermia on drug metabolism and pharmacokinetics, drug response changes during hypothermia have been reported in both clinical and preclinical studies (Table 3).
Table 3.
Current evidence on drug response change in therapeutic hypothermia
| Pharmacological effect | Temperature/subject population | Investigated Drug | Drug response/PD estimates |
|---|---|---|---|
| Muscle relaxant | 34.5°C/patients undergoing surgery | Vecuronium [54] | Recovery time↑, duration of action↑ |
| Muscle relaxant | <35, 35–35.9,36–36.9°C/volunteers | Vecuronium [55] | Response↑, equilibration rate↓ |
| Muscle relaxant | 34°C/healthy volunteers | Atracurium [26] | Response↑, duration of action↑ |
| Muscle relaxant | 34°C/healthy volunteers | Neostigmine [39] | No change in efficacy and duration |
| Muscle relaxant | 30.4°C/neurosurgical patients | Rocuronium[43] | Duration of action↑ |
| Muscle relaxant | 31.9°C/neurological patients | D-tubocurarine [55] | Duration of action↑ |
| Volatile anesthetic | 34, 31°C/children | Isoflurane [57] | Dose requirement↓ 5.1% per °C. MAC↓ |
| Opiate anesthetic | 30°C/dog | Morphine [47] | Hypotension incidence↑ |
| Opiate anesthetic | 30°C/guinea pig ileum | Morphine [58] | IC50↓, the affinity to μ-receptor↓ |
| β-adrenoceptor agonist | 33°C/rats | Isoproterenol [59] | HR↓, CO↓, SV↓, LV dP/dtmax↓ |
| β2-adrenoceptor agonist | 30°C/guinea-pig | Orciprenaline [60] | β1 adrenoceptor sensitivity↑ |
Abbreviations: MAC: Minimum alveolar concentration; IC50: The half maximal inhibitory concentration, HR: Heart rate; CO: Cardiac output; SV: Stroke volume; LV dp/dtmax: Left ventricle contractility.
The drug responses to neuromuscular blockers including vecuronim, atracurium, neostigmine, rocuronium and tubocurarine have been evaluated during hypothermia. Previous study by Heier et al has demonstrated that mild hypothermia increased the duration of action and time for spontaneous recovery from vecuronium-induced neuromuscular blockade in patients undergoing elective surgery [54]. Decreasing core temperature correlated with an increasing duration of action of the second infusion of vecuronium (R2=0.58, p<0.005). There was no difference between hypothermic and normothermic subjects as measured by the steady state concentration producing 50% depression of twitch tension on the train-of-four test. Thus this study has suggested that hypothermia mediated increases in the duration of action of vecuronium was not due to the pharmacodynamics response, but more likely due to a change in pharmacokinetics of vecuronium. Similarly, a previous study by Caldwell et al has also demonstrated the increased duration of action of vecuronium during hypothermia [37]. Hypothermia decreased the rate constant for vecuronium equilibration between plasma and effect site (0.023 min(−1) per C), and increased the slope of the concentration-response relationship by 0.43 per C indicating that recovery of neuromuscular function has been delayed by hypothermia. This study suggested that reduced metabolism and rate of effect site equilibration both contributed to the prolonged response. Previous study by Leslie et al has shown that mild hypothermia at 34°C increased the duration of atracurium significantly [26]. Core hypothermia prolonged the time to recovery of the first twitch in the train-of-four to 10% of its control value (Tl = 10%) after atracurium administration by −60% (p< 0.05), from 44± (SE 4) min to 68 ±(SE 7) min. Beaufort et al has shown that hypothermia at 30.4 ±0.8° C prolonged the duration of action of rocuronium and delayed spontaneous recovery. The altered pharmacokinetics, such as a decreased clearance was a primary contributor [42]. Ham et al reported a prolonged duration of action d-tubocauraine at body temperature of 35.8°C and 31.9°C in neurological patients [55]. Meanwhile, the efficacy of neostigmine as an antagonist of vecuronium induced neuromuscular block was not altered by mild hypothermia at 34°C and clearance of neostigmine was not changed [39]. When temperature was reduced below 30°C, pancuronium neuromuscular block was prolonged because of an increased sensitivity of the neuromuscular junction to pancuronium and delayed biliary and urinary excretion [53]. A 2°C reduction in body temperature may double the duration of neuromuscular blockage. Collectively, these studies suggested that reduction in body temperature to mild and moderate hypothermia may prolong the duration of neuromuscular blockers. It is important for clinical practitioners to be aware of this prolonged response and to increase pharmacodynamics monitoring of these patients in both the cooling and rewarming phases of therapeutic hypothermia. In addition, therapeutic hypothermia produced prolonged response is more likely due to the reduced metabolism and increased drug concentrations during hypothermia. Interested reader can further refer to Heier et al review paper in 2006 which has focused on the response to neuromuscular blocking drugs [56].
Besides neuromuscular blockers, the responses for anesthetics isoflurane and morphine have been evaluated during hypothermia. Decreased isoflurane requirement in children by 5.1% per C in children was estimated by Liu et al. Isoflurane minimum alveolar concentration (MAC) values were 1.69±0.14%, 1.47±0.10%, and 1.22±0. 15% at 37° C, 34° C, and 31° C, respectively [57]. Isoflurane pharmacokinetics was not evaluated in this study. Therefore the pharmacokinetic change related to the effect is unknown. In addition, isoflurane is metabolized by CYP2E1 enzyme, and a decrease in metabolism by CYP2E1 may underlie the prolonged drug response and reduced dosage requirement.
Previous study by Puig et al has shown that the potency of morphine was significantly decreased at 30°C and increased at 40°C when compared with its potency at 37°C in guinea pig ileum [58]. This study demonstrated that there was a large decrease in the affinity of morphine for its receptor as was evidented by a six-fold increase in the dissociation constant at 30°C. This study suggested that the affinity of morphine for the μ-receptor decreased as temperature 30°C. In another animal study by Bansinath et al, morphine response during hypothermia has been evaluated in dog model [47]. After a single intravenous bolus injection of 1 mg/kg, morphine resulted in a significant and sustained decrease in mean arterial pressure in hypothermia, but not in normothermia. The hypotension was observed throughout the experiment in 30°C group but not in the normothermic group. During hypothermia, 10 minutes after morphine administration, the MAP was 35±5 mm Hg vs 114±5 mm Hg in normothermia. The decrease in MAP observed in 30°C was statistically different from 37°C until 3.5 hours. The correlation coefficient between plasma morphine levels and the mean percentage decrease in MAP was 0.98 for the hypothermic group. Similarly, the correlation coefficient for CSF morphine levels and percent decrease in MAP was 0.95 in the same experimental group. Therefore, this drug response change was highly possibly due to the reduced metabolism. Collectively, reduced body temperature in therapeutic hypothermia range prolonged the anesthetic drug responses. Hypotension has been observed as one of the most common side effects for hypothermic patients. Prolonged ICU stay and prolonged respiratory depression may mask and alter the benefit effect from hypothermia. Clinical drug monitoring for side effects of series of anesthetics is warranted.
β-Adrenoceptor response during hypothermia was studied by Han et al. The study has shown that the physiological cardiovascular responses mediated by the β-adrenoceptor were significantly diminished during core hypothermia at 33°C [59]. In this study, the dose dependent positive inotropic and chronotropic effects of isoproterenol observed at 37° were diminished as core temperature lowered to 33°C or below. The lack of ability to increase cardiac output in hypothermia may also partially due to the large increase in total peripheral resistance. The changes of binding affinity or downstream intracellular signaling related to cAMP alteration has been suggested as the underline mechanism of this effect [59]. However, previous study by Williams et al has shown that the positive inotropic responses to orciprenaline of paced left atria and papillary muscles were actually potentiated at the lower temperature at 30°C [60]. A similar hypothermia-induced supersensitivity was observed for the positive chronotropic response of right atria. While the β-adrenoceptor has two types β1 and β2, these studies suggest that hypothermia-induced supersensitivity occurs only at the β1-adrenoceptors not β2, indicating a fundamental temperature-dependent difference between the two receptor types.
In summary, the changes of drug response for neuromuscular blockers, volatile anesthetics, opiates and β-adrenoceptor blockers have been observed during hypothermia. Reduced metabolism and clearance may explain part of the response changes. The reduced affinities to μ-receptor and changes in downstream signaling to the specific effect on β1-adrenoceptors have been reported. Careful clinical pharmacotherapeutic monitoring during hypothermia treatment is necessary to prevent the potential therapy-drug interaction and toxicity caused by the changes in both drug concentration and in drug response during hypothermia.
8. Rewarming
The third step of therapeutic hypothermia is passive rewarming. During rewarming phase, there are possibilities of simulating or restoring physiological functions in homeothermic organism after they have been suppressed by cold. The effect of hypothermia on drug metabolism reversible after rewarming has been suggested. A theoretical time course model of CYP activity during hypothermia and after rewarming was suggested by Tortorici et al [61]. Previous phenytoin study used the pharmacokinetic parameters of rewarming phase as control to compare the drug metabolism change during hypothermia [30]. Phenobarbitone, after the body temperature was allowed to return to normal, the rates of excretion of metabolites (hydroxyphenobarbitone, conjugated hydroxyphenobarbitone and phenobarbitone-N-glucoside) increased substantially (increase 141%, 63% and 114%, respectively) and at the same time, the rate of unchanged phenobarbitone excretion decreased markedly by 48% [40].
It is recommended that the body may be rewarmed slowly at a temperature of 0.3–0.5 °C (1–2° F) every hour to a target temperature of 36°C [14–16, 62]. It may take about eight hours for passively rewarming. The plasma concentration of fentanyl at 6 hours after rewarming was still remain increased compared with baseline (6.3±0.6 ng/mL vs less than 6.0ng/mL, p<0.05) [27]. This suggests that the alterations of drug metabolism may exist during rewarming periods. There is no study currently focused on the rewarming phase, instead many clinical study result actually combined both effects from during hypothermia and after rewarming. For long half-life drugs, the rewarming phase might be even more important.
9. Conclusion
Our current understanding suggests that hypothermia has multiple beneficial and detrimental effects after global ischemia injury. Besides the benefit of hypothermia, the possible side effects associated with hypothermia are unavoidable. The effects of hypothermia on drug metabolism and disposition may increase the probability for unanticipated toxicity, which could limit the putative benefit of this novel therapy. Recent evidence suggests that the specific alterations of drug metabolism and the magnitude of the change observed during hypothermia can be metabolism and elimination route specific. The effect of hypothermia on drug response including muscle relaxant, volatile and opiate anesthetic and β-adrenoceptor agonist have been reported. Pharmacokinetic and pharmacodynamics alteration during hypothermia may both contribute to the effect. Future studies on specific CYP probes metabolism and receptor levels drug response are needed. By defining the impact of mild hypothermia on drug metabolism, disposition and enzyme regulation in experimental models as well as through clinical research in the future, drug dosing during therapeutic hypothermia can be optimized to facilitate the maximal benefits and minimize side effects of this therapeutic intervention.
10. Expert opinion
Therapeutic hypothermia is a proven neuroprotective strategy in multiple animal models and in patient studies for both adult out of hospital cardiac arrest and neonates with hypoxic ischemic encephalopathy. Clinical studies demonstrating neurological benefits have cooled adult patients to 34 to 32°C or 12 to 24 hours, whereas neonates are cooled over the same temperature range for up to 72 hours. Patients who survive to the point of inpatient care are most vulnerable to succumb to this disease during the 24 to 72 hours in which a patient is being cooled and during rewarming period. A wide array of pharmacologic agents are used in these patients, many of which have significant toxicities that may affect ventilator weaning, ability to assess seizure control, assessment of neurocognitive function, and hypotension. Furthermore, many of the enzymes and transporters that affect drug disposition are temperature sensitive, energy dependent processes. Despite this potential for drug-therapy interaction, relatively few studies have been conducted to evaluate the clinical effects of therapeutic hypothermia and no studies have been conducted to determine dosing algorithms for patients during cooling and after rewarming. Based on this need, it is critical for currently ongoing randomized control trials aimed at evaluating the efficacy of therapeutic hypothermia also include sample collection for assessment of drug concentrations as part of the study design.
Based on the currently existing research, there are several conclusions that can be made based on the preponderance of clinical and preclinical data. In general, the effects of therapeutic hypothermia on drug disposition are going to depend on the route of drug elimination. In particular, active processes of drug metabolism and tubular secretion or reabsorption demonstrate reductions in enzymatic or transporter activity. Conversely, passive processes of filtration and possibly blood flow are not significantly altered. Specifically, cytochrome P450 functional activity is reduced during hypothermia. Therefore, the clearance of low extraction drugs is reduced during cooling. Preclinical studies to date suggest that the magnitude of these alterations is greater for CYP isoforms with a narrow binding pocket, such as CYP2D6 and CYP2E1, as compared to large binding pocket P450 isoforms, such as the CYP3A subfamily, thereby, suggesting that the magnitude of change in a metabolism will be CYP isoform and drug specific. Similar to low extraction drugs, the elimination of flow dependent, high extraction ratio drugs also appear to be decreased during hypothermia, although the magnitude of flow alterations is highly temperature dependent and is likely greater in magnitude at temperatures below 32°C. A limited number of preclinical and in vitro studies suggest that active tubular secretion and drug transporter activity is also reduced during therapeutic hypothermia. In contrast, passive renal filtration and drug protein binding do not appear to be significantly altered during cooling.
The aforementioned effects of therapeutic hypothermia on drug metabolism have been supported by clinical studies demonstrating increased drug concentrations in patients during the cooling period. Although drug concentrations are elevated, it is important to recognize that increased concentrations may or may not translate into changes in drug response. Response to some drugs, such as vecuronium are not altered during cooling, therefore, elevated concentrations translate into a prolonged duration of effect. Conversely, in vitro studies have demonstrated significant reductions in morphine receptor response under hypothermic temperatures, thereby, suggesting a potential decrease in response despite increases in morphine concentrations. Drugs with reduced drug responsivity during cooling will be particularly problematic in critically ill patients because many of these drugs are dosed to clinical response. This results in an increased dosing despite overall decreased elimination resulting in further elevations in drug concentrations during cooling. The net effect of these increases in drug concentrations is likely to culminate during rewarming. Depending on the drugs given half-life, drug concentrations will remain elevated until a new steady state is established well into the rewarming period, whereas, full receptor response will return rapidly upon temperature normalization. This suggest that the rewarming period is the most likely timeframe for potential adverse drug effects and warrants the most diligent monitoring for drug toxicity.
In closing, it is clear that a great deal of additional research is needed before a full understanding of the magnitude of the effects of therapeutic hypothermia on drug concentration and responses are known. This void in our knowledge can be met with the incorporation of drug concentration studies within currently ongoing clinical trials in patients receiving therapeutic hypothermia. Additionally, studies in smaller groups of well matched patients evaluating probe drug concentrations will provide additional important information. Ultimately, studies should incorporate pharmacometrics tools in large patient populations to determine specific dosing algorithms for drugs with notable toxicities to guide clinicians in optimal pharmaceutical care in this highly vulnerable patient population.
Abbreviations in the manuscript
- ABC
ATP binding cassette
- ATP
Adenosine triphosphate
- AUC
Area under curve
- CA
Cardiac arrest
- cAMP
Cyclic adenosine monophosphate
- CL
Systemic clearance
- CL’int
Intrinsic clearance
- CLb
Bile clearance
- CLh
hepatic clearance
- CNS
Central nerve system
- CO
Cardiac output
- COMT
Catechol-O-methyltransferase
- Conc
Concentration
- CSF
Cerebrospinal fluid
- CYP
Cytochrome P450
- FD-4
Fluorescein isothiocyanate (FITC)-dextran
- Fu
unbound fraction
- GFR
Glomerular filtration rate
- HIE
Hypoxic ischemic encephalopathy
- HR
Heart rate
- hrs
Hours
- IC50
The half maximal inhibitory concentration
- ICG
Indocyanine green
- ICU
Intensive care unit
- IV
Intravenous
- Ke
Elimination rate
- Km
Michaelis-Menten constant
- LV dp/dtmax
Left ventricle contractility
- M3G
Morphine-3-glucuronide
- M6G
Morphine-6-glucuronide
- MAC
Minimum alveolar concentration
- MAO
Monoamine oxydase
- mins
Minutes
- MRP1
Multidrug resistance protein 1
- MRP2
Multidrug resistance protein 2
- MRT
Mean resident time
- MW
Molecular weight
- OATP
Organic anion transporter
- PGP
P-glycoprotein
- PK/PD
pharmacokinetics/pharmacodynamics
- Q
Blood flow
- SV
Stroke volume
- T1/2
Half-life
- UGT
UDP-galactose transporter
- V1
Volume of distribution in central compartment
- Vd
Volume of distribution
- Vss
Volume of distribution at steady state
Footnotes
Declaration of Interest
S Poloyac is supported by a grant from the National Institute of General Medical Sciences from the National Institute of Health (R01GM073031). The authors have no other conflicts of interest.
Contributor Information
Jiangquan Zhou, Email: jiz51@pitt.edu, Department of Pharmaceutical Sciences, School of Pharmacy, University of Pittsburgh, 3501 Terrace Street, Pittsburgh 15261, Phone: 412-624-1133.
Samuel M. Poloyac, Email: poloyac@pitt.edu, Department of Pharmaceutical Sciences, School of Pharmacy, University of Pittsburgh, 807 Salk Hall, 3501 Terrace Street, Pittsburgh 15261, Phone: 412-624-4595, Fax: 412-624-1850.
References
- 1.Bigelow WG, Lindsay WK, Greenwood WF. Hypothermia; its possible role in cardiac surgery: an investigation of factors governing survival in dogs at low body temperatures. Ann Surg. 1950;132:849–66. doi: 10.1097/00000658-195011000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shann F. Hypothermia for traumatic brain injury: how soon, how cold, and how long? Lancet. 2003;362:1950–1. doi: 10.1016/S0140-6736(03)15083-0. [DOI] [PubMed] [Google Scholar]
- 3.Safar P. Hypothermia and multifaceted therapies for cardiac arrest. Minerva Anestesiol. 1994;60:533–6. [PubMed] [Google Scholar]
- 4.Safar P, Bircher NG. Resuscitative cerebral hypothermia after cardiac arrest. Crit Care Med. 1994;22:1703–4. [PubMed] [Google Scholar]
- 5.Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–56. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 6.Thoresen M, Whitelaw A. Therapeutic hypothermia for hypoxic-ischaemic encephalopathy in the newborn infant. Curr Opin Neurol. 2005;18:111–6. doi: 10.1097/01.wco.0000162850.44897.c6. [DOI] [PubMed] [Google Scholar]
- 7.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;34:557–63. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 8.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–70. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 9.Kochanek PM, Fink EL, Bell MJ, et al. Therapeutic hypothermia: applications in pediatric cardiac arrest. J Neurotrauma. 2009;26:421–7. doi: 10.1089/neu.2008.0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kochanek PM. Bakken Lecture: the brain, the heart, and therapeutic hypothermia. Cleve Clin J Med. 2009;76 (Suppl 2):S8–12. doi: 10.3949/ccjm.76.s2.02. [DOI] [PubMed] [Google Scholar]
- 11.Schwab S, Schwarz S, Spranger M, et al. Moderate hypothermia in the treatment of patients with severe middle cerebral artery infarction. Stroke. 1998;29:2461–6. doi: 10.1161/01.str.29.12.2461. [DOI] [PubMed] [Google Scholar]
- 12.Martinez-Arizala A, Green BA. Hypothermia in spinal cord injury. J Neurotrauma. 1992;9 (Suppl 2):S497–505. [PubMed] [Google Scholar]
- 13.Heinius G, Wladis A, Hahn RG, et al. Induced hypothermia and rewarming after hemorrhagic shock. J Surg Res. 2002;108:7–13. doi: 10.1006/jsre.2002.6470. [DOI] [PubMed] [Google Scholar]
- 14.Oddo M, Schaller MD, Feihl F, et al. From evidence to clinical practice: effective implementation of therapeutic hypothermia to improve patient outcome after cardiac arrest. Crit Care Med. 2006;34:1865–73. doi: 10.1097/01.CCM.0000221922.08878.49. [DOI] [PubMed] [Google Scholar]
- 15.Polderman KH. Application of therapeutic hypothermia in the intensive care unit. Opportunities and pitfalls of a promising treatment modality--Part 2: Practical aspects and side effects. Intensive Care Med. 2004;30:757–69. doi: 10.1007/s00134-003-2151-y. [DOI] [PubMed] [Google Scholar]
- 16.Arpino PA, Greer DM. Practical pharmacologic aspects of therapeutic hypothermia after cardiac arrest. Pharmacotherapy. 2008;28:102–11. doi: 10.1592/phco.28.1.102. [DOI] [PubMed] [Google Scholar]
- 17.Chamorro C, Borrallo JM, Romera MA, et al. Anesthesia and analgesia protocol during therapeutic hypothermia after cardiac arrest: a systematic review. Anesth Analg. 2010;110:1328–35. doi: 10.1213/ANE.0b013e3181d8cacf. [DOI] [PubMed] [Google Scholar]
- 18.Devlin JW, Mallow-Corbett S, Riker RR. Adverse drug events associated with the use of analgesics, sedatives, and antipsychotics in the intensive care unit. Crit Care Med. 2010;38:S231–43. doi: 10.1097/CCM.0b013e3181de125a. [DOI] [PubMed] [Google Scholar]
- 19.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279:1200–5. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 20.Kane-Gill SL, Jacobi J, Rothschild JM. Adverse drug events in intensive care units: risk factors, impact, and the role of team care. Crit Care Med. 2010;38:S83–9. doi: 10.1097/CCM.0b013e3181dd8364. [DOI] [PubMed] [Google Scholar]
- 21.Chernow B. Essentials of Critical Care Pharmacology. Williams and Wilkins; 1989. pp. 77–86. [Google Scholar]
- 22.Cytochrome P450 drug interaction table. Indiana University School of Medicine; [Last accessed Feb24 2011]. Available at: http://medicine.iupui.edu/clinpharm/ddis/table.asp. [Google Scholar]
- 23.Kim RB. Drugs as P-glycoprotein substrates, inhibitors, and inducers. Drug Metab Rev. 2002;34:47–54. doi: 10.1081/dmr-120001389. [DOI] [PubMed] [Google Scholar]
- 24.Kalliokoski A, Niemi M. Impact of OATP transporters on pharmacokinetics. Br J Pharmacol. 2009;158:693–705. doi: 10.1111/j.1476-5381.2009.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pang KS, Rowland M. Hepatic clearance of drugs. I. Theoretical considerations of a “well-stirred” model and a “parallel tube” model. Influence of hepatic blood flow, plasma and blood cell binding, and the hepatocellular enzymatic activity on hepatic drug clearance. J Pharmacokinet Biopharm. 1977;5:625–53. doi: 10.1007/BF01059688. [DOI] [PubMed] [Google Scholar]
- 26.Leslie K, Sessler DI, Bjorksten AR, et al. Mild hypothermia alters propofol pharmacokinetics and increases the duration of action of atracurium. Anesth Analg. 1995;80:1007–14. doi: 10.1097/00000539-199505000-00027. [DOI] [PubMed] [Google Scholar]
- 27.Fritz HG, Holzmayr M, Walter B, et al. The effect of mild hypothermia on plasma fentanyl concentration and biotransformation in juvenile pigs. Anesth Analg. 2005;100:996–1002. doi: 10.1213/01.ANE.0000146517.17910.54. [DOI] [PubMed] [Google Scholar]
- 28.Daemen MJ, Thijssen HH, Vervoort-Peters HT, et al. The effect of pentobarbitone anaesthesia and hypothermia on the hepatic clearance of indocyanine green and S(−)-acenocoumarol in the rat. J Pharm Pharmacol. 1986;38:122–5. doi: 10.1111/j.2042-7158.1986.tb04524.x. [DOI] [PubMed] [Google Scholar]
- 29.Nishida K, Okazaki M, Sakamoto R, et al. Change in pharmacokinetics of model compounds with different elimination processes in rats during hypothermia. Biol Pharm Bull. 2007;30:1763–7. doi: 10.1248/bpb.30.1763. [DOI] [PubMed] [Google Scholar]
- 30.Iida Y, Nishi S, Asada A. Effect of mild therapeutic hypothermia on phenytoin pharmacokinetics. Ther Drug Monit. 2001;23:192–7. doi: 10.1097/00007691-200106000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Tortorici MA, Kochanek PM, Bies RR, et al. Therapeutic hypothermia-induced pharmacokinetic alterations on CYP2E1 chlorzoxazone-mediated metabolism in a cardiac arrest rat model. Crit Care Med. 2006;34:785–91. doi: 10.1097/01.ccm.0000201899.52739.4f. [DOI] [PubMed] [Google Scholar]
- 32.van den Broek MP, Groenendaal F, Egberts AC, et al. Effects of hypothermia on pharmacokinetics and pharmacodynamics: a systematic review of preclinical and clinical studies. Clin Pharmacokinet. 2010;49:277–94. doi: 10.2165/11319360-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 33.Lin JH, Lu AY. Role of pharmacokinetics and metabolism in drug discovery and development. Pharmacol Rev. 1997;49:403–49. [PubMed] [Google Scholar]
- 34.Kumar GN, Surapaneni S. Role of drug metabolism in drug discovery and development. Med Res Rev. 2001;21:397–411. doi: 10.1002/med.1016. [DOI] [PubMed] [Google Scholar]
- 35.Fukuoka N, Aibiki M, Tsukamoto T, et al. Biphasic concentration change during continuous midazolam administration in brain-injured patients undergoing therapeutic moderate hypothermia. Resuscitation. 2004;60:225–30. doi: 10.1016/j.resuscitation.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 36.Hostler D, Zhou J, Tortorici MA, et al. Mild hypothermia alters midazolam pharmacokinetics in normal healthy volunteers. Drug Metab Dispos. 2010;38:781–8. doi: 10.1124/dmd.109.031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caldwell JE, Heier T, Wright PM, et al. Temperature-dependent pharmacokinetics and pharmacodynamics of vecuronium. Anesthesiology. 2000;92:84–93. doi: 10.1097/00000542-200001000-00018. [DOI] [PubMed] [Google Scholar]
- 38.Stearns RA, Chakravarty PK, Chen R, et al. Biotransformation of losartan to its active carboxylic acid metabolite in human liver microsomes. Role of cytochrome P4502C and 3A subfamily members. Drug Metab Dispos. 1995;23:207–15. [PubMed] [Google Scholar]
- 39.Heier T, Clough D, Wright PM, et al. The influence of mild hypothermia on the pharmacokinetics and time course of action of neostigmine in anesthetized volunteers. Anesthesiology. 2002;97:90–5. doi: 10.1097/00000542-200207000-00013. [DOI] [PubMed] [Google Scholar]
- 40.Kadar D, Tang BK, Conn AW. The fate of phenobarbitone in children in hypothermia and at normal body temperature. Can Anaesth Soc J. 1982;29:16–23. doi: 10.1007/BF03007942. [DOI] [PubMed] [Google Scholar]
- 41.Schaible DH, Cupit GC, Swedlow DB, et al. High-dose pentobarbital pharmacokinetics in hypothermic brain-injured children. J Pediatr. 1982;100:655–60. doi: 10.1016/s0022-3476(82)80780-4. [DOI] [PubMed] [Google Scholar]
- 42.Kalser SC, Kelvington EJ, Kunig R, et al. Drug metabolism in hypothermia. Uptake, metabolism and excretion of C14-procaine by the isolated, perfused rat liver. J Pharmacol Exp Ther. 1968;164:396–404. [PubMed] [Google Scholar]
- 43.Beaufort AM, Wierda JM, Belopavlovic M, et al. The influence of hypothermia (surface cooling) on the time-course of action and on the pharmacokinetics of rocuronium in humans. Eur J Anaesthesiol Suppl. 1995;11:95–106. [PubMed] [Google Scholar]
- 44.Carrière V, Berthou F, Baird S, et al. Human cytochrome P450 2E1 (CYP2E1): from genotype to phenotype. Pharmacogenetics. 1996;6:203–11. doi: 10.1097/00008571-199606000-00002. [DOI] [PubMed] [Google Scholar]
- 45.Wittwer E, Kern SE. Role of morphine’s metabolites in analgesia: concepts and controversies. AAPS J. 2006;8:E348–52. doi: 10.1007/BF02854905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Róka A, Melinda KT, Vásárhelyi B, et al. Elevated morphine concentrations in neonates treated with morphine and prolonged hypothermia for hypoxic ischemic encephalopathy. Pediatrics. 2008;121:e844–9. doi: 10.1542/peds.2007-1987. [DOI] [PubMed] [Google Scholar]
- 47.Bansinath M, Turndorf H, Puig MM. Influence of hypo and hyperthermia on disposition of morphine. J Clin Pharmacol. 1988;28:860–4. doi: 10.1002/j.1552-4604.1988.tb03229.x. [DOI] [PubMed] [Google Scholar]
- 48.Jin JS, Sakaeda T, Kakumoto M, et al. Effect of therapeutic moderate hypothermia on multi-drug resistance protein 1-mediated transepithelial transport of drugs. Neurol Med Chir (Tokyo) 2006;46:321–7. doi: 10.2176/nmc.46.321. [DOI] [PubMed] [Google Scholar]
- 49.Smith DA, Jones BC, Walker DK. Design of drugs involving the concepts and theories of drug metabolism and pharmacokinetics. Med Res Rev. 1996;16:243–66. doi: 10.1002/(SICI)1098-1128(199605)16:3<243::AID-MED2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 50.Liu X, Borooah M, Stone J, et al. Serum gentamicin concentrations in encephalopathic infants are not affected by therapeutic hypothermia. Pediatrics. 2009;124:310–5. doi: 10.1542/peds.2008-2942. [DOI] [PubMed] [Google Scholar]
- 51.Satas S, Hoem NO, Melby K, et al. Influence of mild hypothermia after hypoxia-ischemia on the pharmacokinetics of gentamicin in newborn pigs. Biol Neonate. 2000;77:50–7. doi: 10.1159/000014195. [DOI] [PubMed] [Google Scholar]
- 52.Koren G, Barker C, Bohn D, et al. Influence of hypothermia on the pharmacokinetics of gentamicin and theophylline in piglets. Crit Care Med. 1985;13:844–7. doi: 10.1097/00003246-198510000-00014. [DOI] [PubMed] [Google Scholar]
- 53.Miller RD, Agoston S, van der Pol F, et al. Hypothermia and the pharmacokinetics and pharmacodynamics of pancuronium in the cat. J Pharmacol Exp Ther. 1978;207:532–8. [PubMed] [Google Scholar]
- 54.Heier T, Caldwell JE, Sessler DI, et al. Mild intraoperative hypothermia increases duration of action and spontaneous recovery of vecuronium blockade during nitrous oxide-isoflurane anesthesia in humans. Anesthesiology. 1991;74:815–9. doi: 10.1097/00000542-199105000-00003. [DOI] [PubMed] [Google Scholar]
- 55.Ham J, Miller RD, Benet LZ, et al. Pharmacokinetics and pharmacodynamics of d-tubocurarine during hypothermia in the cat. Anesthesiology. 1978;49:324–9. doi: 10.1097/00000542-197811000-00005. [DOI] [PubMed] [Google Scholar]
- 56.Heier T, Caldwell JE. Impact of hypothermia on the response to neuromuscular blocking drugs. Anesthesiology. 2006;104:1070–80. doi: 10.1097/00000542-200605000-00025. [DOI] [PubMed] [Google Scholar]
- 57.Liu M, Hu X, Liu J. The effect of hypothermia on isoflurane MAC in children. Anesthesiology. 2001;94:429–32. doi: 10.1097/00000542-200103000-00011. [DOI] [PubMed] [Google Scholar]
- 58.Puig MM, Warner W, Tang CK, et al. Effects of temperature on the interaction of morphine with opioid receptors. Br J Anaesth. 1987;59:1459–64. doi: 10.1093/bja/59.11.1459. [DOI] [PubMed] [Google Scholar]
- 59.Han YS, Tveita T, Kondratiev TV, et al. Changes in cardiovascular beta-adrenoceptor responses during hypothermia. Cryobiology. 2008;57:246–50. doi: 10.1016/j.cryobiol.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 60.Williams RG, Broadley KJ. Responses mediated via beta 1, but not beta 2-adrenoceptors, exhibit hypothermia-induced supersensitivity. Life Sci. 1982;31:2977–83. doi: 10.1016/0024-3205(82)90064-9. [DOI] [PubMed] [Google Scholar]
- 61.Tortorici MA, Kochanek PM, Poloyac SM. Effects of hypothermia on drug disposition, metabolism, and response: A focus of hypothermia-mediated alterations on the cytochrome P450 enzyme system. Crit Care Med. 2007;35:2196–204. doi: 10.1097/01.ccm.0000281517.97507.6e. [DOI] [PubMed] [Google Scholar]
- 62.Tran BP, McGuire CV, Maloney MA. Use of mild therapeutic hypothermia improves outcomes in cardiac arrest. JAAPA. 2010;23:43–8. doi: 10.1097/01720610-201003000-00008. [DOI] [PubMed] [Google Scholar]