Abstract
Cutaneous T cell lymphomas (CTCL) represent a spectrum of several distinct non-Hodgkin's lymphomas that are characterized by an invasion of the skin by malignant, clonal lymphocytes. Our lab has previously demonstrated that the Protein Kinase C (PKC) β inhibitor Enzastaurin increases apoptosis in malignant lymphocytes of CTCL. These results directly led to a clinical trial for Enzastaurin in CTCL where it was well tolerated and showed modest activity. To ascertain a means of improving the efficacy of Enzastaurin, we investigated complimentary signaling pathways and identified Glycogen Synthase Kinase 3 (GSK3) as important in survival signaling in CTCL. Enzastaurin combined with GSK3 inhibitors demonstrated anenhancement of cytotoxicity. Treatment with a combination of Enzastaurin and the GSK3 inhibitor AR-A014418 resulted in up-regulation of β catenin total protein and β catenin-mediated transcription. Inhibition of β catenin-mediated transcription or shRNA knockdown of β catenin decreased the cytotoxic effects of Enzastaurin plus AR-A014418. In addition, treatment with Enzastaurin and AR-A014418 decreased the mRNA levels and surface expression of CD44. shRNA knockdown of β catenin also restored CD44 surface expression. Our observations provide a rationale for the combined targeting of PKC and GSK3 signaling pathways in CTCL to enhance the therapeutic outcome.
Introduction
Cutaneous T cell lymphomas (CTCL) represent a spectrum of several distinct extranodal non-Hodgkin's lymphomas. These lymphomas are characterized by an invasion of the skin by malignant, clonal CD4+ lymphocytes (Jakob et al., 1996; Rook et al., 1997). Although treatment may achieve remission, early chemotherapeutic treatment of CTCL has not demonstrated survival benefits and no treatments have proven to be curative. Therefore, new therapeutic approaches to this disease are needed.
Our lab has previously demonstrated that treatment with the novel Protein Kinase C β (PKC) inhibitor Enzastaurin increases apoptosis in CTCL (Querfeld et al., 2006). These results directly led to a clinical trial for Enzastaurin in CTCL where it was well tolerated and demonstrated modest biologic activity, suggesting that it may serve as a good platform for combination drug therapies (submitted for publication(Querfeld et al., 2010)).To improve the efficacy of Enzastaurin, we surveyed complementary signaling pathways to identify potential synergistic activity with Enzastaurin. We have identified GSK3 as important in survival signaling in CTCL. GSK3 is a constitutively active serine/threonine kinase involved in a wide variety of cellular processes including glycogen metabolism, transcription, translation, cell cycle and apoptosis (Cohen and Goedert, 2004).
One critical downstream target of both PKC and GSK3 is β catenin. β catenin is a multifunctional protein that has been implicated in a number of biological processes including cell-cell adhesion, embryonic developmentand the Wnt signaling pathway. β catenin is phosphorylated on conserved serine and threonine residues which leads to its ubiquitination and proteasomal degradation. Inhibition of β catenin phosphorylation results in accumulation of cytoplasmic and nuclear β catenin and subsequent binding totranscriptional cofactors such as the TCF/LEF family of proteins (Gumbiner, 1995; He et al., 1998; Mann et al., 1999).
A number of genes involved in apoptosis and survival signaling have been shown to be regulated by β catenin, including CD44(Voutilainen et al., 2006; Wielenga et al., 1999). CD44 is a transmembrane glycoprotein that has been shown to deliver survival signals though a number of intracellular components including PI3K/Akt, Erk, Ras and Lyn (Bates et al., 2001; Bourguignon et al., 2006; Bourguignon et al., 2005; Kothapalli et al., 2008; Lin and Yang-Yen, 2001). Additionally, CD44 increases resistance to cellular stress induced by cytotoxic agents and is able to block p53-dependent cytostatic and apoptotic signals (Godar et al., 2008; Xu et al., 2010).
Our data demonstrates the observation thatco-treatment with Enzastaurin and GSK3 inhibitors results in an increase in apoptosis in part through up-regulation of β catenin levels and modulation of the transcriptional activity of β catenin. To our knowledge this is previously unreported. This increase in β catenin represses expression of the pro-survival factor CD44 at the mRNA and protein level. Knockdown of β catenin restored cell survival as well as expression of CD44. Combination therapy which targets both PKC and GSK3 may offer better clinical options for CTCL.
Results
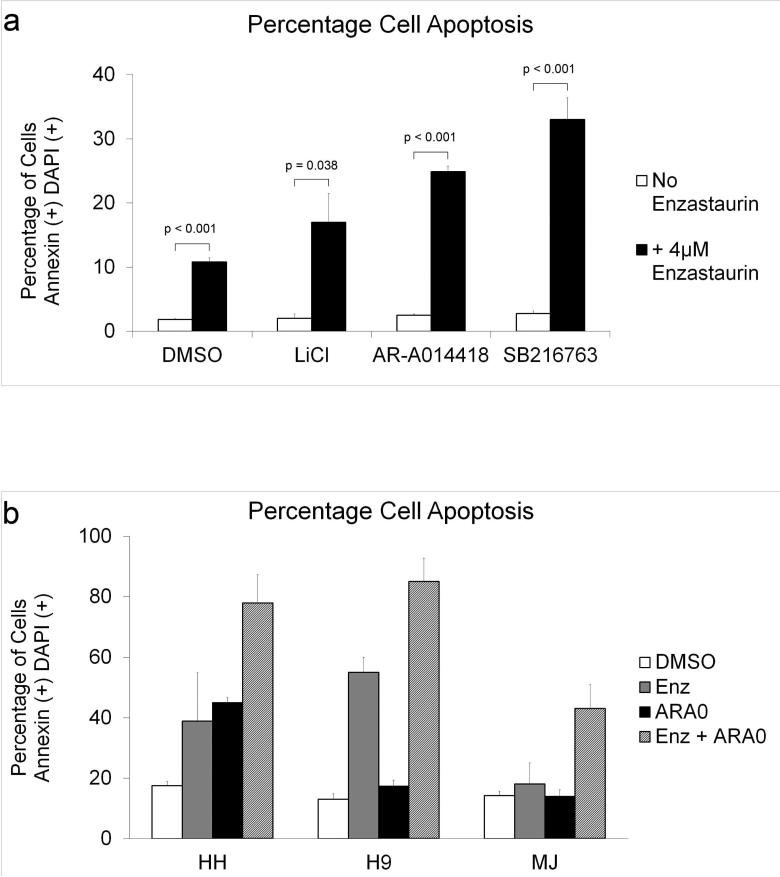
Previous data from our lab indicate that treatment with Enzastaurin results in an increase in apoptosis in HH and HuT-78 CTCL cell lines (Querfeld et al., 2006). Due to the modest efficacy of Enzastaurin in Phase II clinical trial in CTCL, we sought to identify pathways that could enhance the cytotoxicity of Enzastaurin. Recent in vitro and in vivo studies have suggested that the GSK3 signaling pathway is important for survival of malignant cells(Ougolkov et al., 2007; Ougolkov et al., 2005). To test if GSK3 is an important survival factor in CTCL, we treated HuT-78 cells with three structurally distinct GSK3 inhibitors; LiCl, AR-A014418 and SB216763. None of the GSK3 inhibitors alone significantly affected the rate of apoptosis. However, when combined individually with Enzastaurin all three achieved a statistically significant increase in the rate of apoptosis compared to Enzastaurin alone (Figure 1a). AR-A014418 was chosen as a GSK3 inhibitor for remaining experiments due to its high specificity for GSK3 and lack of off-target effects compared to the other two inhibitors (Cohen and Goedert, 2004).
Figure 1. GSK3 Inhibitors Combined with Enzastaurin Increase Apoptosis.
a) HuT-78 cells were cultured in 4μM Enzastaurin, 5mM LiCl, 5μM AR-A014418, 5μM SB216763 or DMSO (vehicle control) and harvested after 5 days. b) HH, H9 and MJ cells were cultured in 4μM Enzastaurin (H9), 8μM Enzastaurin (HH, MJ), 5μM AR-A014418 (H9), 10μM AR-A014418 (MJ), 20μM AR-A014418 (HH), or DMSO (vehicle control) and harvested after 5 days. Detection of Annexin V and DAPI staining for all cells was performed as described previously. Data from three separate experiments was averaged to quantify apoptotic cells (double positive cells) as a percentage of total cells. Error bars represent standard deviation.
The combination of Enzastaurin and AR-A014418 was also examined in other CTCL-derived cell lines to determine if GSK3 inhibition increased the cytotoxicity of Enzastaurin in a similar manner. Enzastaurin combined with AR-A014418 increased apoptosis compared to DMSO control or either drug alone in the cell lines HH, H9 and MJ (Figure 1b). These results demonstrate that the increase in apoptosis induced by this combination of drugs is not restricted to a single cell line.
To determine the therapeutic potential of inhibiting PKC and GSK3, we extended our studies beyond cell lines to include ex vivo samples isolated from CTCL patients. Malignant cells from severalCTCL patients were collected, incubated with the inhibitors and assessed for percentage of cells undergoing apoptosis. The program Calcusyn was used to determine whether the combination of Enzastaurin and AR-A014418 exhibited synergy (http://www.biosoft.com/w/calcusyn.htm). Cells were treated with the inhibitors and the combination index (CI) was calculated. A CI of less than one is interpreted as synergy between the two compounds whereas a CI equal to one suggests additivity. Treatment with the combination of Enzastaurin and AR-A014418 increased apoptosis in a synergistic or additive manner in all patient samples, suggesting that this drug combination holds potential in treating CTCL (Table 1).
Table 1.
CI Values at Different Concentrations of Enzastaurin and AR-A014418 in ex vivo Patient Samples
| Patient | Enzastaurin (uM) | ARA014418 (uM) | CI |
|---|---|---|---|
| P1 | 2 4 |
5 5 |
0.805 0.784 |
| P2 | 2 4 |
5 5 |
0.737 0.825 |
| P3 | 2 4 |
5 5 |
0.872 0.936 |
| P4 | 2 4 |
5 5 |
0.563 0.954 |
| P5 | 2 4 |
5 5 |
0.568 0.801 |
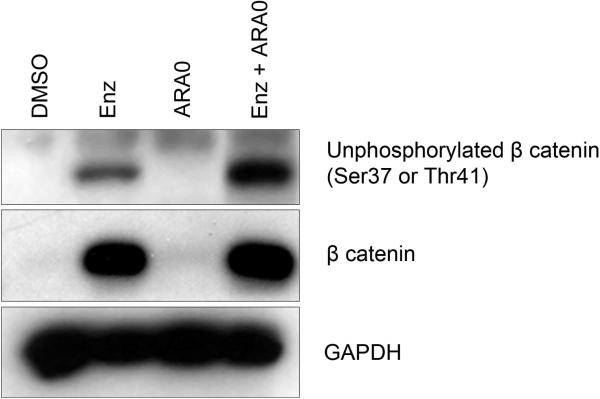
Both PKC and GSK3 can phosphorylateβ catenin and target it for subsequent proteasomal degradation(Chen et al., 2000; Gwak et al., 2006; Raab et al., 2009). Inhibition of β catenin phosphorylation can lead to increased transcriptional activity of β catenin and modulation of gene expression, including a number of genes involved in apoptosis and survival signaling(Clevers and van de Wetering, 1997; He et al., 1998; Mann et al., 1999). To determine if PKC and GSK3 regulate β catenin levels in CTCL, we cultured cells in the presence of Enzastaurin and AR-A014418. Treatment with Enzastaurin alone or Enzastaurin and AR-A014418 resulted in an up-regulation of β catenin protein levels (Figure 2). We also utilized an antibody that detects unphosphorylated β catenin to determine if the phosphorylation state of β catenin was affected by the inhibitors. Treatment with the combination of Enzastaurin and AR-A014418 decreased phosphorylation of β catenin compared to Enzastaurin alone.
Figure 2. Enzastaurin Combined with AR-A014418 Increases Unphosphorylated (Active) β catenin Levels.
HuT-78 cells were cultured instandard drug conditions of DMSO, 4 μM Enzastaurin, 5 μM AR-A014418 or the combination of Enzastaurin and AR-A014418 and lysates were prepared at 24 hours. Immunoblots were performed as described previously. Blot is representative of three separate experiments.
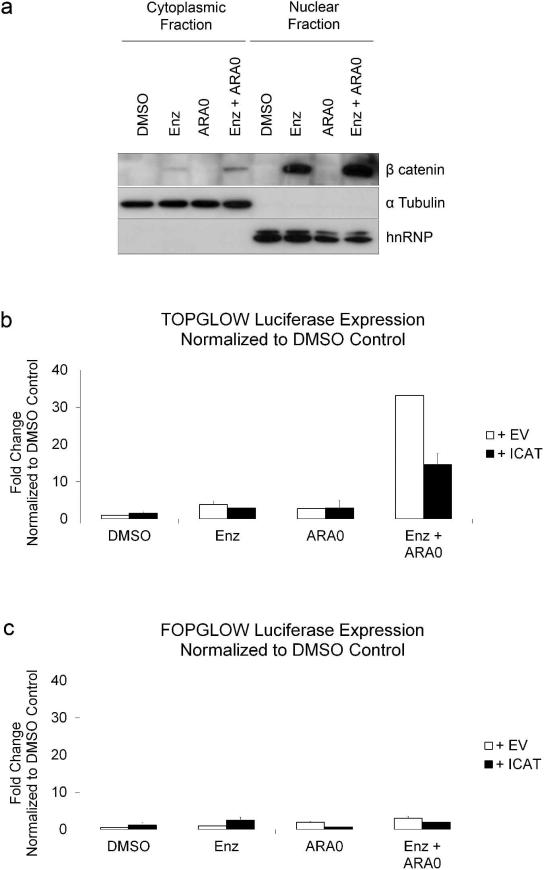
Unphosphorylated β catenin is targeted to the nucleus to form a nuclear complex with TCF / LEF transcription factors (Eastman and Grosschedl, 1999; Huber et al., 1996). To determine if the combination of inhibitors increased the nuclear localization and transcriptional activity of β catenin, cells were incubated with the two inhibitors and cells were fractionated to separate nuclear and cytoplasmic fractions. α Tubulin was used as a cytoplasmic marker and hnRNP A0 was used as a nuclear marker. Treatment with Enzastaurin and AR-A014418resulted in an increase of nuclear β catenin compared to DMSO control or either inhibitor alone (Figure 3a). To examine if inhibitor treatment affected β catenin-mediated transcription, we used a TCF / β catenin luciferase reporter. Cells were transfected with the reporter and treated with the inhibitors Enzastaurin and AR-A014418. Neither Enzastaurin nor AR-A014418 alone significantly increased luciferase activity (Figure 3b). However, the combination of both inhibitors resulted in an increase in luciferase activity. Cells were also transiently transfected with ICAT, a protein which represses TCF / β catenin -mediated transactivation via inhibition of the interaction between β catenin and TCF (Tago et al., 2000). ICAT expression decreased luciferase activity compared to vector control in cells treated with Enzastaurin and AR-A014418. In a separate experiment, cells were transiently transfected with a control luciferase reporter, FOPGLOW (mutant TCF binding sites) and no significant difference in activity was observed between cells treated with DMSO control or any of the inhibitors (Figure 3c).
Figure 3. Enzastaurin Combined with AR-A014418 Increases Nuclear Localization and Transcriptional Acitivity of β catenin.
a) HuT-78 cells were cultured in the standard drug conditions of DMSO, Enzastaurin or AR-A014418 and harvested at 24 hours. Cell were fractionated and resultant lysates were immunoblotted with indicated antibodies.b)HuT-78 cells were transfected with 3 μg TOPGLOW luciferase reporter (Millipore, Billerica, MA) and either PC3DNA or ICAT vector, treated with inhibitors and harvested after 24 hours. c)HuT-78 cells were transfected with 3 μg FOPGLOW luciferase reporter (Millipore, Billerica, MA) and either PC3DNA or ICAT vector, treated with inhibitors and harvested after 24 hours. Luciferase reporter assays were carried out using manufacturer's instructions (Promega, Madison, WI).
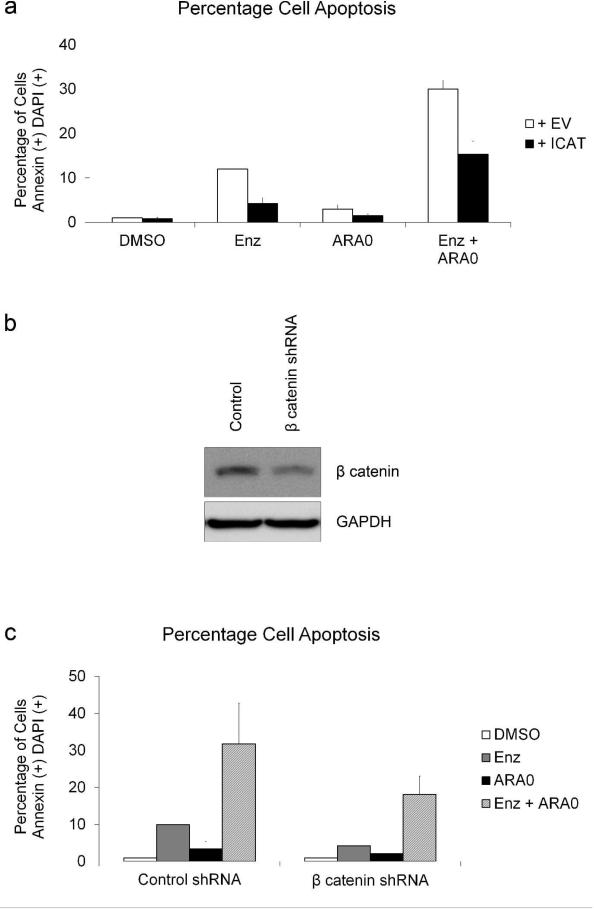
To determine if β catenin-mediated gene regulationis important in modulating cell survival we transiently transfected HuT-78 cells with either the ICAT vector or an empty vector control, treated with the inhibitors and examined the percentage of cells undergoing apoptosis. In cells co-treated with Enzastaurin and AR-A014418, ICAT expression decreased apoptosis compared to vector control (Figure 4a). This suggests that β catenin-mediated gene regulation is in part responsible for the activation of apoptosis in cells treated with Enzastaurin and AR-A014418. To confirm these results, we knocked down β catenin using an shRNA-expressing lentivirus. Cells were transduced with either control lentivirus or β catenin shRNA lentivirus and selected in puromycin. Cells were then treated with Enzastaurin and AR-A014418 and β catenin protein was examined by immunoblot. Total β catenin levels were decreased in cells transduced with the β catenin shRNA lentivirus compared to cells transduced with control lentivirus (Figure 4b). Apoptosis was also measuredin the β catenin knockdown and control cells. A decrease in the percentage of cells undergoing apoptosis when treated with Enzastaurin and AR-A014418 was observed in the β catenin knockdown cells compared to control cells (Figure 4c). Similarly, β catenin levels were also knocked down in the CTCL cell lines HH, H9 and MJ. Knockdown of β catenin levels in all three cell lines resulted in a decrease in the percentage of cells undergoing apoptosis when treated with Enzastaurin and AR-A014418 compared to control cells (Supplementary Figure S2 and S3 online).
Figure 4. β catenin-mediated Transcription Increases Apoptosis in CTCL.
a)HuT-78 cells were transfected with 3 μg GFP expressing vector and either PC3DNA or ICAT vector. Detection of Annexin V and DAPI staining was performed as described previously. Data from three separate experiments was used to quantify double positive cells as a percentage of total cells. Error bars represent standard deviation. b) β catenin expression was knocked down in HuT-78 cells using an shRNA-expressing lentivirus. Cells were then treated with inhibitors and β catenin protein was examined by immunoblot after 24 hours. c) Detection of Annexin V and DAPI staining was performed on HuT-78 cells transduced with lentivirus as described previously. Data from three separate experiments was used to quantify double positive cells as a percentage of total cells. Error bars represent standard deviation.
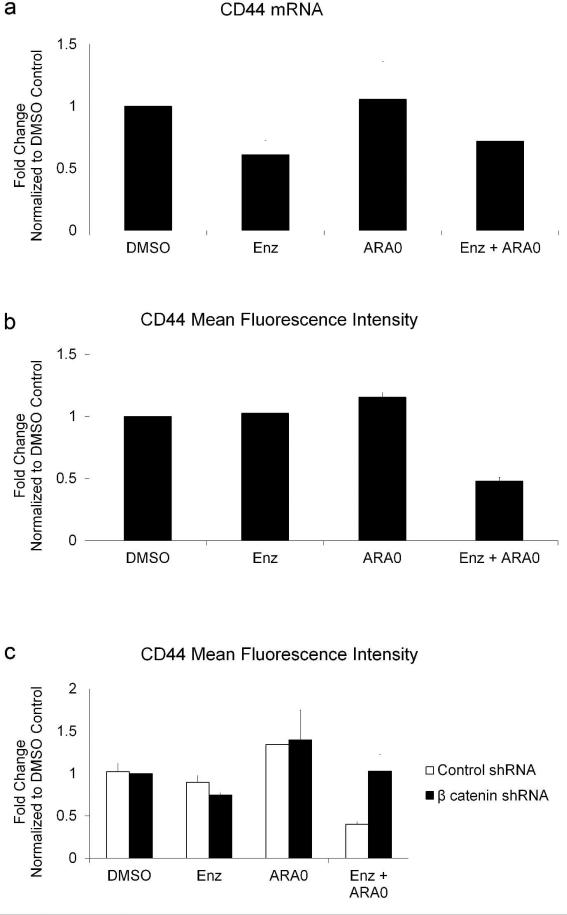
β catenin can modulate the transcription of several genes involved in survival signaling, including CD44. We examined mRNA levels of CD44 in cells treated with Enzastaurin and AR-A014418 to determine if the increase in β catenin levels resulted in adecrease in gene expression. Treatment with Enzastaurin or the combination of Enzastaurin and AR-A014418 resulted in a decrease in CD44 mRNA levels (Figure 5a). To determine if treatment with the inhibitors nonspecifically decreases all transcriptional targets of β catenin, we examined the effect of Enzastaurin and AR-A014418 on c-Myc and Cyclin D1. Treatment with the two inhibitors did not significantly affect expression of c-Myc or Cyclin D1 (data not shown), suggesting that co-treatment with Enzastaurin and AR-A014418 modulates only a subset of β catenin responsive genes.
Figure 5. Enzastaurin Combined with AR-A014418 Modulates Expression of CD44.
a)RNA was isolated from HuT-78 cells treated with inhibitors and data from three separate experiments was used to calculate the change in gene expression as a percentage of DMSO control. Error bars represent standard deviation.b)HuT-78 cells were stained with antibodies that detect CD44 and examined by flow cytometry. Cells in late apoptosis bind antibodies non-specifically and were therefore gated out during analysis. Error bars represent standard deviation from three separate experiments. c) HuT-78 cells transduced with lentiviruses were stained with antibodies against CD44 and examined by flow cytometry. Error bars represent standard deviation from three separate experiments.
To determine if this decrease in CD44 mRNA resulted in lower surface expression of CD44, cells were treated with the two inhibitors and examined by flow cytometry. Surface expression of CD44 decreased in cells treated with the two inhibitors compared to cells treated with either inhibitor alone or DMSO control (Figure 5b). To confirm that the observed decrease in CD44 surface expression was mediated by β catenin, β catenin was knocked down and cells were treated with the two inhibitors. Knockdown of β catenin resulted in a restoration of surface CD44 levels, suggesting that β catenin was indeed responsible for the drug-induceddecrease in CD44 (Figure 5c).
Discussion
The goal of cancer therapeutics is to eliminate cancer cells with minimal damage to nonmalignant cells. The combination of Enzastaurin and AR-A014418 seeks to achieve this goal by inhibiting complementary survival pathways important for tumor cell survival and allows for lower doses of the individual drugs that may be less toxic to nonmalignant cells. Treatment with Enzastaurin and AR-A014418 results in enhanced apoptosisin CTCL cell lines as well as in patient samples, suggesting that GSK3 and PKC signaling pathways play important roles in CTCL. Treatment with Enzastaurin and AR-A014418 resulted ina decrease in β catenin phosphorylation, an accumulation of cytoplasmic and nuclear β catenin and an increase in transcriptional activity. This increase in transcriptionally active β catenin resulted inan increased rate of apoptosis andtranscriptional changes in CD44. Together, this points to the combination of PKC and GSK3 inhibition as a potential treatment for CTCL.
Although Enzastaurin primarily affects PKC, Enzastaurin has been shown to inhibit other AGC(cAMP-dependent protein kinase/protein kinase G/protein kinase C) family kinases involved in survival signaling, including Akt and p90RSK(Graff et al., 2005). We have not ruled out the possibility that the Enzastaurin-mediated increase in apoptosis observed in CTCL may be due in part to additional factors outside of the inhibition of PKC. Additional studies will be needed to better define the critical targets of Enzastaurin.
β catenin has been shown to be over-expressed in some CTCL patients (Bellei et al., 2006; Bellei et al., 2004). β catenin accumulation was seen in 31% of the MF samples and 70% of the SS samples in one study and 46% of MF cases in another study (Bellei et al., 2006; Bellei et al., 2004). However, the β catenin observed in these tumor samples was not found in the nucleus and there was no detectable expression of β catenin / TCF target genes (Bellei et al., 2004). This suggests that the mere presence of β catenin will not necessarily trigger apoptosis in CTCL. Translocation to the nucleus and transcriptional activity appear to be important in activating apoptosis. It is also likely that the specific cofactors that bind to β catenin also play a role. In addition to members of the TCF/LEF family of transcription factors, β catenin can associate with a number of other cofactors to modulate transcription, including p300, CBP, histone deacetylases and FOXO family proteins (Billin et al., 2000; Hecht et al., 2000; Hoogeboom et al., 2008; Takemaru and Moon, 2000). The expression of ICAT may disrupt the interaction of β catenin with other proteins besides TCF / LEF family members, such as p300 (Daniels and Weis, 2002).
β-catenin has been shown to not only up-regulate expression of TCF / β catenin target genes but also repress gene expression (Deng et al., 2002; Deng et al., 2004). For example, β catenin has been shown previously to inhibit transcription factors involved in survival signaling, such as NF-κB (Deng et al., 2002; Deng et al., 2004). Although some studies have suggested that β catenin may positively regulate the expression of CD44, β catenin appears to repress the expression of CD44 in the context of Enzastaurin and AR-A014418 treatment in CTCL cells.
Although Enzastaurin alone was able to decrease mRNA levels of CD44, only the combination of Enzastaurin and AR-A014418 was able to decrease surface expression of CD44. The control of CD44 expression is very complex and likely influenced by a number of factors, including splicing and posttranslational regulation. Expression of specific isoforms of CD44 can be regulated by PI3K and PKC (Fichter et al., 1997). β catenin can not only affect CD44 mRNA expression, but has been shown to also modulate splicing of CD44 isoforms (Goncalves et al., 2008). We used primer probe sets and flow cytometry antibodies that could detect all splice variants and isoforms of CD44, respectively. Treatment with Enzastaurin and AR-A014418 may only be inhibiting the surface expression of a subset of all CD44 isoforms. Further experiments will need to be conducted to determine the specific isoforms of CD44 affected by the combination of Enzastaurin and AR-A014418.
All different subtypes and stages of CTCL have been shown to express CD44 (Orteu et al., 1997). Additionally, CD44 functions in PKC-regulated migration of T cells (Fanning et al., 2005). Inhibition of PKC by Enzastaurin as well as the induced decrease of CD44 may be a particularly effective therapeutic againstthe skin-homing malignant lymphocytes of CTCL.
The concentrations of both Enzastaurin and AR-A014418 used in these experiments have been previously shown to be at or below achievable levels in vivo. Phase I clinical trials showed that steady-state plasma concentrations of up to 8μM Enzastaurin are achievable in patients(Graff et al., 2005). Although no pharmacokinetic data for AR-A014418 exists for humans, blood plasma concentrations of 3.75μM AR-A014418 have been measured with oral dosing of 1 μmol/kg in Sprague–Dawley rats and much higher dosages (10 fold) been used with no reported dose limiting toxicities (Martins et al., 2010)(Medina and Avila, 2010)(Gould et al., 2004).
The combination of Enzastaurin and AR-A014418 has the potential to affect a number of important signaling pathways. In vivo experiments in mice will be needed to address if the combination of Enzastaurin with a GSK3 inhibitor activates synergistic toxicities or if it has other off-target effects. For example, Enzastaurin is able to inhibit both natural and antibody-dependent cellular cytotoxicity of NK cells against tumor targets, and therefore may suppress NK cell-mediated activity against tumor cells(Ogbomo et al., 2011).Interestingly, treatment of NK cells with a GSK3 inhibitor reversed Enzastaurin-mediated inhibition of NK cell cytotoxicity. In vivo experiments will be needed to address these and other potential positive and negative off-target effects.
Understanding the role of PKC and GSK3 in survival signaling in CTCL will provide insight into the biology of CTCL as well as define rational targets for therapy. Our observations point to the importance of targeting these two pathways in CTCL and the possibility of an improvement in therapeutic outcome.
Materials and Methods
Cell lines and cell culture
HH, H9, HuT-78 and MJcells were grown in RPMI 1640 (Invitrogen, Baltimore, MD) supplemented with 10% fetal bovine serum (MJ in 20%), 2mM glutamine, 100 U/mL penicillin, 100 mg/mL streptomycin, and 2.5 mg/mL amphotericin B. For primary samples, IRB approval was obtained for this study, and each patient completed consent forms. Blood samples were collected from CTCL patients and peripheral blood mononuclear cells (PBMCs) were isolated using BD Vacutainer CPT tubes (BD Biosciences, San Jose, CA). CD4 positive cells were then isolated using manufacturer's instructions for the EasySep immuno-magnetic cell separation kit (Stemcell, BC, Canada).
Antibodies and Reagents
ICAT vector was a gift from the Gottardi Lab. Antibodies against β catenin and active β catenin were purchased from Millipore (Temecula, CA). Antibodies against α tubulin and hnRNP A0 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). CD44 antibodies were purchased from BD Biosciences. All other antibodies were purchased from Cell Signaling. Enzastaurin was a gift from Eli Lilly & Co (Indianapolis, IN). Annexin DAPI staining was performed according to the manufacturer's instructions (BD Pharmingen, San Diego, CA). Flow cytometry data was recorded by using a DakoCytomation CyAn machine, and samples analyzed by using FCS Express.
Cell Viability Assay
Cell viability assays were carried out using MTS reagent from Promega according to the manufacturer's instructions (Madison, WI). The data are expressed as percentage of DMSO control cells.
Transfection
HH and HuT-78 cells were transiently transfected using an Amaxa Nucleofector I device (Lonza, Cologne, Germany). Five million HuT-78 cells were electroporated in 0.1 mL of Solution V using the T-01 program with 3 μg of the appropriate vector.
Luciferase assay
Cells were transiently transfected with 3 μg of plasmid vector and incubated at 37°C for 24 hours. Lysates were prepared 24 hours post-transfection using the Promega Luciferase Assay System according to the manufacturer's instructions (Promega, Madison, WI). Luminescence was measured for 15 seconds using a BioTek Synergy HT plate reader (BioTek, Winooski, VT). Luminescence was normalized to protein concentration of sample.
Cell fractionation
Cells were washed twice in phosphate buffered saline (PBS) and resuspended in 1 mL Buffer A [10 mM HEPES, pH 7.5, 10 mM KCl, 1 mM EDTA, 1 mM DTT, 1 mM MgCl2, 5% v/v glycerol, 0.1 mM Na3VO4] for 10 min on ice. 25 μL Buffer B [10 mM HEPES, pH 7.5, 10 mM KCl, 1 mM EDTA, 1 mM DTT, 1 mM MgCl2, 5% v/v glycerol, 0.1 mM Na3VO4, 10% v/v Nonidet P-40] was added and lysates were spun down at 4,000 rpm for 10 min at 4°C. Supernatant was collected (cytoplasmic fraction) and stored. Remaining pellet was washed twice with 1 mL Buffer C [10 mM HEPES, pH 7.5, 10 mM KCl, 1 mM DTT, 1 mM MgCl2] and resuspended in 500 μL of Buffer D [20 mM HEPES, pH 7.5, 0.4M NaCl, 1 mM EDTA, 1 mM DTT, 1 mM MgCl2, 25% v/v glycerol]. Lysates were incubated for 15 min on ice with intermittent vortexing and then spun down at 15,000 × g for 5 min at 4°C. Supernatants were collected (nuclear fraction) and stored.
rt-PCR
RNA lysates were prepared using RNeasy Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Reverse transcriptase step was run on Eppendorf Master Cycle Thermocycler (Eppendorf, Hauppauge, NY).Real time PCR was performed using the TaqMan Gene Expression Assay (Applied Biosystems, Foster City, CA) and run on 7500 Fast Real Time PCR Machine (Applied Biosystems, Foster City, CA). Samples were run in triplicate. rt-PCR was performed a minimum of three independent times for each gene. RNA expression was normalized to three independent control genes using geNorm (http://medgen.ugent.be/~jvdesomp/genorm/).
Immunoblotting and immunoprecipitation
Lysates were prepared and resuspended in SDS loading buffer, heated to 95°C for 5 min, separated by 10% SDS-PAGE and transferred to Immobilon membranes. Membranes were blocked in BLOK Casein in TBS (G Biosciences, St. LouisMO) for 1 h at room temperature, incubated overnight in BLOK containing the appropriate primary antibodies at 4°C, washed three times in TBST, and incubated with secondary antibodies in BLOK for 1 hour at room temperature. For detection, membranes were washed three times in TBST and detected with ECL Plus (Pierce, Rockford, IL).
Lentivirus production and transduction
Lentivirus were produced using the Lentiphos HT kit (Clontech, Mountain View, CA) and GIPZ Lentiviral shRNAmir constructs according to the manufacturer's instructions (Open Biosystems, Huntsville, AL). To transduce the cells, one million cells were plated per well in a 6-well plate with 5 mL of viral supernatant and 5 μL of polybrene. Cells were spun for 2h at 3,200 rpm at room temperature.
Supplementary Material
Figure S1. HuT-78 cells were cultured in 4 μM Enzastaurin, 5 mM LiCl, 5 μM AR-A014418, 5 μM SB216763 or DMSO (vehicle control) and harvested after 5 days. Detection of Annexin V and DAPI staining was performed as described previously. Data shown in representative of three independent experiments.
Figure S2. Detection of Annexin V and DAPI staining was performed on HH, H9 and MJ cells transduced with lentivirus as described previously. Data from three separate experiments was used to quantify double positive cells as a percentage of total cells. Error bars represent standard deviation.
Figure S3. β catenin expression was knocked down in HH, H9 and MJ cells using an shRNA-expressing lentivirus. Cells were then treated with inhibitors and β catenin protein was examined by immunoblot after 24 hours.
Acknowledgements
We would like to thank Dr. Cara Gottardi for her gift of the ICAT vector as well as for her scientific guidance. We would also like to thank Dr. Fotini Gounari and David Pavkovich for critical reading of this manuscript.
Abbreviations used
- CTCL
cutaneous T-cell lymphoma
- PKC
Protein Kinase C
- GSK3
Glycogen Synthase Kinase 3
Footnotes
Conflict of Interest
Mark Rovedo has received an honorarium for presenting his data to Eli Lilly. Steve Rosen is an unpaid consultant of Eli Lilly.
References
- Bates RC, Edwards NS, Burns GF, Fisher DE. A CD44 survival pathway triggers chemoresistance via lyn kinase and phosphoinositide 3-kinase/Akt in colon carcinoma cells. Cancer Res. 2001;61:5275–83. [PubMed] [Google Scholar]
- Bellei B, Cota C, Amantea A, Muscardin L, Picardo M. Association of p53 Arg72Pro polymorphism and beta-catenin accumulation in mycosis fungoides. Br J Dermatol. 2006;155:1223–9. doi: 10.1111/j.1365-2133.2006.07527.x. [DOI] [PubMed] [Google Scholar]
- Bellei B, Pacchiarotti A, Perez M, Faraggiana T. Frequent beta-catenin overexpression without exon 3 mutation in cutaneous lymphomas. Mod Pathol. 2004;17:1275–81. doi: 10.1038/modpathol.3800181. [DOI] [PubMed] [Google Scholar]
- Billin AN, Thirlwell H, Ayer DE. Beta-catenin-histone deacetylase interactions regulate the transition of LEF1 from a transcriptional repressor to an activator. Mol Cell Biol. 2000;20:6882–90. doi: 10.1128/mcb.20.18.6882-6890.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon LY, Gilad E, Brightman A, Diedrich F, Singleton P. Hyaluronan-CD44 interaction with leukemia-associated RhoGEF and epidermal growth factor receptor promotes Rho/Ras co-activation, phospholipase C epsilon-Ca2+ signaling, and cytoskeleton modification in head and neck squamous cell carcinoma cells. J Biol Chem. 2006;281:14026–40. doi: 10.1074/jbc.M507734200. [DOI] [PubMed] [Google Scholar]
- Bourguignon LY, Gilad E, Rothman K, Peyrollier K. Hyaluronan-CD44 interaction with IQGAP1 promotes Cdc42 and ERK signaling, leading to actin binding, Elk-1/estrogen receptor transcriptional activation, and ovarian cancer progression. J Biol Chem. 2005;280:11961–72. doi: 10.1074/jbc.M411985200. [DOI] [PubMed] [Google Scholar]
- Chen RH, Ding WV, McCormick F. Wnt signaling to beta-catenin involves two interactive components. Glycogen synthase kinase-3beta inhibition and activation of protein kinase C. J Biol Chem. 2000;275:17894–9. doi: 10.1074/jbc.M905336199. [DOI] [PubMed] [Google Scholar]
- Clevers H, van de Wetering M. TCF/LEF factor earn their wings. Trends Genet. 1997;13:485–9. doi: 10.1016/s0168-9525(97)01305-x. [DOI] [PubMed] [Google Scholar]
- Cohen P, Goedert M. GSK3 inhibitors: development and therapeutic potential. Nat Rev Drug Discov. 2004;3:479–87. doi: 10.1038/nrd1415. [DOI] [PubMed] [Google Scholar]
- Daniels DL, Weis WI. ICAT inhibits beta-catenin binding to Tcf/Lef-family transcription factors and the general coactivator p300 using independent structural modules. Mol Cell. 2002;10:573–84. doi: 10.1016/s1097-2765(02)00631-7. [DOI] [PubMed] [Google Scholar]
- Deng J, Miller SA, Wang HY, Xia W, Wen Y, Zhou BP, et al. beta-catenin interacts with and inhibits NF-kappa B in human colon and breast cancer. Cancer Cell. 2002;2:323–34. doi: 10.1016/s1535-6108(02)00154-x. [DOI] [PubMed] [Google Scholar]
- Deng J, Xia W, Miller SA, Wen Y, Wang HY, Hung MC. Crossregulation of NF-kappaB by the APC/GSK-3beta/beta-catenin pathway. Mol Carcinog. 2004;39:139–46. doi: 10.1002/mc.10169. [DOI] [PubMed] [Google Scholar]
- Eastman Q, Grosschedl R. Regulation of LEF-1/TCF transcription factors by Wnt and other signals. Curr Opin Cell Biol. 1999;11:233–40. doi: 10.1016/s0955-0674(99)80031-3. [DOI] [PubMed] [Google Scholar]
- Fanning A, Volkov Y, Freeley M, Kelleher D, Long A. CD44 cross-linking induces protein kinase C-regulated migration of human T lymphocytes. Int Immunol. 2005;17:449–58. doi: 10.1093/intimm/dxh225. [DOI] [PubMed] [Google Scholar]
- Fichter M, Hinrichs R, Eissner G, Scheffer B, Classen S, Ueffing M. Expression of CD44 isoforms in neuroblastoma cells is regulated by PI 3-kinase and protein kinase C. Oncogene. 1997;14:2817–24. doi: 10.1038/sj.onc.1201127. [DOI] [PubMed] [Google Scholar]
- Godar S, Ince TA, Bell GW, Feldser D, Donaher JL, Bergh J, et al. Growth-inhibitory and tumor- suppressive functions of p53 depend on its repression of CD44 expression. Cell. 2008;134:62–73. doi: 10.1016/j.cell.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves V, Matos P, Jordan P. The beta-catenin/TCF4 pathway modifies alternative splicing through modulation of SRp20 expression. RNA. 2008;14:2538–49. doi: 10.1261/rna.1253408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TD, Einat H, Bhat R, Manji HK. AR-A014418, a selective GSK-3 inhibitor, produces antidepressant-like effects in the forced swim test. Int J Neuropsychopharmacol. 2004;7:387–90. doi: 10.1017/S1461145704004535. [DOI] [PubMed] [Google Scholar]
- Graff JR, McNulty AM, Hanna KR, Konicek BW, Lynch RL, Bailey SN, et al. The protein kinase Cbeta-selective inhibitor, Enzastaurin (LY317615.HCl), suppresses signaling through the AKT pathway, induces apoptosis, and suppresses growth of human colon cancer and glioblastoma xenografts. Cancer Res. 2005;65:7462–9. doi: 10.1158/0008-5472.CAN-05-0071. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Signal transduction of beta-catenin. Curr Opin Cell Biol. 1995;7:634–40. doi: 10.1016/0955-0674(95)80104-9. [DOI] [PubMed] [Google Scholar]
- Gwak J, Cho M, Gong SJ, Won J, Kim DE, Kim EY, et al. Protein-kinase-C-mediated beta-catenin phosphorylation negatively regulates the Wnt/beta-catenin pathway. J Cell Sci. 2006;119:4702–9. doi: 10.1242/jcs.03256. [DOI] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–12. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- Hecht A, Vleminckx K, Stemmler MP, van Roy F, Kemler R. The p300/CBP acetyltransferases function as transcriptional coactivators of beta-catenin in vertebrates. EMBO J. 2000;19:1839–50. doi: 10.1093/emboj/19.8.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogeboom D, Essers MA, Polderman PE, Voets E, Smits LM, Burgering BM. Interaction of FOXO with beta-catenin inhibits beta-catenin/T cell factor activity. J Biol Chem. 2008;283:9224–30. doi: 10.1074/jbc.M706638200. [DOI] [PubMed] [Google Scholar]
- Huber O, Korn R, McLaughlin J, Ohsugi M, Herrmann BG, Kemler R. Nuclear localization of beta-catenin by interaction with transcription factor LEF-1. Mech Dev. 1996;59:3–10. doi: 10.1016/0925-4773(96)00597-7. [DOI] [PubMed] [Google Scholar]
- Jakob T, Neuber K, Altenhoff J, Kowalzick L, Ring J. Stage-dependent expression of CD7, CD45RO, CD45RA and CD25 on CD4-positive peripheral blood T-lymphocytes in cutaneous T-cell lymphoma. Acta Derm Venereol. 1996;76:34–6. doi: 10.2340/00015555763436. [DOI] [PubMed] [Google Scholar]
- Kothapalli D, Flowers J, Xu T, Pure E, Assoian RK. Differential activation of ERK and Rac mediates the proliferative and anti-proliferative effects of hyaluronan and CD44. J Biol Chem. 2008;283:31823–9. doi: 10.1074/jbc.M802934200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YH, Yang-Yen HF. The osteopontin-CD44 survival signal involves activation of the phosphatidylinositol 3-kinase/Akt signaling pathway. J Biol Chem. 2001;276:46024–30. doi: 10.1074/jbc.M105132200. [DOI] [PubMed] [Google Scholar]
- Mann B, Gelos M, Siedow A, Hanski ML, Gratchev A, Ilyas M, et al. Target genes of beta-catenin-T cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc Natl Acad Sci U S A. 1999;96:1603–8. doi: 10.1073/pnas.96.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins DF, Rosa AO, Gadotti VM, Mazzardo-Martins L, Nascimento FP, Egea J, et al. The Antinociceptive Effects of AR-A014418, a Selective Inhibitor of Glycogen Synthase Kinase-3 Beta, in Mice. J Pain. 2010 doi: 10.1016/j.jpain.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Medina M, Avila J. Glycogen synthase kinase-3 (GSK-3) inhibitors for the treatment of Alzheimer's disease. Curr Pharm Des. 2010;16:2790–8. doi: 10.2174/138161210793176581. [DOI] [PubMed] [Google Scholar]
- Ogbomo H, Biru T, Michaelis M, Loeschmann N, Doerr HW, Cinatl J., Jr The anti-tumoral drug enzastaurin inhibits natural killer cell cytotoxicity via activation of glycogen synthase kinase-3beta. Biochem Pharmacol. 2011;81:251–8. doi: 10.1016/j.bcp.2010.09.026. [DOI] [PubMed] [Google Scholar]
- Orteu CH, Li W, Allen MH, Smith NP, Barker JN, Whittaker SJ. CD44 variant expression in cutaneous T-cell lymphoma. J Cutan Pathol. 1997;24:342–9. doi: 10.1111/j.1600-0560.1997.tb00802.x. [DOI] [PubMed] [Google Scholar]
- Ougolkov AV, Bone ND, Fernandez-Zapico ME, Kay NE, Billadeau DD. Inhibition of glycogen synthase kinase-3 activity leads to epigenetic silencing of nuclear factor kappaB target genes and induction of apoptosis in chronic lymphocytic leukemia B cells. Blood. 2007;110:735–42. doi: 10.1182/blood-2006-12-060947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ougolkov AV, Fernandez-Zapico ME, Savoy DN, Urrutia RA, Billadeau DD. Glycogen synthase kinase-3beta participates in nuclear factor kappaB-mediated gene transcription and cell survival in pancreatic cancer cells. Cancer Res. 2005;65:2076–81. doi: 10.1158/0008-5472.CAN-04-3642. [DOI] [PubMed] [Google Scholar]
- Querfeld C, Kuzel TM, Kim YH, Porcu P, Duvic M, Musiek A, et al. Multicenter phase II trial of enzastaurin in patients with relapsed or refractory advanced cutaneous T-cell lymphoma. Leuk Lymphoma. 2010 doi: 10.3109/10428194.2011.572265. [DOI] [PubMed] [Google Scholar]
- Querfeld C, Rizvi MA, Kuzel TM, Guitart J, Rademaker A, Sabharwal SS, et al. The selective protein kinase C beta inhibitor enzastaurin induces apoptosis in cutaneous T-cell lymphoma cell lines through the AKT pathway. J Invest Dermatol. 2006;126:1641–7. doi: 10.1038/sj.jid.5700322. [DOI] [PubMed] [Google Scholar]
- Raab MS, Breitkreutz I, Tonon G, Zhang J, Hayden PJ, Nguyen T, et al. Targeting PKC: a novel role for beta-catenin in ER stress and apoptotic signaling. Blood. 2009;113:1513–21. doi: 10.1182/blood-2008-05-157040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook AH, Gottlieb SL, Wolfe JT, Vowels BR, Sood SS, Niu Z, et al. Pathogenesis of cutaneous T-cell lymphoma: implications for the use of recombinant cytokines and photopheresis. Clin Exp Immunol. 1997;107(Suppl 1):16–20. [PubMed] [Google Scholar]
- Tago K, Nakamura T, Nishita M, Hyodo J, Nagai S, Murata Y, et al. Inhibition of Wnt signaling by ICAT, a novel beta-catenin-interacting protein. Genes Dev. 2000;14:1741–9. [PMC free article] [PubMed] [Google Scholar]
- Takemaru KI, Moon RT. The transcriptional coactivator CBP interacts with beta-catenin to activate gene expression. J Cell Biol. 2000;149:249–54. doi: 10.1083/jcb.149.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voutilainen KA, Anttila MA, Sillanpaa SM, Ropponen KM, Saarikoski SV, Juhola MT, et al. Prognostic significance of E-cadherin-catenin complex in epithelial ovarian cancer. J Clin Pathol. 2006;59:460–7. doi: 10.1136/jcp.2005.029876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wielenga VJ, Smits R, Korinek V, Smit L, Kielman M, Fodde R, et al. Expression of CD44 in Apc and Tcf mutant mice implies regulation by the WNT pathway. Am J Pathol. 1999;154:515–23. doi: 10.1016/S0002-9440(10)65297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Stamenkovic I, Yu Q. CD44 attenuates activation of the hippo signaling pathway and is a prime therapeutic target for glioblastoma. Cancer Res. 2010;70:2455–64. doi: 10.1158/0008-5472.CAN-09-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. HuT-78 cells were cultured in 4 μM Enzastaurin, 5 mM LiCl, 5 μM AR-A014418, 5 μM SB216763 or DMSO (vehicle control) and harvested after 5 days. Detection of Annexin V and DAPI staining was performed as described previously. Data shown in representative of three independent experiments.
Figure S2. Detection of Annexin V and DAPI staining was performed on HH, H9 and MJ cells transduced with lentivirus as described previously. Data from three separate experiments was used to quantify double positive cells as a percentage of total cells. Error bars represent standard deviation.
Figure S3. β catenin expression was knocked down in HH, H9 and MJ cells using an shRNA-expressing lentivirus. Cells were then treated with inhibitors and β catenin protein was examined by immunoblot after 24 hours.