Abstract
The auditory experience is crucial for the normal development and maturation of brain structure and the maintenance of the auditory pathways. The specific aims of this review are (i) to provide a brief background of the synaptic morphology of the endbulb of Held in hearing and deaf animals; (ii) to argue the importance of this large synaptic ending in linking neural activity along ascending pathways to environmental acoustic events; (iii) to describe how the re-introduction of electrical activity changes this synapse; and (iv) to examine how changes at the endbulb synapse initiate trans-synaptic changes in ascending auditory projections to the superior olivary complex, the inferior complex, and the auditory cortex.
Keywords: endbulb of Held, cochlear nucleus, cochlear implant, superior olivary complex, inferior colliculus, auditory cortex
1. Introduction
Auditory experience has long been known to play a critical role in the developing mammalian auditory system. Neural activity is important for normal construction and maintenance of auditory structure and function (Parks et al., 2004; Shepherd et al., 2006; Walmsley et al., 2006, Ryugo and Limb, 2009; Sanes and Bao, 2009). It influences the refinement of the genetic blueprint for circuitry including axonal distribution, pruning, and synapse formation (Leake et al., 1988; Baker et al., 2010). The absence of auditory stimulation introduces a series of pathological and atrophic changes that include more widespread distributions of axonal projections (Leake et al., 1988), abnormal projections (Moore and Kitzes, 1985; Nordeen et al., 1983a,b), delayed maturation (Sanes,1993; Kandler, 2004), and language impairments (Robbins, 2006). The effects of the re-introduction of auditory activity through electrical stimulation on synaptic morphology and function has been studied using models of congenital deafness that include both hereditary deafness and chemical deafening, surgical ablation of the organ of Corti, and acoustic trauma. Collectively, these data emphasize the malleability of auditory synapses in response to variations in the acoustic environment.
In the present report, we review the evidence addressing the pathological consequences of sensory deprivation and the restorative influences brought about by the introduction of neural activity through cochlear implantation. The main focus concerns morphological plasticity observed at the level of the auditory nerve in the cochlear nucleus (CN). Not surprisingly, changes at these synapses have consequences further up the system at the superior olivary complex (Schwartz and Higa, 1982; Russell and Moore, 1995; Kapfer et al., 2002; Sanes and Bao, 2009; Tirko et al., 2009), inferior colliculus (Snyder et al., 1995, 2000), and auditory cortex (Heid et al., 1998; Klinke et al., 1999, 2001; Kral et al., 2000, 2001). We will discuss several observations that have advanced our knowledge of synaptic plasticity and expanded our understanding of the effect that functional manipulations have on the auditory system.
Synapses are definable not only by presynaptic characteristics at the release site such as synaptic vesicle size and shape, transmitter chemicals, neuromodulators, and transporter molecules but also by the postsynaptic co mposition of transmitter receptor subunits, shape and curvature of the postsynaptic density (PSD), ion channels, and associated second messenger and retrograde signaling systems. Moreover, there must also be consideration of size and distribution of the terminal, target compartment (e.g., cell body, dendritic shaft, spine), and location of the cell bodies that give rise to the projection. Proper transmission of acoustic signals from neuron to neuron depends in large part on the precise spatial arrangement of these factors at the release site. The corollary to this notion is that abnormalities of synaptic structure will impair signal transmission, thereby corrupting the neural representation of the acoustic stimulus.
Our ability to hear and understand sound commences with the receptor sensory organ of the ear, the cochlea. For the processing of sound, neural activity in the central nervous system must be tightly coupled to acoustic events. The hair cell receptors within the cochlea transduce sound energy into neural signals in auditory nerve fibers that are conveyed to the CN (Kiang et al., 1965). The CN receives all incoming auditory information and gives rise to the ascending auditory pathways. Different sounds are revealed by distinctive characteristics in their time-varying features. Thus, timing of neural activity within the central auditory pathways must not only be time-locked to the external sound stimuli but also to the evoked patterns of activity transmitted along the ascending pathways. Synchrony along the various parallel pathways is essential, and aberrations in these pathways will corrupt processing and disturb how sound is perceived.
Auditory nerve fibers are the major source of excitation to cells of the ventral CN (Koerber et al., 1966). In the anteroventral cochlear nucleus (AVCN), myelinated auditory nerve fibers give rise to large, axosomatic synaptic endings known as endbulbs of Held (Held,1893; Ramón y Cajal, 1909; Lorente de Nó, 1981). Endbulbs have a calyx-like appearance where the end of the fiber is marked by the emergence of several thick, gnarled branches that divide repeatedly to form an elaborate arborization of en passant and terminal swellings to embrace the postsynaptic spherical bushy cell (SBC, Ryugo and Fekete, 1982). These endbulbs are among the largest synaptic endings in the brain (Lenn and Reese, 1966), and one-to-three endbulbs selectively contact a single SBC (Brawer and Morest, 1975; Cant and Morest, 1979; Ryugo and Sento, 1991; Ryugo and Fekete, 1982; Nicol and Walmsley, 2002). They contain up to 2,000 release sites (Ryugo et al., 1996) and transmit activity with high fidelity to the postsynaptic SBC (Pfeiffer, 1966; Babalian et al., 2003). The size and evolutionary conservation of endbulbs among vertebrates emphasize its importance in enabling spike activity to be yoked in time to acoustic events (Fig. 1).
Figure 1.
Examples of endbulbs of Held from various terrestrial vertebrates. This important axosomatic terminal is found in turtles (Browner and Marbey, 1988), alligator lizards (Szpir et al., 1990), chicken (Jhaveri and Morest, 1982), barn owl (Carr and Boudreau, 1991), mouse (Limb and Ryugo, 2000), guinea pig (Tsuji and Liberman, 1997), cat (Ryugo et al., 1996, 1998), rhesus monkey (Ryugo, unpublished observations), and human (Adams, 1986). The large size and evolutionary conservation of this synaptic terminal indicates it importance in hearing (From Ryugo and Parks, 2003).
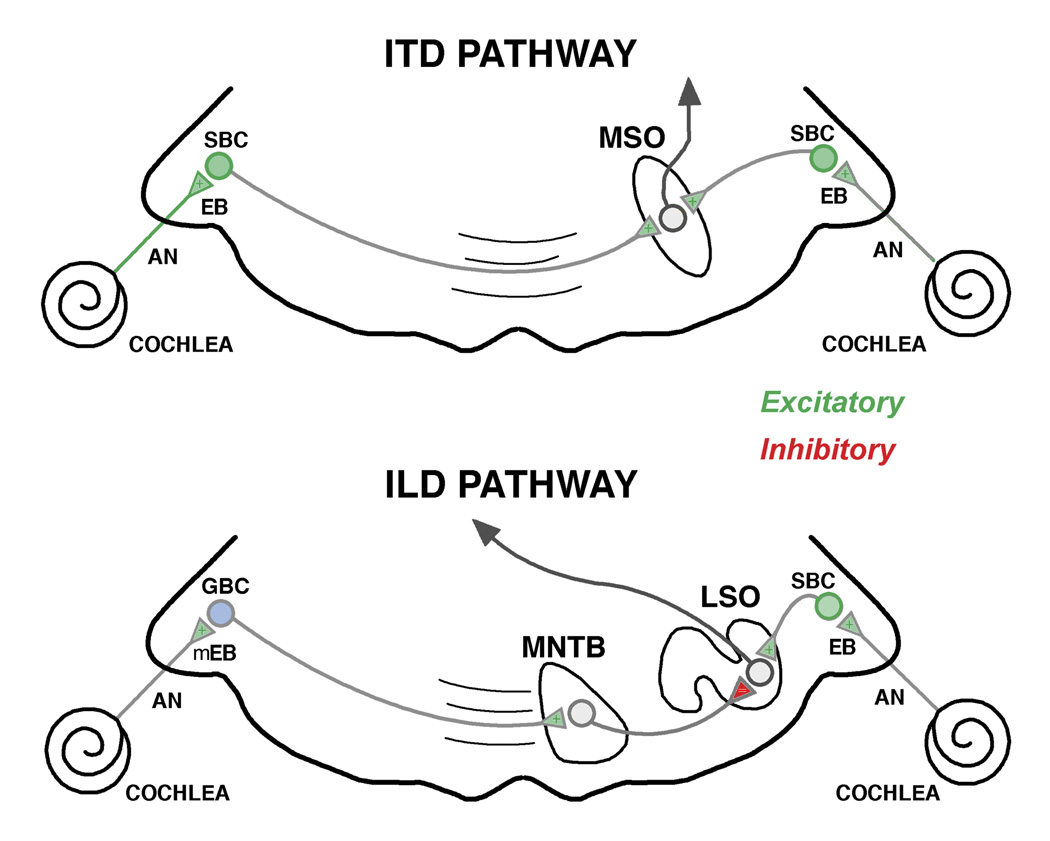
The endbulb synapse has been implicated in the pathway that processes the precise timing features of sound that is crucial for binaural hearing (Yin and Chan, 1990; Fitzpatrick et al., 1997). SBCs send projections from the AVCN to the superior olivary complex (Cant and Casseday, 1986). These projections terminate onto ipsilateral neurons of the lateral superior olive and bilaterally upon bipolar neurons of the medial superior olive (MSO). In the MSO, inputs from the right CN terminate on the dendrites facing the right, whereas inputs from the left CN terminate on the dendrites facing left. The MSO is the first nucleus to process synaptic input from both ears (Fig. 2).
Figure 2.
Simplified schematic diagram emphasizing the timing pathway involved in binaural sound localization. The interaural timing difference (ITD) pathway relies primarily on endbulb (EB) input from the auditory nerve (AN), SBCs (green), and their projection to the MSO to achieve coincidence detection. The interaural level difference (ILD) pathway utilizes GBCs (pale blue) by way of the MNTB, and SBCs, to converge in the LSO. Abbreviations: Auditory nerve, AN; EB, endbulb; GBC, globular bushy cell; LSO, lateral superior olive; mEB, modified endbulb; MNTB, medial nucleus of the trapezoid body; MSO, medial superior olive; SBC, spherical bushy cell. Green indicates excitatory action, whereas red indicates inhibitory action. (Adapted from Tollin, 2003).
Interaural time differences (ITDs) are crucial for localizing sounds on the horizontal plane. The ITD pathway utilizes the difference in arrival of a sound at the two ears to place a sound source. The general concept for this binaural sensitivity is the coincidence detection model where neurons would only respond when binaural excitatory inputs converged simultaneously (Jeffress, 1948). In this model, an array of MSO neurons receive systematically arranged inputs, each with a delay line such that it would only respond if a sound source were in a particular position off the midline. Some neurons would respond when the sound was directly in front of the animal, whereas others would respond when the sound moved away from the midline. A delay line would compensate for a progressive shift in time of arrival at the two ears. The system is sensitive to differences in the range of tens of microseconds, so it is clear that precision in synaptic transmission is required (Grothe, 2000; McAlpine et al., 2001). The endbulb, therefore, is not only important for processing important timing cues for sound localization but also for time-varying cues in speech such as voice onset, stressed syllables, gaps, and amplitude modulation (Moiseff and Konishi, 1981; Blackburn and Sachs, 1990). The point of this discussion is to emphasize that endbulbs and the timing of neural activity are linked and highly important to the proper processing and perception of sound. Moreover, the endbulb is highly advantageous as a research tool because it is prominent and readily recognizable for experimental study.
2. Synaptic Morphology of the Endbulb
2.1. Normal Hearing Animals
During postnatal development, the endbulb of Held begins with the formation of a solid, spoon-shaped growth cone and culminates in a highly branched axosomatic arborization (Ryugo and Fekete, 1982). Each endbulb can form approximately 500–2,000 synapses onto the postsynaptic SBC (Ryugo et al., 1996). This feature in particular suggests a highly secure synaptic interface to maintain the temporal fidelity of all incoming signals to the SBC (Romand, 1978; Oertel, 1983; Manis and Marx, 1991). The structure of this giant synaptic terminal has been extensively studied to learn about synapse formation, its target specificity, and its reaction to deafness (Gulley et al., 1977; Neises et al., 1982; Ryugo and Fekete, 1982; Carr and Boudreau, 1996; Limb and Ryugo, 2000; Ryugo et al., 1997, 1998; Oleskevich, et al., 2004).
One of the most striking morphological features observed in the normal neonatal endbulb has been the interdigitation of the membranes of the postsynaptic SBC and the presynaptic endbulb (Jhaveri and Morest, 1982; Neises et al., 1982; Carr and Boudreau, 1996; Ryugo et al., 2006). The abundant but transient surface contact between both endbulb and SBC has no known function but resembles certain epithelial cell specializations (e.g., pedicel processes of the kidney, striated duct cells of the submandibular gland, or basal processes of marginal cells of the stria vascularis) where cell surface is maximized by vigorous infoldings. Such hypertrophied surface contact for endbulbs would facilitate the exchange of chemical signals involved in the stabilization of some endbulbs and elimination of others or any other developmental process yet to be determined. It is estimated that on a dozen endbulbs contact a SBC in neonatal cats but that by adulthood, this number has been reduced to only two endbulbs per SBC (Ryugo et al., 2006).
In normal hearing cats the endbulb arborization onto the SBC has been shown to vary systematically with respect to the average level of spike discharges received from auditory nerve fibers having low or high levels of activity (Ryugo et al., 1996). Endbulbs from fibers having high levels of activity (e.g., high (>18 s/s) spontaneous discharge rates and low thresholds for evoked responses) exhibit modest levels of branching with relatively large en passant and terminal swellings. In contrast, endbulbs from fibers having relatively low levels of activity (e.g., low (<18 s/s) spontaneous discharge rates and high thresholds for evoked responses) exhibit highly elaborate branching with relatively small en passant and terminal swellings. The differences in branching complexity were confirmed by statistically significant differences in fractal values. Moreover, the larger swellings on the highly active endbulbs resembled the swollen endings of overactive terminals where it was speculated that the swelling was caused by the fusion of synaptic vesicles (Heuser and Reese, 1973; Boyne et al., 1975; Burwen and Satir, 1977). Using electron microscopy to study synaptic ultrastructure, endbulbs receiving relatively low levels of spike discharges were associated with larger PSDs, whereas those exhibiting high rates of spike discharges exhibited smaller PSDs (Ryugo et al., 1996). These data are consistent with observations from rats exposed to repetitive tones or silence; stimulated animals exhibited endbulbs with smaller PSDs compared to those of animals exposed to silence (Rees et al., 1985). The synapse structure of endbulbs is clearly plastic and subject to activity-related change.
2.2. Congenitally Deaf Animals
The endbulb synapse has been studied in congenitally deaf white cats to determine the extent to which sound influences its growth (Ryugo et al., 1997, 1998). Due to the elaborate form of the endbulb, changes in morphology should be evident and quantitative. Moreover, given that variations in endbulb morphology were already apparent in normal hearing cats where differences could be attributed to disparities in spike discharge rates, we predicted that in the extreme case of congenital deafness, there should be definable and obvious abnormalities.
Single unit recordings in the auditory nerve of congenitally deaf white cats revealed several important features (Ryugo et al., 1998). First, in completely deaf cats, there was virtually no spontaneous activity and no evoked activity. Second, in hard of hearing cats (thresholds >60 dB), there was spontaneous activity, but elevated in distribution. Spontaneous activity in general was similar to that of normal hearing cats, but the upper range of spontaneous activity was extended (>150 s/s). In some auditory nerve fibers, spontaneous discharges exceeded 180 s/s. These data on activity were consistent with inner ear histology: deaf animals exhibited no organ of Corti, whereas hard of hearing animals exhibited a full complement of hair cells but showed signs of hydrops with an outward bulging Reissner’s membrane (Ryugo et al., 1998).
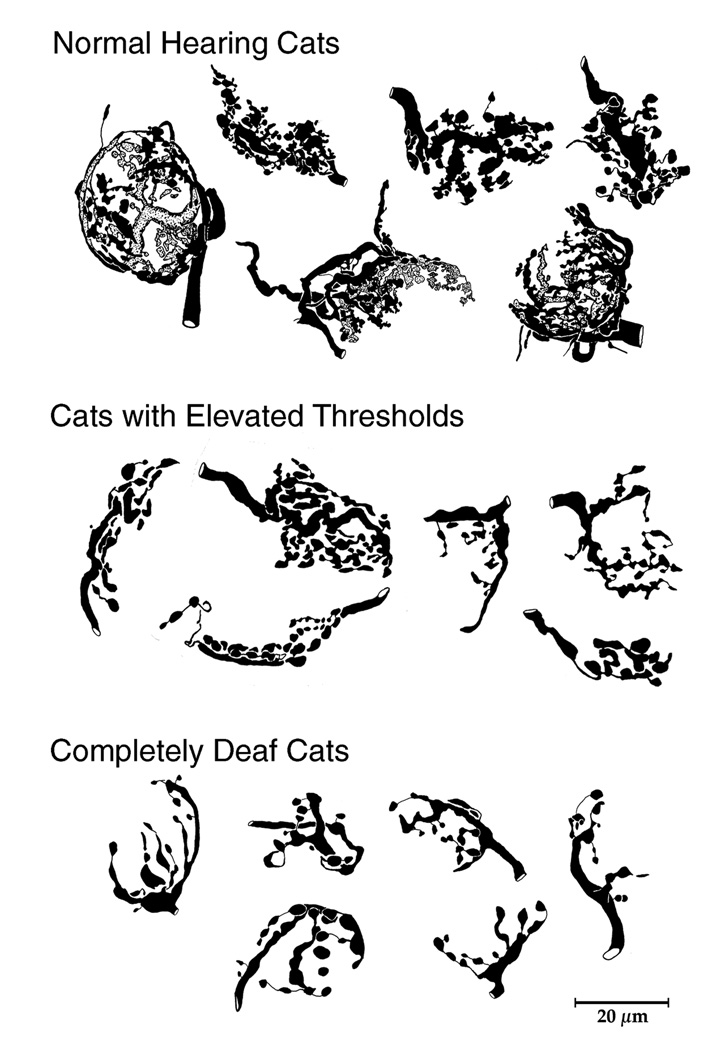
The most obvious structural correlate in the CN was that the endbulbs contact significantly smaller postsynaptic SBCs (Saada et al., 1996; O’Neil et al., 2010; Ryugo et al., 2010). Second, the degree of endbulb arborization was graded in arborization complexity with respect to hearing threshold. In completely deaf cats, the extent and complexity of endbulb branching were much less and there were significantly fewer swellings (Ryugo et al., 1997, 1998). These features were quantified by fractal analysis and counts, respectively (Fig. 3). The graded nature of this effect was revealed by the intermediate position of endbulbs from cats with some but diminished hearing. Cats with elevated thresholds exhibited statistically different fractal values from completely deaf cats and normal hearing cats. Normal hearing cats displayed the most elaborate and complex endbulb arborizations.
Figure 3.
Light microscopic drawing tube reconstructions of HRP-labeled endbulbs collected from cats. Note how the branching complexity is positively correlated to hearing status (From Ryugo et al., 1998).
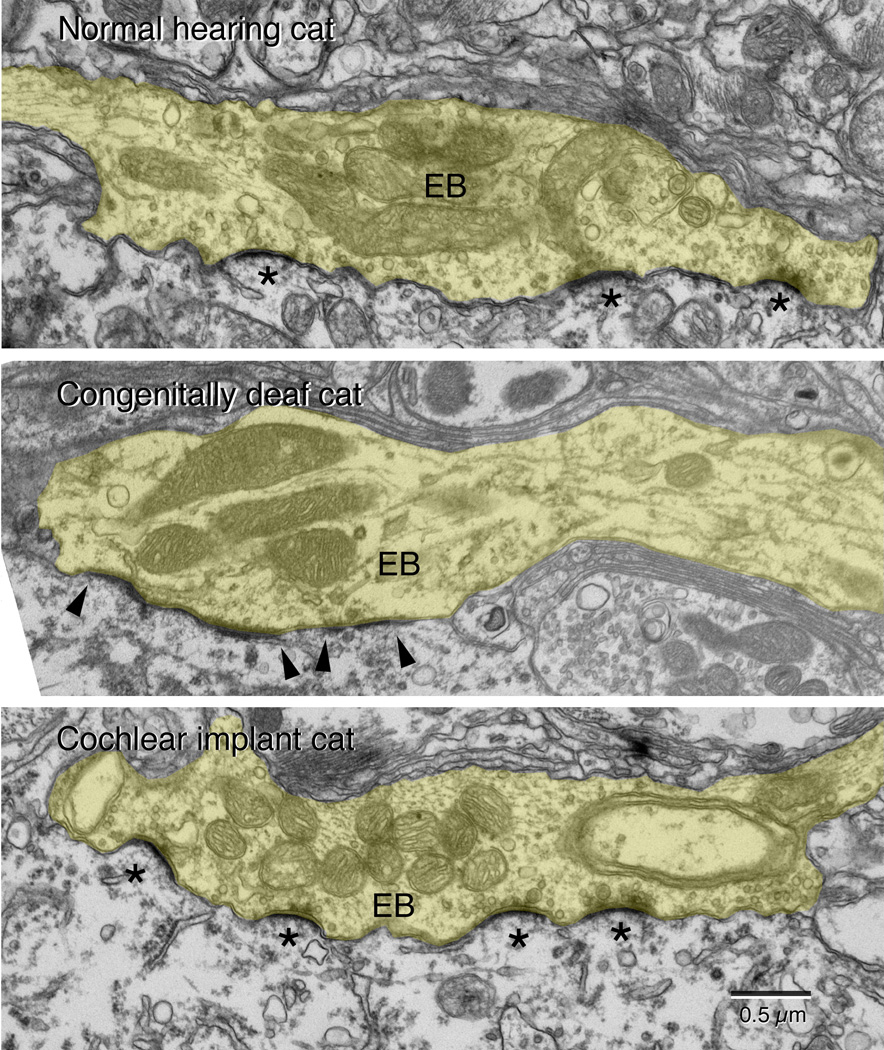
When these endbulbs were examined at greater resolution with an electron microscope, additional features are shown to be affected by hearing loss (Ryugo et al., 1997, 1998). In normal hearing cats, endbulbs give rise to numerous punctate, dome-shaped PSDs (Fig. 4A). Surrounding the PSD were accumulations of round, clear synaptic vesicles. In contrast, endbulbs of congenitally deaf white cats exhibited a flattening and hypertrophy of the PSDs (Fig. 4B). Moreover, there was a striking increase in synaptic vesicle density near the release site (Baker et al., 2010). The endbulb synapses from cats that were not deaf but suffering from hearing loss exhibited features that were intermediate between those of normal hearing and completely deaf cats (Ryugo et al., 1998). That is, the PSDs were intermediate in size and curvature.
Figure 4.
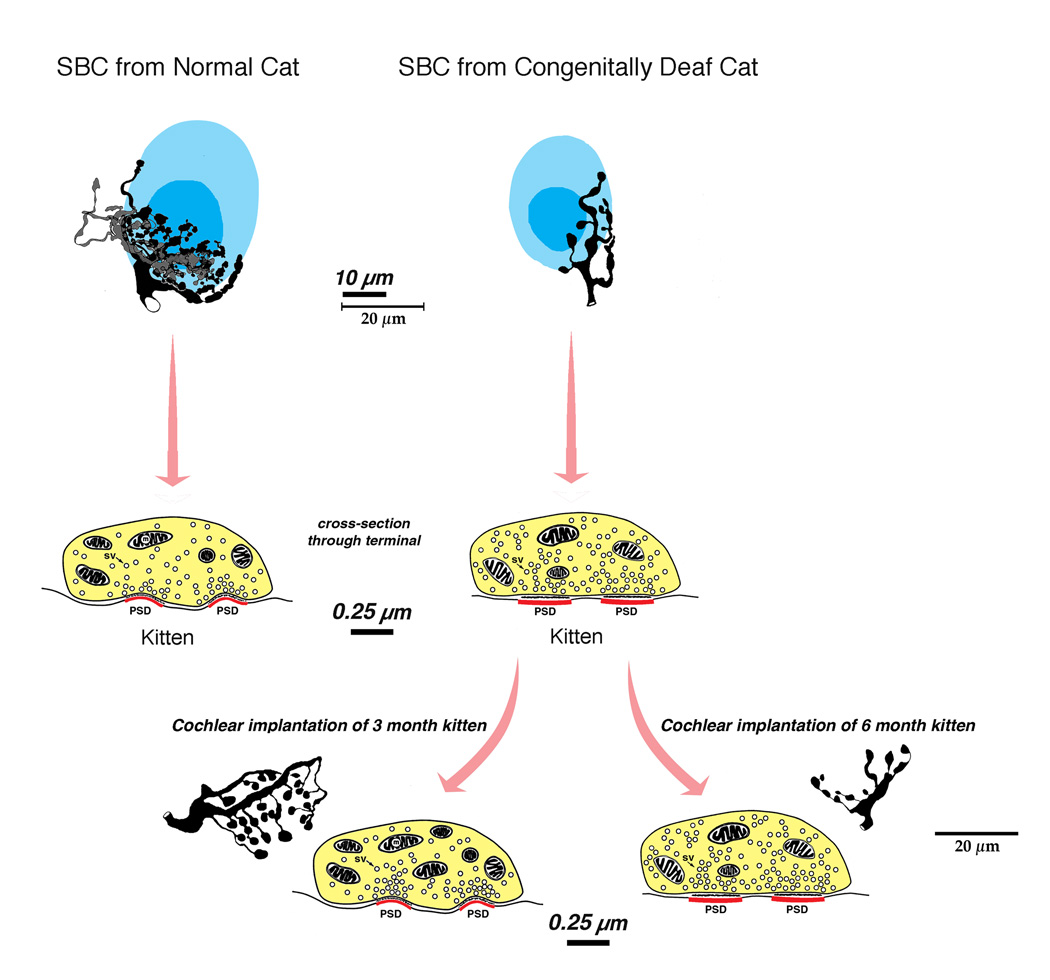
Representative electron micrographs of endbulbs (EB, tinted yellow) taken from a normal hearing cat, a congenitally deaf cat, and a congenitally deaf cat stimulated electrically through a cochlear implant. The cats are matched in age. This series of micrographs illustrate that stimulation of the auditory nerve via a cochlear implant restores the structure of the synapse, specifically with respect to the size and curvature of the postsynaptic density (*) and the return of synaptic vesicle density adjacent to the synapse. (From Ryugo et al., 2005).
Deafness and hearing loss caused abnormalities in endbulb branching, soma size, and synapse morphology that were statistically different between cohorts (Ryugo et al., 1998; Redd et al., 2000). Abnormalities in synaptic transmission do not just occur with complete deafness. There has been transmission abnormalities reported in DBA/2J mice with hearing loss (Wang and Manis, 2005, 2006). These changes reveal that auditory synapses are highly sensitive to the quantity and quality of simulation. The presence of transmission irregularities from the presynaptic endbulb to the postsynaptic SBC could introduce jitter or perhaps even transmission failure. Such interruptions would diminish the precise processing of timing information. The implication is that even hearing loss will produce difficulties beyond elevated thresholds.
Light and electron microscopic analyses were conducted on the synaptic inputs to the somata of SBCs from congenitally deaf and normal hearing cats and mice. Normally, approximately 40% of axosomatic terminals appear to be inhibitory because they exhibit non-round synaptic vesicles or they stain for the inhibitory neurotransmitters, GABA or glycine (Adams and Mugnaini, 1987; Kolston et al., 1992). Based on the changes in endbulb structure and size as a result of deafness, it might be predicted that non-auditory nerve endings would expand into the territory vacated by shrinking auditory nerve endings. Instead, there is a selective loss of inhibitory terminals (Asako et al., 2005). This change in the composition of endings on SBCs in profoundly deaf animals is expected to have a significant effect on auditory processing of information transmitted through hearing aids or cochlear implants. The loss of inhibitory endings could also lead to increased excitability caused by a “release” of inhibition, a phenomenon that might underlie some forms of tinnitus.
The congenitally deaf white cat model used in these studies manifests a type of cochleosaccular degeneration that causes sensorineural hearing loss, mimicking the Scheibe deformity in humans. That is, the deafness is apparently caused by a collapse of Reissner’s membrane, which obliterates the scala media and the organ of Corti (Scheibe, 1882,1885; Bosher and Hallpike, 1965,1967; Deol, 1970; Suga and Hattler, 1970; Mair, 1973; Brighton et al., 1991). The question could be asked whether the synaptic changes observed in these deaf cats are due to loss of neural activity in the auditory nerve, or whether they are part of the genetic syndrome and unrelated to spike activity. Several arguments can be presented to counter this concern. First, ototoxic deafening produces a similar flattening and hypertrophy of the PSD (Ryugo et al., 2010). Second, in Shaker-2 mice whose deafness is caused by a mutation of the myosin 15 gene that leads to loss of hair cell stereocilia, changes in endbulb form (Limb and Ryugo, 2000) and synaptic structure (Lee et al., 2003) resemble those of the congenitally deaf white cat. Third, similar pathologic changes in endbulb morphology have been inferred in the congenitally deaf guinea pig (Gulley et al., 1978). Because non-feline animals with deafness caused by independent means and cats of normal genetics but deafened by drugs all show these same synaptic anomalies, we can attribute the synaptic pathology, at least in part, to the lack of auditory nerve activity.
Congenital deafness does not restrict its effects to the auditory nerve and CN (Mair, 1973; Rebillard et al., 1981a,b; West and Harrison,1973; Larsen and Kirchhoff, 1992; Saada et al., 1996). Alterations in cell size and number, receptive field properties, and laminar organization are expressed at higher nuclei of the auditory system including the superior olivary complex (Schwartz and Higa, 1982), inferior colliculus (Snyder et al., 1995, 2000; Heid et al., 1997), and auditory cortex (Heid et al., 1998; Klinke et al., 1999, 2001; Kral et al., 2000, 2001). Thus, alterations and plasticity at the endbulb synapse are reflections of a wider range of possible change throughout the central auditory system initiated by hearing loss and deafness.
3. Synaptic Plasticity: Implications for Cochlear Implants
3.1. Restoration of the Endbulb Synapse
Cochlear implants are electronic neural prostheses that are able to restore functional hearing to most individuals who are profoundly deaf or severely hard of hearing. Cochlear implants achieve their effects through bypassing the nonfunctioning auditory receptors of the inner ear and directly stimulating the auditory nerve (Rauschecker and Shannon, 2002). It is estimated that over 188,000 people worldwide benefit from cochlear implantation (http://www.nidcd.nih.gov/health/hearing/coch.asp). Individuals who lose hearing after developing speech and congenitally deaf children who receive implants early in life are the best candidates for cochlear implants, although the level of benefit varies widely from one individual to the next. Young children under the age of 2 years who exhibit nonsyndromic sensorineural deafness are also excellent candidates (Waltzman et al., 1994, 1997). Because children who receive implants at progressively older ages tend to perform more poorly, it is hypothesized that uncorrected congenital deafness leads to irreversible abnormalities throughout the central auditory system.
The synaptic changes in auditory nerve endings associated with congenital deafness present an interesting test for thinking about sensory deprivation and brain plasticity. Could the synaptic abnormalities in the CN represent the key to disrupting auditory processing throughout the central auditory system? Would the re-introduction of spike discharges in the auditory nerve serve to reverse or prevent the synaptic abnormalities?
Miniaturized cochlear implants (Clarion II implants donated by Advanced Bionics Corporation) were implanted into 3-month old, congenitally deaf white kittens. The implants utilized a 6-electrode array. After a short period of recovery, each kitten was stimulated 7 hours a day, 5 days a week for 2–3 months. The device utilized a speech processor identical to that used with human patients (Kretzmer et al., 2004). During the period of stimulation animals learned to approach their food bowl in response to a specific “call” showing that the animals were processing signals of biological relevance. Synapse restoration was evident on the side of stimulation where the small size and dome-shaped curvature of the PSD returned (Ryugo et al., 2005).
The plasticity of the endbulb-SBC synapse is notably different from that of the modified endbulb - globular bushy cell (GBC) synapse (Redd et al., 2000). Deafness induced an assortment of synaptic abnormalities that included a reduction in the size but not branching complexity of the modified endbulbs, reduction in GBC size, a flattening and slight but statistically significant hypertrophy of PSDs, and loss of extracellular cisternae between pre- and postsynaptic structures. The same stimulation parameters that restored endbulb synapses on SBCs had surprisingly different effects on modified endbulb synapses: synapses of the electrically stimulated ipsilateral auditory nerve showed no recovery (O’Neil et al., 2011).
The differential effects of deafness and electrical stimulation on the synapses of spherical and globular bushy cells emphasize biological variability even within the CN. Auditory stimulation with a unilateral cochlear implant restores the structure of the ITD pathway but does not restore the initial stages of the interaural level difference (ILD) pathway. This observation raises intriguing questions about the specific mechanisms by which cochlear implants mediate hearing. Even with the apparent restoration of endbulb synapses, individuals with bilateral cochlear implants have trouble processing interaural time differences, whereas paradoxically, they are able to utilize interaural level differences (van Hoesel, 2004, 2007). There remains a discrepancy between clinical observations and the differential restoration of binaural pathways by electrical stimulation through cochlear implantation.
Glutamate is the major excitatory neurotransmitter of the central synapses of myelinated auditory nerve fibers mediating rapid transmission (Hunter et al., 1993; Safieddine and Wenthold, 1997; Hollmann and Heinemann, 1994; Hackney et al., 1996; Wang et al., 1998; Petralia et al., 2000; Ryugo and Parks, 2003). Vesicular glutamate transporter 1 (Vglut1) functions to package glutamate into synaptic vesicles (Wojcik et al., 2004; Zhou et al., 2007) and is a marker for excitatory projections (Altschuler et al., 1984; Martin, 1985; Schweitzer et al., 1991; Zhou et al., 2007). Within the CN, auditory nerve terminals immunostain predominantly for Vglut1, whereas the distribution of Vglut1 after unilateral deafening was decreased in the side ipsilateral to that which was deafened (Zeng et al., 2009). The appearance of nests of swellings around SBC somata (Fig. 5) was reminiscent of HRP-labeled endbulbs (Ryugo and Fekete, 1982; Ryugo et al., 1997, 1998; Tsuji and Liberman, 1997).
Figure 5.
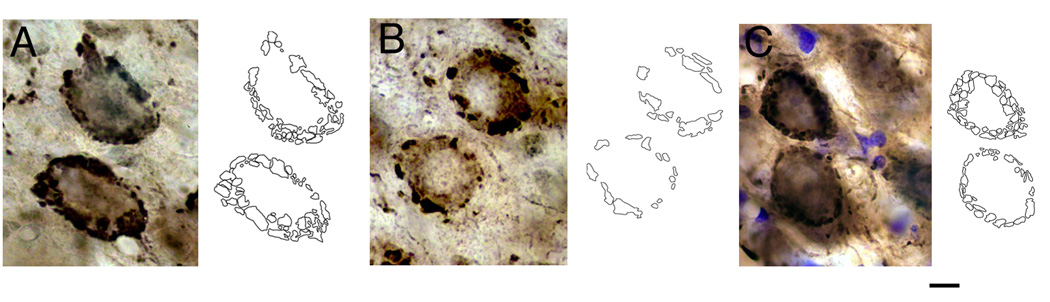
Photomicrographs of endbulbs of cats stained by antibodies directed against the vesicular glutamate transporter (Vglut1), alongside corresponding drawings to illustrate the individual swellings that were drawn and measured. (A) Representative endbulbs from a normal hearing cat. Note the high density of small swellings that encircle the equator of the cell body. (B) Typical staining from a congenitally deaf cat, where the number of swellings around the cell body is greatly reduced. These images of Vglut1-stained endbulbs resemble those of HRP-labeled endbulbs illustrated in Figure 3. (C) Usual images of endbulbs from congenitally deaf cat that received 2–3 months of electrical stimulation through a cochlear implant. The increased number of swellings around the cell body implies that activity restored the endbulb arborization. Scale bar equals 10 µm.
Antibodies directed against Vglut1 were used to identify endbulbs in the AVCN of cats. Endbulb morphology was analyzed from cats having normal hearing, congenital deafness, or congenital deafness and electrical stimulation by way of a unilateral cochlear implant. The goal was to assess to what extent electrical stimulation restored the morphology of these prominent terminal endings. Because the tissue was counterstained using cresyl violet, the cells could be identified by their axosomatic embrace of swellings and by cytologic criteria (Osen, 1969; Cant and Morest, 1979). Aggregates of boutons completely surrounding the equator of the SBC were analyzed (Fig. 5). Those from normal hearing cats contained on average 17.2 ± 4.45 boutons with an average size of 7.76 ± 4.0 sq. micrometers. In contrast, those of congenitally deaf cats contained an average of 12.44 ± 2.9 boutons with an average size of 5.0 ± 2.35 sq. micrometers. Electrical stimulation of the auditory nerve fibers via cochlear implants resulted in a restoration of bouton number (mean, 17.6 ± 2.4) but not bouton size (mean, 5.25 ± 1.2 sq. micrometers). These data imply that activity also restores endbulb arborization complexity (Fig. 7).
Figure 7.
Summary diagram illustrating endbulb plasticity under conditions of normal hearing, congenital deafness, and congenital deafness with cochlear implantation. Deafness results in a reduction in terminal arborization. The endbulb synapses of deaf animals lose their dome shape and hypertrophy, and synaptic vesicle density increases. Electrical stimulation through a cochlear implant in young but not older cats restores synaptic morphology. Synapses regain their dome shape and punctate distribution, and synaptic vesicle density over the release site returns to normal. The endbulb itself does not regain its highly branched arborization, nor do the swellings return to the small size typical of those in hearing cats.
It seems intuitive that the benefits derived from cochlear implants should be mediated by the health of the spiral ganglion cells because cochlear implants could not function without the presence of some surviving cells. The structural restoration of endbulb synapses, however, was not related to ganglion cell number (26,130 – 48,192) or mean ganglion cell size (112.6 – 244.4 sq. micrometers). The most important variable was the age at onset of ipsilateral electrical stimulation: stimulation commencing at three months of age had a positive effect on synapse recovery, whereas stimulation commencing at six months of age had no effect (Chen et al., 2010). Variables that influence implant success (e.g., open speech recognition) include cause of deafness and duration of deafness (Nadol et al., 1989; Moore et al., 1997; Linthicum and Fayad, 2009). Other possible variables are the degree of auditory nerve myelination, peripheral process survival, proximity of stimulating electrodes to spiral ganglion neurons, amount of scar tissue produced by surgical trauma and cochlear implantation, and blood supply to spiral ganglion neurons. The issue of how many neurons are required to produce maximum benefit from a cochlear implant remains an open question.
3.2. Bilateral effects of unilateral implantation
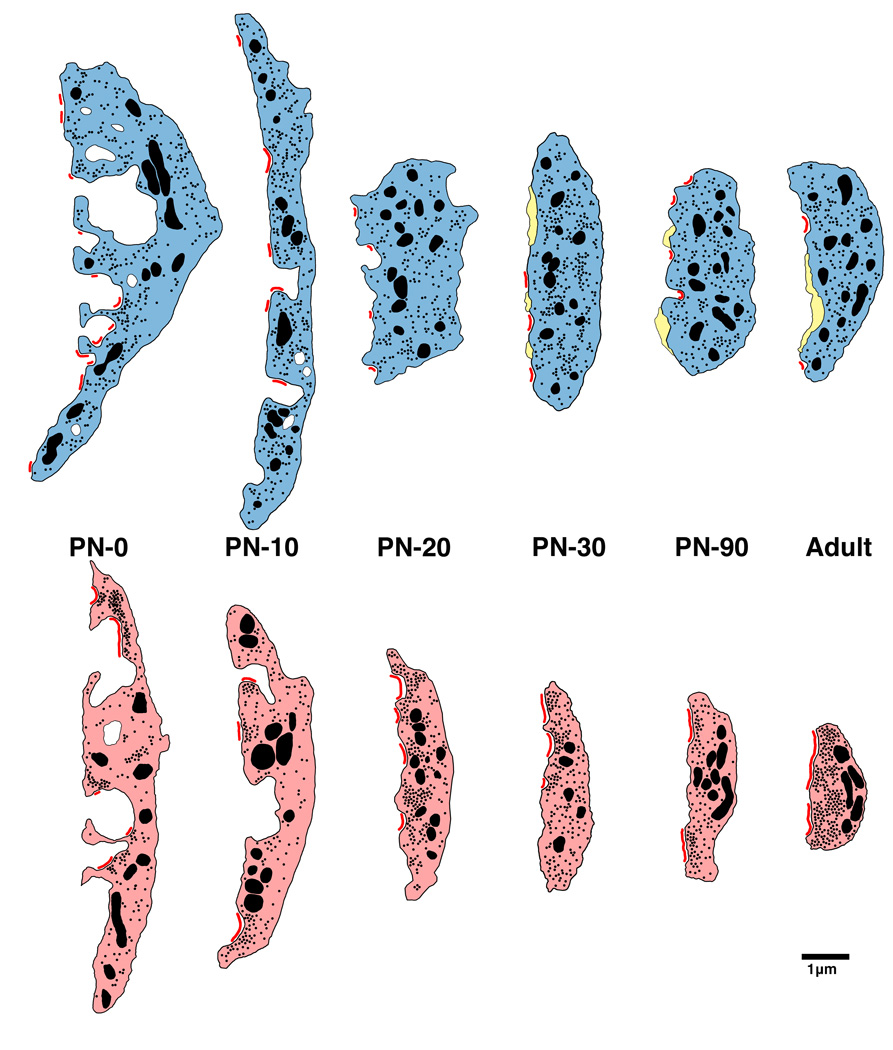
Restoration of auditory activity by unilateral electrical stimulation in deaf cats resulted in improvements in temporal processing at the level of the cortex (Klinke et al., 1999), the inferior colliculus (Vollmer et al., 2005) and the CN (Ryugo et al., 2005). In a separate study, three and six month old congenitally deaf cats received unilateral cochlear implants and were stimulated for 10–19 weeks. Animals learned to approach their food bowl for a food reward in response to a specific acoustic stimulus (a bugle playing Adjutant’s Call). The cats ignored other bugle calls. Upon examination of the endbulb synapse, restoration was seen in animals implanted at 3 months of age but not at 6 months of age (Fig. 4C). The endbulb synapses of the contralateral CN in the 3-month old implanted cats also exhibited synapses with more normal structural features demonstrating that electrical stimulation with a cochlear implant can help preserve central auditory synapses through direct and indirect pathways. Because newborn kittens destined to be deaf (e.g., histologic verification of an absent organ of Corti) already exhibit statistically significant hypertrophy of PSDs (Fig. 6), we can safely use the term “restore” when referring to the effect of cochlear implantation on synaptic structure (Baker et al., 2010).
Figure 6.
Schematic of endbulb development in normal hearing (top) and deaf (bottom) cats. At birth, endbulb profiles have a highly convoluted membrane abutting the SBC, which gradually becomes smoother with age. Endbulbs of deaf animals are on average smaller than those found in age-matched normal hearing cats. The number of PSDs (red) at birth in deaf animals is less than half that of normal. Mature endbulbs of both deaf and normal animals have the same number of PSDs, the PSDs of deaf animals are longer and flatter than the normal convex PSDs. Deaf endbulbs exhibit an increase in synaptic vesicle (black dots) density near the PSDs. No differences were seen with respect to mitochondria (large black) size or volume fraction between the two groups. Endbulbs of normal cats begin to develop intermembraneous cisternae (yellow) around postnatal day 10, whereas endbulbs from deaf cats rarely develop them. (From Baker et al., 2010).
3.3. Critical Periods
The concept of the critical period has been applied to explain biological phenomena that occur or are most severely affected over a brief period of time during development. Examples of such developmental events are exemplified by “imprinting” (Lorenz, 1935), cortical barrel plasticity (Van der Loos and Woolsey, 1973; Weller and Johnson, 1975), birdsong acquisition (Konishi, 1985), and functional maturation of auditory cortex (Chang and Merzenich, 2003; Zhou et al., 2008). Reports that young children receiving cochlear implants gained far superior benefit compared to that of older children and adults also hinted strongly at a critical period (Quittner and Steck, 1991; Waltzman et al., 1993; Gantz et al., 1994; Tyler and Summerfield, 1996). In congenitally deaf cats, electrical stimulation was reported to recruit auditory cortical responses contingent upon its commencement before 6 months of age (Klinke et al., 2001; Kral et al., 2002). The structural foundation for these observations may be attributable to the fact that 3-month old cochlear implant recipient exhibited somewhat restored auditory nerve synapses, whereas 6-month old cochlear implant recipients did not. The developmental period preceding puberty (cats reach puberty at six months of age) appears most favorable for implant-induced synaptic plasticity, and the restoration of endbulb synapses is hypothesized to have facilitated the proper delivery of afferent signals to the forebrain in a timely, coherent, and synchronized way.
3.4. Chemical Deafening and Cochlear Implantation
Ototoxically deafened animals in comparison to congenitally deaf animals exhibited the characteristically hypertrophied PSDs (Ryugo et al., 2010). After electrical stimulation for a period of up to twelve months the auditory nerve synapse demonstrated recovery with a size that was statistically identical to that of normal hearing animals. However, other aspects of synapse morphology were dissimilar to that seen in heredity deafened and stimulated animals (Ryugo et al., 2010). These studies that used ototoxic deafening of normal hearing cats have reported small but positive effects of electrical stimulation on CN cell size (Lustig et al., 1994; Leake et al., 1999; Stakhovskaya et al., 2008), whereas others using similar methods (and often from the same lab) show no effects (Hultcrantz et al., 1991; Ni et al., 1993; Coco et al., 2007). Electrical stimulation of auditory nerve fibers via cochlear implants had no effect on the size of the SBC neurons in deaf white cats (O’Neil et al., 2010). In summary, the two models of congenital deafness—hereditary versus ototoxic—are not identical and are associated with different morphological consequences.
4. Trans-synaptic Changes in the Auditory Pathway
Electrical stimulation of the auditory nerve via cochlear implantation rescued many of the synaptic abnormalities associated with congenital deafness, including PSD size, distribution, and curvature, and synaptic vesicle density. The restored synapses, however, were not completely normal because intermembraneous cisternae that tend to flank release sites did not return (O’Neil et al., 2010), and restoration was not uniform across all cell types in the CN (O’Neil et al., 2011). Accordingly, electrically evoked ABRs differed from those in normal cats. Evoked peaks in the ABR waveform whose height and sharpness are indicative of synchronous ascending volleys, while more prominent in implanted cats compared to that of unstimulated congenitally deaf cats, were nonetheless delayed and flattened. These defects in the ABR waveform suggest a reduction of synchrony in the evoked responses, perhaps caused by transmission jitter or transmission failure along the ascending pathways (e.g., the ITD and ILD circuits).
Medial Superior Olive (MSO). Excitatory inputs to MSO neurons are segregated such that ipsilateral input innervates lateral dendrites and contralateral inputs innervate medial dendrites (Smith et al., 1993; Russell et al., 1995; Kapfer et al., 2002). These neurons function as a “coincidence detector” for processing ITDs (Grothe and Sanes, 1994; Brand et al., 2002; Carr et al., 2004). In addition, inhibitory inputs tend to be confined to the MSO cell bodies of mammals specialized for low frequency hearing (e.g., gerbil, cat, chinchilla) that are thought to “fine tune” the timing of inputs from the separate ears (Werthat et al., 2008; Couchman et al., 2010). This topographical arrangement differs in MSO cells of mammals specialized for high frequency hearing (e.g., rat, opossum, bat, juvenile gerbil) where there is an equal excitatory-inhibitory synapse distribution on both cell somata and dendrites. Inhibitory input to the MSO arise from the medial and lateral nucleus of the trapezoid body (MNTB, LNTB, Cant and Hyson, 1992; Kuwabara and Zook, 1992; Grothe and Sanes, 1993) and function to adjust the output signal of MSO neurons (Brand et al., 2002; Pecka et al., 2008). The spatial distribution of these excitatory and inhibitory inputs is sensitive to developmental abnormalities within the acoustic environment.
Deafness causes a bilateral disruption in the spatially segregated inputs to the MSO principal neurons as seen in mammals with low frequency hearing. In congenitally deaf animals, inhibitory input at the cell somata is significantly less than what is observed in hearing controls (Kapfer et al., 2002; Tirko et al., 2009). This change in axosomatic inhibition was inferred by a loss of staining for gephyrin, an anchoring protein for the glycine receptor (Kapfer et al., 2002) and the migration of terminals containing flattened and pleomorphic synaptic vesicles (indicative of inhibitory synapses) away from the cell body and out onto the dendrites (Tirko et al., 2009). Cochlear implantation of the congenitally deaf cat and 2–3 months of stimulation resulted in a restoration of inhibitory input onto the cell somata and a return of excitatory inputs to the dendrites (Tirko et al., 2009).
Lateral Superior Olive (LSO). The LSO is involved in the processing of ILDs (Tollin, 2003). The ILD circuit measures the difference in sound level or intensity between the two ears, since the ear further from the sound source receives a relatively softer sound due to the “shadow” effect of the head. This binaural nucleus also plays a central role in sound localization, specifically in the processing of high frequency sounds. ILDs are encoded by integrating both excitatory and inhibitory input. The LSO receives excitatory input from ipsilateral SBCs and inhibitory input from the ipsilateral MNTB that is excited by input from the contralateral globular bushy cells (Boudreau and Tsuchitani, 1968, Moore and Caspary, 1983; Sanes et al., 1987; Sanes and Rubel, 1988; Sanes, 1990; Wu and Kelly, 1992; Kandler et al., 2009).
Inputs to the LSO are tonotopically organized and aligned so that a single neuron is excited and inhibited by the same sound frequency (Kandler et al., 2009). Within the LSO, there is a remarkable degree of synaptic reorganization involving experience dependent plasticity that is important for normal auditory development (Sanes and Takacs, 1993; Kandler and Friauf, 1995; Sanes and Friauf, 2000; Kapfer et al., 2002; Kim and Kandler, 2003). In gerbils, development of the MNTB-LSO pathway begins with synaptic pruning of MNTB axon terminals in the LSO and a decrease in the spread of LSO dendrites occurring after hearing onset (Sanes and Siverls, 1991; Sanes et al., 1992; Rietzel and Friauf, 1998; Kandler et al., 2009). Pruning depends in large part on auditory experience where GABA and glycinergic synapses are essential for the formation of precise tonotopic maps (Kim and Kandler, 2003; Gillespie et al., 2005; Kandler et al., 2009).
Deafness-associated synaptic alterations occur throughout the auditory brain stem and an imbalance of excitation and inhibition are reflected in neuronal response profiles (Bledsoe et al., 1995; Francis and Manis, 2000; Kaltenbach and Afman, 2000; Mossop et al., 2000; Syka et al., 2000). The types of change vary with respect to cell type as well as cause and extent of deafness. A decrease in excitatory transmission to the LSO may be a result of a change in total synaptic contacts, distribution of the inputs, presynaptic release, postsynaptic cell response, or any combination above (Buras et al., 2006). The fact is that any change in one location can result in widespread trans-synaptic change elsewhere.
Inferior Colliculus (IC). The IC is a large midbrain structure that has three principal subdivisions and a complex organization. There is a dorsal and lateral cortex that forms a “rind” around a central nucleus that forms the core (Morest and Oliver, 1984; Winer, 2005). The rind receives inputs from auditory as well as nonauditory sources (Robards, 1979; Ryugo et al., 1981). The central nucleus is tonotopically organized and receives ascending auditory inputs from many sources including cochlear nuclei, superior olivary complex, and nuclei of the lateral lemniscus as well as descending inputs from the auditory cortex and superior colliculus (Roth et al., 1978; Adams, 1979; Andersen et al., 1980). It is the main brain stem station through which nearly all ascending projections must pass before reaching the auditory forebrain. A rudimentary tonotopic organization within the IC has been shown to exist in long-term deafened animals (Snyder et al., 1990; Shepherd et al., 1999). This organization is established and maintained in the absence of auditory input (e.g., deafness) revealing the power of the genetic blueprint (Young and Rubel, 1986; Friauf and Kandler, 1990). Acute deafness did not increase temporal dispersion in spike timing to electric pulse trains in the auditory nerve nor impair ITD sensitivity (Shepherd and Javel, 1997; Sly et al., 2007; Hancock et al., 2010). Congenital deafness, however, did reduce ITD sensitivity in the responses of IC units. Single unit data in the IC showed that half as many neurons in the congenitally deaf cat showed ITD sensitivity to low pulse trains when compared to the acutely deafened animals. In neurons that showed ITD tuning, the tuning was found to be broader and more variable (Hancock et al., 2010). These findings reveal that ITD sensitivity is seriously affected by congenital deafness.
Auditory Cortex (AC). Deafness leads to structural and functional change throughout the central auditory system (Ferrara and Halnan, 1983; McMullen and Glaser, 1988; McMullen et al., 1988; Kral et al., 2000, 2001). In congenitally deaf cats, the AC does not receive any sound evoked input but it does display some rudimentary representation of cochleotopy and ITDs (Hartmann et al., 1997; Tillein et al., 2010). Investigations on the functional deficits of the auditory cortex in adult deaf cats were conducted where electrically evoked synaptic currents in cortical layers were compared between congenitally deaf and normal hearing animals (Kral et al., 2000). Marked functional deficits were found in the AC of the congenitally deaf cat attributed to result from abnormalities of the corticocortical and thalamocortical projections. Auditory experience through electrical stimulation was found to be necessary for recruitment and maturation of the AC, and such experiences expanded the functional area of AC over that of animals who did not receive meaningful stimulation (Klinke et al., 1999, 2001). Successful restoration of auditory synapses depended on early intervention in the life of the deaf animal and reflected the kinds of beneficial clinical outcomes in humans that reinforced the idea of a critical period in development (Busby et al., 1992,1993; Tyler et al., 1997; Busby and Clark, 1999) as well as cats (Klinke et al., 2001; O’Neil et al., 2010).
5. Concluding Remarks
Auditory experience has been shown to influence the structure and function of the central auditory system from birth through adulthood. Neural activity in the form of spike discharges is necessary for the initial formation of precise synaptic structure, tonotopic organization, and proper distribution of terminals in the ascending auditory pathway. Abnormalities in the organization of both excitatory and inhibitory connections are produced by deafness, hearing loss, and/or abnormal auditory environments. The pathologic changes in the developing organism become permanent unless normal activity is restored within an age-dependent critical period. The reestablishment of activity in the auditory system through electrical stimulation results in a remarkable recovery of the involved synapses and circuit organization.
Acknowledgements
The authors are supported by NIH grant DC000232, a grant from Advanced Bionics Corporation, and a Life Science Research Award from New South Wales, Australia.
Abbreviations
- AVCN
anteroventral cochlear nucleus
- CN
cochlear nucleus
- IC
inferior colliculus
- ILD
interaural level difference
- ITD
interaural time difference
- LSO
lateral superior olive
- MSO
medial superior olive
- MNTB
medial nucleus of the trapezoid body
- SBC
spherical bushy cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JC. Ascending projections to the inferior colliculus. J. Comp. Neurol. 1979;183:519–538. doi: 10.1002/cne.901830305. [DOI] [PubMed] [Google Scholar]
- Adams JC. Neuronal morphology in the human cochlear nucleus. Arch. Otolaryngol. Head Neck Surg. 1986;112:1253–1261. doi: 10.1001/archotol.1986.03780120017003. [DOI] [PubMed] [Google Scholar]
- Adams JC, Mugnaini E. Patterns of glutamate decarboxylase immunostaining in the feline cochlear nuclear complex studied with silver enhancement and electron microscopy. J. Comp. Neurol. 1987;262:375–401. doi: 10.1002/cne.902620305. [DOI] [PubMed] [Google Scholar]
- Altschuler RA, Wenthold RJ, Schwartz AM, Haser WG, Curthoys NP, Parakkal MH, Fex J. Immunocytochemical localization of glutaminase-like immunoreactivity in the auditory nerve. Brain Res. 1984;291:173–178. doi: 10.1016/0006-8993(84)90667-x. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Roth GL, Aitkin LM, Merzenich MM. The efferent projections of the central nucleus and the pericentral nucleus of the inferior colliculus in the cat. J. Comp. Neurol. 1980;194:649–662. doi: 10.1002/cne.901940311. [DOI] [PubMed] [Google Scholar]
- Asako M, Holt AG, Griffith RD, Buras ED, Altschuler RA. Deafness-related decreases in glycine-immunoreactive labeling in the rat cochlear nucleus. J. Neurosci. Res. 2005;81:102–109. doi: 10.1002/jnr.20542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babalian AL, Ryugo DK, Rouiller EM. Discharge properties of identified cochlear neurons and auditory nerve fibers in response to repetitive electrical stimulation of the auditory nerve. Exp. Brain Res. 2003;153:452–460. doi: 10.1007/s00221-003-1619-x. [DOI] [PubMed] [Google Scholar]
- Baker CA, Montey KL, Pongstaporn T, Ryugo DK. Postnatal development of the endbulb of Held in congenitally deaf cats. Front. Neuroanat. 2010;4:1–14. doi: 10.3389/fnana.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn CC, Sachs MS. The representations of the steady-state vowel sound/e/ in the discharge patterns of cat anteroventral cochlear nucleus neurons. J. Neurophysiol. 1990;63:1191–1212. doi: 10.1152/jn.1990.63.5.1191. [DOI] [PubMed] [Google Scholar]
- Bledsoe SC, Jr, Nagase S, Miller JM, Altschuler RA. Deafness-induced plasticity in the mature central auditory system. Neuroreport. 1995;7:225–229. [PubMed] [Google Scholar]
- Bosher SK, Hallpike CS. Observations on the histological features, development and pathogenesis of the inner ear degeneration of the deaf white cat. Proc. R. Soc. Lond., B, Biol. Sci. 1965;162:147–170. doi: 10.1098/rspb.1965.0030. [DOI] [PubMed] [Google Scholar]
- Bosher SK, Hallpike CS. Observations on the histogenesis of the inner ear degeneration of the deaf white cat and its possible relationship to the aetiology of certain unexplained varieties of human congenital deafness. J. Laryngol. Otol. 1967;80:222–235. doi: 10.1017/s0022215100065191. [DOI] [PubMed] [Google Scholar]
- Boudreau JC, Tsuchitani C. Binaural interaction in the cat superior olive S segment. J. Neurophysiol. 1968;31:442–454. doi: 10.1152/jn.1968.31.3.442. [DOI] [PubMed] [Google Scholar]
- Boyne AW, Bohan TP, Williams TH. Changes in cholinergic synaptic vesicle populations and the ultrastructure of the nerve terminal membranes of Narcine brasiliensis electron organ stimulated to fatigue in vivo. J. Cell Biol. 1975;67:814–825. doi: 10.1083/jcb.67.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A, Behrend O, Marquardt T, McAlpine D, Grothe B. Precise inhibition is essential for microsecond interaural time difference coding. Nature. 2002;417:543–547. doi: 10.1038/417543a. [DOI] [PubMed] [Google Scholar]
- Brawer JR, Morest DK. Relations between auditory nerve endings and cell types in the cat‘s anteroventral cochlear nucleus seen with the Golgi method and Nomarski optics. J. Comp. Neurol. 1975;160:491–506. doi: 10.1002/cne.901600406. [DOI] [PubMed] [Google Scholar]
- Brighton P, Ramesar R, Winship I. Hearing impairment and pigmentary disturbance. Ann. N.Y. Acad. Sci. 1991;630:152–166. doi: 10.1111/j.1749-6632.1991.tb19584.x. [DOI] [PubMed] [Google Scholar]
- Browner RH, Marbey D. The nucleus magnocellularis in the red-eared turtle, Chrysemys scripta elegans: eighth nerve endings and neuronal types. Hear Res. 1988;33:257–271. doi: 10.1016/0378-5955(88)90156-6. [DOI] [PubMed] [Google Scholar]
- Buras ED, Holt AG, Griffith RD, Asako M, Altschuler RA. Changes in glycine immunoreactivity in the rat superior olivary complex following deafness. J. Comp. Neurol. 2006;494:179–189. doi: 10.1002/cne.20795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwen SJ, Satir BH. Plasma membrane folds on the mast cell surface and their relationship to secretory activity. J. Cell Biol. 1977;74:690–697. doi: 10.1083/jcb.74.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busby PA, Clark GM. Gap detection by early-deafened cochlear-implant subjects. J. Acoust. Soc. Am. 1999;105:1841–1852. doi: 10.1121/1.426721. [DOI] [PubMed] [Google Scholar]
- Busby PA, Tong YC, Clark GM. Psychophysical studies using a multiple-electrode cochlear implant in patients who were deafened early in life. Audiology. 1992;31:95–111. doi: 10.3109/00206099209072905. [DOI] [PubMed] [Google Scholar]
- Busby PA, Tong YC, Clark GM. Electrode position, repetition rate, speech perception by early-and late-deafened cochlear implant patients. J. Acoust. Soc. Am. 1993;93:1058–1067. doi: 10.1121/1.405554. [DOI] [PubMed] [Google Scholar]
- Cant NB, Casseday JH. Projections from the anteroventral cochlear nucleus to the lateral and medial superior olivary nuclei. J. Comp. Neurol. 1986;247:457–476. doi: 10.1002/cne.902470406. [DOI] [PubMed] [Google Scholar]
- Cant NB, Hyson RL. Projections from the lateral nucleus of the trapezoid body to the medial superior olivary nucleus in the gerbil. Hear Res. 1992;58:26–34. doi: 10.1016/0378-5955(92)90005-8. [DOI] [PubMed] [Google Scholar]
- Cant NB, Morest DK. The bushy cells in the anteroventral cochear nucleus of the cat. A study with the electron microscope. Neuroscience. 1979;4:1925–1945. doi: 10.1016/0306-4522(79)90066-6. [DOI] [PubMed] [Google Scholar]
- Carr CE, Boudreau RE. Central projections of auditory nerve fibers in the barn owl. J. Comp. Neurol. 1991;314:306–318. doi: 10.1002/cne.903140208. [DOI] [PubMed] [Google Scholar]
- Carr CE, Boudreau RE. Development of the time coding pathways in the auditory brainstem of the barn owl. J. Comp. Neurol. 1996;373:467–483. doi: 10.1002/(SICI)1096-9861(19960930)373:4<467::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Carr CE, Koppl C. Coding interaural time differences at low best frequencies in the barn owl. J. Physiol. 2004;98:99–112. doi: 10.1016/j.jphysparis.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Chang EF, Merzenich MM. Environmental noise retards auditory cortical development. Science. 2003;300:498–502. doi: 10.1126/science.1082163. [DOI] [PubMed] [Google Scholar]
- Chen I, Limb CJ, Ryugo DK. The effect of cochlear-implant-mediated electrical stimulation on spiral ganglion cells in congenitally deaf white cats. J. Assoc. Res. Otolaryngol. 2010;11:587–603. doi: 10.1007/s10162-010-0234-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coco A, Epp SB, Fallon JB, Xu J, Millard RE, Shepherd RK. Does cochlear implantation and electrical stimulation affect residual hair cells and spiral ganglion neurons? Hear Res. 2007;225:60–70. doi: 10.1016/j.heares.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couchman K, Grothe B, Felmy F. Medial superior olivary neurons receive surprisingly few excitatory and inhibitory inputs with balanced strength and short-term dynamics. J. Neurosci. 2010;30:17111–17121. doi: 10.1523/JNEUROSCI.1760-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deol MS. The relationship between abnormalities of pigmentation and of the inner ear. Proc. R. Soc. Lond. B., Biol. Sci. 1970;175:201–217. doi: 10.1098/rspb.1970.0019. [DOI] [PubMed] [Google Scholar]
- Ferrara ML, Halnan CR. Congenital structural brain defects in the deaf dalmation. Vet. Rec. 1983;112:344–346. doi: 10.1136/vr.112.15.344. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick DC, Batra R, Stanford TR, Kuwada S. A neuronal population code for sound localization. Nature. 1997;388:871–874. doi: 10.1038/42246. [DOI] [PubMed] [Google Scholar]
- Francis HW, Manis PB. Effects of deafferentation on the electrophysiology of ventral cochlear nucleus neurons. Hear Res. 2000;149:91–105. doi: 10.1016/s0378-5955(00)00165-9. [DOI] [PubMed] [Google Scholar]
- Friauf E, Kandler K. Auditory projections to the inferior colliculus of the rat are present by birth. Neurosci. Lett. 1990;120:58–61. doi: 10.1016/0304-3940(90)90167-8. [DOI] [PubMed] [Google Scholar]
- Gantz BJ, Tyler RS, Woodworth GG, Tye-Murray N, Fryauf-Bertschy H. Results of multichannel cochlear implants in congenital and acquired prelingual deafness in children: five-year follow-up. Am. J. Otol. 1994 Suppl 2:1–7. [PubMed] [Google Scholar]
- Gillespie DC, Kim G, Kandler K. Inhibitory synapses in the developing auditory system are glutamatergic. Nat. Neurosci. 2005;8:332–338. doi: 10.1038/nn1397. [DOI] [PubMed] [Google Scholar]
- Grothe B. The evolution of temporal processing in the medial superior olive, an auditory brainstem structure. Prog. Neurobiol. 2000;61:581–610. doi: 10.1016/s0301-0082(99)00068-4. [DOI] [PubMed] [Google Scholar]
- Grothe B, Sanes DH. Bilateral inhibition by glycinergic afferents in the medial superior olive. J. Neurophysiol. 1993;69:1192–1196. doi: 10.1152/jn.1993.69.4.1192. [DOI] [PubMed] [Google Scholar]
- Grothe B, Sanes DH. Synaptic inhibition influences the temporal coding properties of medial superior olivary neurons: an in vitro study. J. Neurosci. 1994;(3 Pt 2):1701–1709. doi: 10.1523/JNEUROSCI.14-03-01701.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulley RL, Landis DM, Reese TS. Internal organization of membranes at endbulbs of Held in the anteroventral cochlear nucleus. J. Comp. Neurol. 1978;180:707–741. doi: 10.1002/cne.901800405. [DOI] [PubMed] [Google Scholar]
- Gulley RL, Wenthold RJ, Neises GR. Remodeling of neuronal membranes as an early response to deafferentation. A freeze-fracture study. J. Cell Biol. 1977;75:837–850. doi: 10.1083/jcb.75.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney CM, Osen KK, Ottersen OP, Storm-Mathisen J, Manjaly G. Immunocytochemical evidence that glutamate is a neurotransmitter in the cochlear nerve: a quantitative study in the guinea-pig anteroventral cochlear nucleus. Eur. J. Neurosci. 1996;8:79–91. doi: 10.1111/j.1460-9568.1996.tb01169.x. [DOI] [PubMed] [Google Scholar]
- Hancock K, Noel V, Ryugo DK, Delgutte B. Neural coding of ITD with bilateral cochlear implants: Effects of congenital deafness. J. Neurosci. 2010 doi: 10.1523/JNEUROSCI.3213-10.2010. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann R, Shepherd RK, Heid S, Klinke R. Response of the primary auditory cortex to electrical stimulation of the auditory nerve in the congenitally deaf white cat. Hear Res. 1997;112:115–133. doi: 10.1016/s0378-5955(97)00114-7. [DOI] [PubMed] [Google Scholar]
- Heid S, Hartmann R, Klinke R. A model for prelingual deafness, the congenitally deaf white cat—population statistics and degenerative changes. Hear Res. 1998;115:101–112. doi: 10.1016/s0378-5955(97)00182-2. [DOI] [PubMed] [Google Scholar]
- Heid S, Jahn-Siebert TK, Klinke R, Hartmann R, Langner G. Afferent projection patterns in the auditory brainstem in normal and congenitally deaf white cats. Hear Res. 1997;110:191–199. doi: 10.1016/s0378-5955(97)00074-9. [DOI] [PubMed] [Google Scholar]
- Held H. Die centrale Gehorleitung. Arch. Anat. Physiol. Anat. Abt. 1893;17:201–248. [Google Scholar]
- Heuser JE, Reese TS. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J. Cell Biol. 1973;57:315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu. Rev. Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Hultcrantz M, Snyder R, Rebscher S, Leake P. Effects of neonatal deafening and chronic intracochlear electrical stimulation on the cochlear nucleus in cats. Hear Res. 1991;54:272–280. doi: 10.1016/0378-5955(91)90121-o. [DOI] [PubMed] [Google Scholar]
- Hunter C, Petralia RS, Vu T, Wenthold RJ. Expression of AMPA-selective glutamate receptor subunits in morphologically defined neurons of the mammalian cochlear nucleus. J. Neurosci. 1993;13:1932–1946. doi: 10.1523/JNEUROSCI.13-05-01932.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffress LA. A place theory of sound localization. J. Comp. Physiol. Psychol. 1948;41:35–39. doi: 10.1037/h0061495. [DOI] [PubMed] [Google Scholar]
- Jhaveri S, Morest DK. Sequential alterations of neuronal architecture in nucleus magnocellularis of the developing chicken: a Golgi study. Neuroscience. 1982;7:837–853. doi: 10.1016/0306-4522(82)90046-x. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Afman CE. Hyperactivity in the dorsal cochlear nucleus after intense sound exposure and its resemblance to tone-evoked activity: a physiological model for tinnitus. Hear Res. 2000;140:165–172. doi: 10.1016/s0378-5955(99)00197-5. [DOI] [PubMed] [Google Scholar]
- Kandler K. Activity-dependent organization of inhibitory circuits: lessons from the auditory system. Curr. Opin. Neurobiol. 2004;14:96–104. doi: 10.1016/j.conb.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Kandler K, Clause A, Noh J. Tonotopic reorganization of developing auditory brainstem circuits. Nat. Neurosci. 2009;12:711–717. doi: 10.1038/nn.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler K, Friauf E. Development of glycinergic and glutamatergic synaptic transmission in the auditory brainstem of perinatal rats. J. Neurosci. 1995;15:6890–6904. doi: 10.1523/JNEUROSCI.15-10-06890.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapfer C, Seidl AH, Schweizer H, Grothe B. Experience-dependent refinement of inhibitory inputs to auditory coincidence-detector neurons. Nat. Neurosci. 2002;5:247–253. doi: 10.1038/nn810. [DOI] [PubMed] [Google Scholar]
- Kiang NYS, Watanabe T, Thomas EC, Clark LF.Discharge Patterns of Single Fibers in the Cat‘s Auditory Nerve 1965. **Cambridge (Mass.)M.I.T. Press [Google Scholar]
- Kim G, Kandler K. Elimination and strengthening of glycinergic/GABAergic connections during tonotopic map formation. Nat. Neurosci. 2003;6:282–290. doi: 10.1038/nn1015. [DOI] [PubMed] [Google Scholar]
- Klinke R, Hartmann R, Heid S, Tillein J, Kral A. Plastic changes in the auditory cortex of congenitally deaf cats following cochlear implantation. Audiol. Neurootol. 2001;6:203–206. doi: 10.1159/000046833. [DOI] [PubMed] [Google Scholar]
- Klinke R, Kral A, Heid S, Tillein J, Hartmann R. Recruitment of the auditory cortex in congenitally deaf cats by long-term cochlear electrostimulation. Science. 1999;285:1729–1733. doi: 10.1126/science.285.5434.1729. [DOI] [PubMed] [Google Scholar]
- Koerber KC, Pfeiffer RR, Warr WB, Kiang NY. Spontaneous spike discharges from single units in the cochlear nucleus after destruction of the cochlea. Exp. Neurol. 1966;16:119–130. doi: 10.1016/0014-4886(66)90091-4. [DOI] [PubMed] [Google Scholar]
- Kolston J, Osen KK, Hackney CM, Ottersen OP, Storm-Mathisen J. An atlas of glycine-and GABA-like immunoreactivity and colocalization in the cochlear nuclear complex of the guinea pig. Anat. Embryol (Berl) 1992;186:443–465. doi: 10.1007/BF00185459. [DOI] [PubMed] [Google Scholar]
- Konishi M. Birdsong: From behavior to neuron. Ann. Rev. Neurosci. 1985;8:125–170. doi: 10.1146/annurev.ne.08.030185.001013. [DOI] [PubMed] [Google Scholar]
- Kral A, Hartmann R, Tillein J, Heid S, Klinke R. Congenital auditory deprivation reduces synaptic activity within the auditory cortex in a layer-specific manner. Cereb. Cortex. 2000;10:714–726. doi: 10.1093/cercor/10.7.714. [DOI] [PubMed] [Google Scholar]
- Kral A, Hartmann R, Tillein J, Heid S, Klinke R. Delayed maturation and sensitive periods in the auditory cortex. Audiol. Neurootol. 2001;6:346–362. doi: 10.1159/000046845. [DOI] [PubMed] [Google Scholar]
- Kral A, Hartmann R, Tillein J, Heid S, Klinke R. Hearing after congenital deafness: central auditory plasticity and sensory deprivation. Cereb. Cortex. 2002;12:797–807. doi: 10.1093/cercor/12.8.797. [DOI] [PubMed] [Google Scholar]
- Kretzmer EA, Meltzer NE, Haenggeli CA, Ryugo DK. An animal model for cochlear implants. Arch. Otolaryngol. Head Neck Surg. 2004;130:499–508. doi: 10.1001/archotol.130.5.499. [DOI] [PubMed] [Google Scholar]
- Kuwabara N, Zook JM. Projections to the medial superior olive from the medial and lateral nuclei of the trapezoid body in rodents and bats. J. Comp. Neurol. 1992;324:522–538. doi: 10.1002/cne.903240406. [DOI] [PubMed] [Google Scholar]
- Larsen SA, Kirchhoff TM. Anatomical evidence of synaptic plasticity in the cochlear nuclei of white-deaf cats. Exp. Neurol. 1992;115:151–157. doi: 10.1016/0014-4886(92)90240-q. [DOI] [PubMed] [Google Scholar]
- Leake PA, Hradek GT. Cochlear pathology of long term neomycin induced deafness in cats. Hear Res. 1988;33:11–33. doi: 10.1016/0378-5955(88)90018-4. [DOI] [PubMed] [Google Scholar]
- Leake PA, Hradek GT, Snyder RL. Chronic electrical stimulation by a cochlear implant promotes survival of spiral ganglion neurons after neonatal deafness. J. Comp. Neurol. 1999;412:543–562. doi: 10.1002/(sici)1096-9861(19991004)412:4<543::aid-cne1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Lee DJ, Cahill HB, Ryugo DK. Effects of congenital deafness in the cochlear nuclei of Shaker-2 mice: an ultrastructural analysis of synapse morphology in the endbulbs of Held. J. Neurocytol. 2003;32:229–243. doi: 10.1023/B:NEUR.0000010082.99874.14. [DOI] [PubMed] [Google Scholar]
- Lenn NJ, Reese TS. The fine structure of nerve endings in the nucleus of the trapezoid body and the ventral cochlear nucleus. Am. J. Anat. 1966;118:375–389. doi: 10.1002/aja.1001180205. [DOI] [PubMed] [Google Scholar]
- Limb CJ, Ryugo DK. Development of primary axosomatic endings in the anteroventral cochlear nucleus of mice. J. Assoc. Res. Otolaryngol. 2000;1:103–119. doi: 10.1007/s101620010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linthicum FH, Jr, Fayad JN. Spiral ganglion cell loss is unrelated to segmental cochlear sensory system degeneration in humans. Otol. Neurotol. 2009;30:418–422. doi: 10.1097/mao.0b013e31819a8827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente de Nó R. The Primary Acoustic Nuclei. New York: Raven Press; 1981. [Google Scholar]
- Lorenz K. Der Kumpan in der Umwelt des Vogels. Der Artgenosse als auslosendes Moment sozialer Verhaltensweisen. J fur Ornithologie. 1935:83. [Google Scholar]
- Lustig LR, Leake PA, Snyder RL, Rebscher SJ. Changes in the cat cochlear nucleus following neonatal deafening and chronic intracochlear electrical stimulation. Hear Res. 1994;74:29–37. doi: 10.1016/0378-5955(94)90173-2. [DOI] [PubMed] [Google Scholar]
- Mair IW. Hereditary deafness in the white cat. Acta. Otolaryngol. 1973 Suppl 314:1–48. [PubMed] [Google Scholar]
- Manis PB, Marx SO. Outward currents in isolated central cochlear nucleus neurons. J. Neurosci. 1991;11:2865–2880. doi: 10.1523/JNEUROSCI.11-09-02865.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MR. Evidence for an excitatory amino acid as the transmitter of the auditory nerve in the in vitro mouse cochlear nucleus. Hear Res. 1985;20:215–220. doi: 10.1016/0378-5955(85)90026-7. [DOI] [PubMed] [Google Scholar]
- McAlpine D, Jiang D, Palmer AR. A neural code for low-frequency sound localization in mammals. Nat. Neurosci. 2001;4:396–401. doi: 10.1038/86049. [DOI] [PubMed] [Google Scholar]
- McMullen NT, Glaser EM. Auditory cortical responses to neonatal deafening: pyramidal neuron spine loss without changes in growth or orientation. Exp. Brain Res. 1988;72:195–200. doi: 10.1007/BF00248516. [DOI] [PubMed] [Google Scholar]
- McMullen NT, Goldberger B, Suter CM, Glaser EM. Neonatal deafening alters nonpyramidal dendrite orientation in auditory cortex: a computer microscope study in the rabbit. J. Comp. Neurol. 1988;267:92–106. doi: 10.1002/cne.902670107. [DOI] [PubMed] [Google Scholar]
- Moiseff A, Konishi M. Neuronal and behavioral sensitivity to binaural time differences in the owl. J. Neurosci. 1981;1:40–48. doi: 10.1523/JNEUROSCI.01-01-00040.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MJ, Caspary DM. Strychnine blocks binaural inhibition in lateral superior olivary neurons. J. Neurosci. 1983;3:237–242. doi: 10.1523/JNEUROSCI.03-01-00237.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DR, Kitzes LM. Projections from the cochlear nucleus to the inferior colliculus in normal and neonatally cochlea-ablated gerbils. J. Comp. Neurol. 1985;24:180–195. doi: 10.1002/cne.902400208. [DOI] [PubMed] [Google Scholar]
- Moore JK, Niparko JK, Miller MR, Perazzo LM, Linthicum FH. Effect of adult-onset deafness on the human central auditory system. Ann. Otol. Rhino. Laryngol. 1997;106:385–390. doi: 10.1177/000348949710600505. [DOI] [PubMed] [Google Scholar]
- Morest DK, Oliver DL. The neuronal architecture of the inferior colliculus in the cat: defining the functional anatomy of the auditory midbrain. J. Comp. Neurol. 1984;222:209–236. doi: 10.1002/cne.902220206. [DOI] [PubMed] [Google Scholar]
- Mossop JE, Wilson MJ, Caspary DM. Moore D.R., Down-regulation of inhibition following unilateral deafening. Hear Res. 2000;147:183–187. doi: 10.1016/s0378-5955(00)00054-x. [DOI] [PubMed] [Google Scholar]
- Nadol JB, Jr, Young YS, Glynn RJ. Survival of spiral ganglion cells in profound sensorineural hearing loss: implications for cochlear implantation. Ann. Otol. Rhinol. Laryngol. 1989;98:411–416. doi: 10.1177/000348948909800602. [DOI] [PubMed] [Google Scholar]
- Neises GR, Mattox DE, Gulley RL. The maturation of the end bulb of Held in the rat anteroventral cochlear nucleus. Anat. Rec. 1982;204:271–279. doi: 10.1002/ar.1092040312. [DOI] [PubMed] [Google Scholar]
- Ni D, Seldon HL, Shepherd RK, Clark GM. Effect of chronic electrical stimulation on cochlear nucleus neuron size in normal hearing kittens. Acta. Otolaryngol. 1993;113:489–497. doi: 10.3109/00016489309135851. [DOI] [PubMed] [Google Scholar]
- Nicol MJ, Walmsley B. Ultrastructural basis of synaptic transmission between endbulbs of held and bushy cells in the rat cochlear nucleus. J. Physiol. 2002;539:713–723. doi: 10.1113/jphysiol.2001.012972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordeen KW, Killackey HP, Kitzes LM. Ascending projections to the inferior colliculus following unilateral cochlear ablation in the neonatal gerbil, Meriones unguiculatus. J. Comp. Neurol. 1983a;214:144–153. doi: 10.1002/cne.902140204. [DOI] [PubMed] [Google Scholar]
- Nordeen KW, Killackey HP, Kitzes LM. Ascending projections to the inferior colliculus following unilateral cochlear ablation in the adult gerbil, Meriones unguiculatus. J. Comp. Neurol. 1983b;214:131–143. doi: 10.1002/cne.902140204. [DOI] [PubMed] [Google Scholar]
- Oertel D. Synaptic responses and electrical properties of cells in brain slices of the mouse anteroventral cochlear nucleus. J. Neurosci. 1983;3:2043–2053. doi: 10.1523/JNEUROSCI.03-10-02043.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleskevich S, Youssoufian M, Walmsley B. Presynaptic plasticity at two giant auditory synapses in normal and deaf mice. J. Physiol. 2004;560(Pt 3):709–719. doi: 10.1113/jphysiol.2004.066662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil JN, Limb CJ, Baker CA, Ryugo DK. Bilateral effects of unilateral cochlear implantation in congenitally deaf cats. J. Comp. Neurol. 2010;518:2382–2404. doi: 10.1002/cne.22339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil JN, Limb CJ, Flores K, Rosenblatt A, Ryugo DK. Effects of unilateral cochlear implantation on auditory nerve synapses and globular bushy cells in congenitally deaf white cats. Assn. Res. Otolaralyngol. Abst. 2011 [Google Scholar]
- Osen KK. Cytoarchitecture of the cochlear nuclei in the cat. J. Comp. Neurol. 1969;136:453–484. doi: 10.1002/cne.901360407. [DOI] [PubMed] [Google Scholar]
- Parks TN, Rubel EW, Popper AN, Fay RR, editors. Plasticity of the Auditory System. New York: Springer; 2004. [Google Scholar]
- Pecka M, Brand A, Behrend O, Grothe B. Interaural time difference processing in the mammalian medial superior olive: the role of glycinergic inhibition. J. Neurosci. 2008;28:6914–6925. doi: 10.1523/JNEUROSCI.1660-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Rubio ME, Wang YX, Wenthold RJ. Differential distribution of glutamate receptors in the cochlear nuclei. Hear Res. 2000;147:59–69. doi: 10.1016/s0378-5955(00)00120-9. [DOI] [PubMed] [Google Scholar]
- Pfeiffer RR. Anteroventral cochlear nucleus: wave forms of extracellularly recorded spike potentials. Science. 1966;154:667–668. doi: 10.1126/science.154.3749.667. [DOI] [PubMed] [Google Scholar]
- Quittner AL, Steck JT. Predictors of cochlear implant use in children. Am. J. Otol. 1991;(12 Suppl):89–94. [PubMed] [Google Scholar]
- Ramón y Cajal S. Histologie du systeme nerveux de l’homme et des vertebres. Maloine, Paris. 1909 [Google Scholar]
- Rauschecker JP, Shannon RV. Sending sound to the brain. Science. 2002;295:1025–1029. doi: 10.1126/science.1067796. [DOI] [PubMed] [Google Scholar]
- Rebillard M, Rebilliard G, Pujol R. Variability of the hereditary deafness in the white cat I. Physiology. Hear Res. 1981a;5:179–187. doi: 10.1016/0378-5955(81)90044-7. [DOI] [PubMed] [Google Scholar]
- Rebillard M, Rebilliard G, Pujol R. Variability of the hereditary deafness in the white cat. II. Histology. Hear Res. 1981b;5:189–200. doi: 10.1016/0378-5955(81)90045-9. [DOI] [PubMed] [Google Scholar]
- Redd EE, Pongstaporn T, Ryugo DK. The effects of congenital deafness on auditory nerve synapses and globular bushy cells in cats. Hear Res. 2000;147:160–174. doi: 10.1016/s0378-5955(00)00129-5. [DOI] [PubMed] [Google Scholar]
- Rees S, Guldner FH, Aitkin L. Activity dependent plasticity of postsynaptic density structure in the ventral cochlear nucleus of the rat. Brain Res. 1985;325:370–374. doi: 10.1016/0006-8993(85)90343-9. [DOI] [PubMed] [Google Scholar]
- Rietzel HJ, Friauf E. Neuron types in the rat lateral superior olive and developmental changes in the complexity of their dendritic arbors. J. Comp. Neurol. 1998;390:20–40. [PubMed] [Google Scholar]
- Robards MJ. Somatic neurons in the brainstem and neocortex projecting to the external nucleus of the inferior colliculus: an anatomical study in the opposum. J. Comp. Neurol. 1979;184:547–565. doi: 10.1002/cne.901840308. [DOI] [PubMed] [Google Scholar]
- Robbins AM. Language development in children with cochlear implants. In: Waltzman SB, Roland JR Jr, editors. Cochlear Implants. New York: Thieme Medical Publishers; 2006. pp. 153–166. [Google Scholar]
- Romand R. Survey of intracellular recording in the cochlear nucleus of the cat. Brain Res. 1978;148:43–65. doi: 10.1016/0006-8993(78)90377-3. [DOI] [PubMed] [Google Scholar]
- Roth GL, Aitkin LM, Andersen RA, Merzenich MM. Some features of the spatial organization of the central nucleus of the inferior colliculus of the cat. J. Comp. Neurol. 1978;182:661–680. doi: 10.1002/cne.901820407. [DOI] [PubMed] [Google Scholar]
- Russell FA, Moore DR. Afferent reorganization within the superior olivary complex of the gerbil: development and induction by neonatal, unilateral cochlear removal. J. Comp. Neurol. 1995;352:607–625. doi: 10.1002/cne.903520409. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Baker CA, Montey KL, Chang LY, Coco A, Fallon JB, Shepherd RK. Synaptic plasticity after chemical deafening and electrical stimulation of the auditory nerve in cats. J. Comp. Neurol. 2010;518:1046–1063. doi: 10.1002/cne.22262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryugo DK, Fekete DM. Morphology of primary axosomatic endings in the anteroventral cochlear nucleus of the cat: a study of the endbulbs of Held. J. Comp. Neurol. 1982;210:239–257. doi: 10.1002/cne.902100304. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Kretzmer EA, Niparko JK. Restoration of auditory nerve synapses in cats by cochlear implants. Science. 2005;310:1490–1492. doi: 10.1126/science.1119419. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Limb CJ. Brain plasticity: The impact of the environment on the brain as it relates to cochlear implants. In: Niparko JK, Kirk KI, Mellon NK, Robbins AM, Tucci DL, Wilson BS, editors. Cochlear Implants: Principles and Practices. 2nd Edition. Philasdelphia: Lippincott Williams & Wilkins; 2009. pp. 19–37. [Google Scholar]
- Ryugo DK, Montey KL, Wright AL, Bennett ML, Pongstaporn T. Postnatal development of a large auditory nerve terminal: the endbulb of Held in cats. Hear Res. 2006:216–217. 100–115. doi: 10.1016/j.heares.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Parks TN. Primary innervation of the avian and mammalian cochlear nucleus. Brain Res. Bull. 2003;60:435–456. doi: 10.1016/s0361-9230(03)00049-2. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Pongstaporn T, Huchton DM, Niparko JK. Ultrastructural analysis of primary endings in deaf white cats: morphologic alterations in endbulbs of Held. J. Comp. Neurol. 1997;385:230–244. doi: 10.1002/(sici)1096-9861(19970825)385:2<230::aid-cne4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Rosenbaum BT, Kim PJ, Niparko JK, Saada AA. Single unit recordings in the auditory nerve of congenitally deaf white cats: morphological correlates in the cochlea and cochlear nucleus. J. Comp. Neurol. 1998;397:532–548. doi: 10.1002/(sici)1096-9861(19980810)397:4<532::aid-cne6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Sento S. Synaptic connections of the auditory nerve in cats: relationship between endbulbs of held and spherical bushy cells. J. Comp. Neurol. 1991;305:35–48. doi: 10.1002/cne.903050105. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Willard FH, Fekete DM. Differential afferent projections to the inferior colliculus from the cochlear nucleus in the albino mouse. Brain Res. 1981;210:342–349. doi: 10.1016/0006-8993(81)90907-0. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Wu MM, Pongstaporn T. Activity-related features of synapse morphology: a study of endbulbs of Held. J. Comp. Neurol. 1996;365:141–158. doi: 10.1002/(SICI)1096-9861(19960129)365:1<141::AID-CNE11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Saada AA, Niparko JK, Ryugo DK. Morphological changes in the cochlear nucleus of congenitally deaf white cats. Brain Res. 1996;736:315–328. doi: 10.1016/0006-8993(96)00719-6. [DOI] [PubMed] [Google Scholar]
- Safieddine S, Wenthold RJ. The glutamate receptor subunit delta1 is highly expressed in hair cells of the auditory and vestibular systems. J. Neurosci. 1997;17:7523–7531. doi: 10.1523/JNEUROSCI.17-19-07523.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes DH. An in vitro analysis of sound localization mechanisms in the gerbil lateral superior olive. 1990;10:3494–3506. doi: 10.1523/JNEUROSCI.10-11-03494.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes DH. The development of synaptic function and integration in the central auditory system. J. Neurosci. 1993;13:2627–2637. doi: 10.1523/JNEUROSCI.13-06-02627.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes DH, Bao S. Tuning up the developing auditory CNS. Curr. Opin Neurobiol. 2009;19:188–199. doi: 10.1016/j.conb.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes DH, Friauf E. Development and influence of inhibition in the lateral superior olivary nucleus. Hear Res. 2000;147:46–58. doi: 10.1016/s0378-5955(00)00119-2. [DOI] [PubMed] [Google Scholar]
- Sanes DH, Geary WA, Wooten GF, Rubel EW. Quantitative distribution of the glycine receptor in the auditory brain stem of the gerbil. J. Neurosci. 1987;7:3793–3802. doi: 10.1523/JNEUROSCI.07-11-03793.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes DH, Markowitz S, Bernstein J, Wardlow J. The influence of inhibitory afferents on the development of postsynaptic dendritic arbors. J. Comp. Neurol. 1992;321:637–644. doi: 10.1002/cne.903210410. [DOI] [PubMed] [Google Scholar]
- Sanes DH, Rubel EW. The ontogeny of inhibition and excitation in the gerbil lateral superior olive. J. Neurosci. 1988;8:682–700. doi: 10.1523/JNEUROSCI.08-02-00682.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes DH, Siverls V. Development and specificity of inhibitory terminal arborizations in the central nervous system. J. Neurobiol. 1991;22:837–854. doi: 10.1002/neu.480220805. [DOI] [PubMed] [Google Scholar]
- Sanes DH, Takacs C. Activity-dependent refinement of inhibitory connections. Eur. J. Neurosci. 1993;5:570–574. doi: 10.1111/j.1460-9568.1993.tb00522.x. [DOI] [PubMed] [Google Scholar]
- Scheibe A. A case of deaf-mutism, with auditory atrophy and anomalies of development in the membraneous labyrinth of both ears. Arch. Otolaryngol. 1882;21:12–22. [Google Scholar]
- Scheibe A. Bildungsanomalien im Hautigen Labyrinth bei Taubstummheit. Z. Ohrenheilk. 1885;27:95–99. [Google Scholar]
- Schwartz IR, Higa JF. Correlated studies of the ear and brainstem in the deaf white cat: changes in the spiral ganglion and the medial superior olivary nucleus. Acta. Otolaryngol. 1982;93:9–18. doi: 10.3109/00016488209130847. [DOI] [PubMed] [Google Scholar]
- Schweitzer L, Jensen KF, Jannssen R. Glutamate neurotoxicity in rat auditory system: cochlear nuclear complex. Neurotoxicol. Teratol. 1991;13:189–193. doi: 10.1016/0892-0362(91)90010-t. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Javel E. Electrical stimulation of the auditory nerve. I. Correlation of physiological responses with cochlear status. Hear Res. 1997;108:112–144. doi: 10.1016/s0378-5955(97)00046-4. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Javel E. Electrical stimulation of the auditory nerve: II. Effect of stimulus waveshape on single fibre response properties. Hear Res. 1999;130:171–188. doi: 10.1016/s0378-5955(99)00011-8. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Meltzer NE, Fallon JB, Ryugo DK. Consequences of deafness and electrical stimulation on the peripheral and central auditory system. In: Waltzman SB, Roland JT, editors. Cochlear Implants. New York: Thieme Medical PUblishers Inc.; 2006. pp. 25–39. [Google Scholar]
- Sly DJ, Heffer LF, White MW, Shepherd RK, Birch MG, Minter RL, Nelson NE, Wise AK, O’Leary SJ. Deafness alters auditory nerve fibre responses to cochlear implant stimulation. Eur. J. Neurosci. 2007;26:510–522. doi: 10.1111/j.1460-9568.2007.05678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PH, Joris PX, Yin TC. Projections of physiologically characterized spherical bushy cell axons from the cochlear nucleus of the cat: evidence for delay lines to the medial superior olive. J. Comp. Neurol. 1993;331:245–260. doi: 10.1002/cne.903310208. [DOI] [PubMed] [Google Scholar]
- Snyder R, Leake P, Rebscher S, Beitel R. Temporal resolution of neurons in cat inferior colliculus to intracochlear electrical stimulation: effects of neonatal deafening and chronic stimulation. J. Neurophysiol. 1995;73:449–467. doi: 10.1152/jn.1995.73.2.449. [DOI] [PubMed] [Google Scholar]
- Snyder RL, Rebscher SJ, Cao KL, Leake PA, Kelly K. Chronic intracochlear electrical stimulation in the neonatally deafened cat. I: Expansion of central representation. Hear Res. 1990;50:7–33. doi: 10.1016/0378-5955(90)90030-s. [DOI] [PubMed] [Google Scholar]
- Snyder RL, Vollmer M, Moore CM, Rebscher SJ, Leake PA, Beitel RE. Responses of inferior colliculus neurons to amplitude-modulated intracochlear electrical pulses in deaf cats. 2000;84:166–183. doi: 10.1152/jn.2000.84.1.166. [DOI] [PubMed] [Google Scholar]
- Stakhovskaya O, Hradek GT, Snyder RL, Leake PA. Effects of age at onset of deafness and electrical stimulation on the developing cochlear nucleus in cats. Hear Res. 2008;243:69–77. doi: 10.1016/j.heares.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga F, Hattler KW. Physiological and histopathological correlates of hereditary deafness in animals. Laryngoscope. 1970;80:81–104. doi: 10.1288/00005537-197001000-00007. [DOI] [PubMed] [Google Scholar]
- Syka J, Popelar J, Kvasnak E, Astl J. Response properties of neurons in the central nucleus and external and dorsal cortices of the inferior colliculus in guinea pig. Exp. Brain Res. 2000;133:254–266. doi: 10.1007/s002210000426. [DOI] [PubMed] [Google Scholar]
- Szpir MR, Sento S, Ryugo DK. Central projections of cochlear nerve fibers in the alligator lizard. J. Comp. Neurol. 1990;295:530–547. doi: 10.1002/cne.902950403. [DOI] [PubMed] [Google Scholar]
- Tillein J, Hubka P, Syed E, Hartmann R, Engel AK, Kral A. Cortical representation of interaural time difference in congenital deafness. Cereb. Cortex. 2010;20:492–506. doi: 10.1093/cercor/bhp222. [DOI] [PubMed] [Google Scholar]
- Tirko NN, Pongstaporn T, Ryugo DK. Synaptic organization of MSO principal neurons in hearing, deaf, and cochlear-implanted cats. Abstr. Midwinter Res. Meet. Assoc. Res. Otolaryngol. 2009 [Google Scholar]
- Tollin DJ. The lateral superior olive: a functional role in sound source localization. Neuroscientist. 2003;9:127–143. doi: 10.1177/1073858403252228. [DOI] [PubMed] [Google Scholar]
- Tsuji J, Liberman MC. Intracellular labeling of auditory nerve fibers in guinea pig: central and peripheral projections. J. Comp. Neurol. 1997;381:188–202. [PubMed] [Google Scholar]
- Tyler R, Parkinson AJ, Fryauf-Bertchy H, Lowder MW, Parkinson WS, Gantz BJ, Kelsay DM. Speech perception by prelingually deaf children and postlingually deaf adults with cochlear implant. Scand. Audiol. 1997 Suppl 46:65–71. [PubMed] [Google Scholar]
- Tyler RS, Summerfield AQ. Cochlear implantation: relationships with research on auditory deprivation and acclimatization. Ear Hear. 1996;17:38S–50S. doi: 10.1097/00003446-199617031-00005. [DOI] [PubMed] [Google Scholar]
- Van der Loos H, Woolsey TA. Somatosensory cortex: structural alterations following early injury to sense organs. Science. 1973;179:395–398. doi: 10.1126/science.179.4071.395. [DOI] [PubMed] [Google Scholar]
- van Hoesel RJ. Exploring the benefits of bilateral cochlear implants. Aud. Neurootol. 2004;9:234–246. doi: 10.1159/000078393. [DOI] [PubMed] [Google Scholar]
- van Hoesel RJ. Sensitivity to binaural timing in bilateral cochlear implant users. J. Acoust. Soc. Am. 2007;121:2192–2206. doi: 10.1121/1.2537300. [DOI] [PubMed] [Google Scholar]
- Vollmer M, Leake PA, Beitel RE, Rebscher SJ, Snyder RL. Degradation of temporal resolution in the auditory midbrain after prolonged deafness is reversed by electrical stimulation of the cochlea. J. Neurophysiol. 2005;93:3339–3355. doi: 10.1152/jn.00900.2004. [DOI] [PubMed] [Google Scholar]
- Walmsley B, Berntson A, Leao RN, Fyffe RE. Activity-dependent regulation of synaptic strength and neuronal excitability in central auditory pathways. J. Physiol. 2006;572(Pt2):313–321. doi: 10.1113/jphysiol.2006.104851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltzman SB, Cohen NL, Gomolin RH, Green JE, Shapiro WH, Hoffman RA, Roland JT., Jr Open-set speech perception in congenitally deaf children using cochlear implants. Am. J. Otol. 1997 18;:342–349. [PubMed] [Google Scholar]
- Waltzman SB, Cohen NL, Gomolin RH, Shapiro WH, Ozdamar SR, Hoffman RA. Long-term results of early cochlear implantation in congenitally and prelingually deafened children. Am. J. Otol. 1994;15 Suppl 2:9–13. [PubMed] [Google Scholar]
- Waltzman SB, Cohen NL, Shapiro WH. The benefits of cochlear implantation in the geriatric population. Otolaryngol. Head Neck Surg. 1993;108:329–333. doi: 10.1177/019459989310800404. [DOI] [PubMed] [Google Scholar]
- Wang Y, Manis PB. Synaptic transmission at the cochlear nucleus endbulb synapse during age-related hearing loss in mice. J. Neurophysiol. 2005;94:1814–1824. doi: 10.1152/jn.00374.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Manis PB. Temporal coding by cochlear nucleus bushy cells in DBA/2J mice with early onset hearing loss. J. Assoc. Res. Otolaryngol. 2006;7:412–424. doi: 10.1007/s10162-006-0052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TX, Wenthold RJ, Ottersen OP, Petralia RS. Endbulb synapses in the anteroventral cochlear nucleus express a specific subset of AMPA-type glutamate receptor subunits. J. Neurosci. 1998;18:1148–1160. doi: 10.1523/JNEUROSCI.18-03-01148.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller WL, Johnson JL. Barrels in cerebral cortex by receptor disruption in newborn, but not in five-day-old mice (Cricetidoe and Muridae) Brain Res. 1975;83:504–508. doi: 10.1016/0006-8993(75)90844-6. [DOI] [PubMed] [Google Scholar]
- Werthat F, Alexandrova O, Grothe B, Koch U. Experience-dependent refinement of the inhibitory axons projecting to the medial superior olive. Dev. Neurobiol. 2008;68:1454–1462. doi: 10.1002/dneu.20660. [DOI] [PubMed] [Google Scholar]
- West CD, Harrison JM. Transneuronal cell atrophy in the congenitally deaf white cat. J. Comp. Neurol. 1973;151:377–398. doi: 10.1002/cne.901510406. [DOI] [PubMed] [Google Scholar]
- Winer JA. Decoding the auditory corticofugal systems. Hear Res. 2005;207:1–9. doi: 10.1016/j.heares.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Wojcik SM, Rhee JS, Herzog E, Sigler A, Jahn R, Takamori S, Brose N, Rosenmund C. An essential role for vesicular glutamate transporter 1 (VGLUT1) in postnatal development and control of quantal size. Proc. Natl. Acad. Sci. 2004;101:158–163. doi: 10.1073/pnas.0401764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SH, Kelly JB. Physiological evidence for ipsilateral inhibition in the lateral superior olive: synaptic responses in mouse brain slice. Hear Res. 1994;73:57–64. doi: 10.1016/0378-5955(94)90282-8. [DOI] [PubMed] [Google Scholar]
- Yin DC, Chan JC. Interaural time sensitivity in medial superior olive of cat. J. Neurophysiol. 1990;64:465–488. doi: 10.1152/jn.1990.64.2.465. [DOI] [PubMed] [Google Scholar]
- Young SR, Rubel EW. Embryogenesis of arborization pattern and topography of individual axons in N. laminaris of the chicken brain stem. J. Comp. Neurol. 1986;254:425–459. doi: 10.1002/cne.902540402. [DOI] [PubMed] [Google Scholar]
- Zeng C, Nannapaneni N, Zhou J, Hughes LF, Shore S. Cochlear damage changes the distribution of vesicular glutamate transporters associated with auditory and nonauditory inputs to the cochlear nucleus. J. Neurosci. 2009;29:4210–4217. doi: 10.1523/JNEUROSCI.0208-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Nagarajan N, Mossop BJ, Merzenich MM. Influences of un-modulated acoustic inputs on functional maturation and critical-period plasticity of the primary auditory cortex. Neuroscience. 2008;154:390–396. doi: 10.1016/j.neuroscience.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Nannapaneni N, Shore S. Vesicular glutamate transporters 1 and 2 are differentially associated with auditory nerve and spinal trigeminal inputs to the cochlear nucleus. J. Comp. Neurol. 2007;500:777–787. doi: 10.1002/cne.21208. [DOI] [PubMed] [Google Scholar]