Abstract
Cytokine-induced stimulation of p38 mitogen activated protein kinase (MAPK) has been shown to influence behaviorally-relevant pathophysiologic pathways including monoamine neurotransmission and neuroendocrine function and thus may contribute to behavioral changes that occur during chronic administration of the innate immune cytokine, interferon (IFN)-alpha. Accordingly, in the current study, phosphorylation (activation) of intracellular p38 MAPK in peripheral blood lymphocytes was analyzed by flow cytometry every 2 hours for 12 hours following the initial injection of IFN-alpha in eleven patients with chronic hepatitis C. Hourly assessments of plasma concentrations of adrenocorticotropic hormone, cortisol and interleukin-6 were also obtained. Symptoms of depression and fatigue were measured at baseline and after 4 and 12 weeks of IFN-alpha treatment. Acute administration of IFN-alpha significantly increased the percentage of lymphocytes staining positive for intracellular phosphorylated p38 (p-p38). IFN-alpha-induced increases in p-p38 were significantly greater in patients that developed clinically significant depressive symptoms [Montgomery Asberg Depression Rating Scale (MADRS) score ≥15] during the first 12 weeks of IFN-alpha treatment. Increases in the percentage of p-p38-positive lymphocytes following the first IFN-alpha injection also highly correlated with depression severity at weeks 4 (r=0.85, p=0.001) and 12 (r=0.70, p=0.018). Similar relationships were observed for fatigue. Examination of relationships between p-p38 induction and factors previously reported to predict IFN-alpha-induced depressive symptoms revealed strong associations of p-p38 with baseline MADRS (r=0.82, p=0.002) and cortisol responses to the initial injection of IFN-alpha (r=0.91, p=0.000). Taken together, these findings indicate that sensitivity of p38 MAPK signaling pathways to immune stimulation is associated with depressive symptoms during chronic IFN-alpha treatment.
Keywords: interferon-alpha, p38 mitogen activated protein kinase, cytokines, depression, fatigue, flow cytometry, cortisol, innate immunity
Introduction
Activation of the innate immune system has been shown to produce behavioral alterations that are mediated in part by changes in monoamine metabolism and the hypothalamic-pituitary-adrenal (HPA) axis (Dantzer et al. 2008; Miller et al. 2009). For example, administration of the innate immune cytokine, interferon (IFN)-alpha, for infectious diseases and cancer induces high rates of depression and fatigue, and alters both monoamines and HPA axis function. More specifically, IFN-alpha increases cerebrospinal fluid (CSF) cytokines which negatively correlate with CSF concentrations of the serotonin metabolite, 5-hydroxyindoleacetic acid (5-HIAA), that, in turn, correlate with depression (Raison et al. 2009). In addition, polymorphisms in the serotonin transporter have been associated with IFN-alpha-induced depression severity (Bull et al. 2009; Lotrich et al. 2009). IFN-alpha has also been shown to inhibit glucocorticoid receptor (GR) function and lead to flattening of the diurnal cortisol curve, which correlates with depression and fatigue (Hu et al. 2009; Raison et al. 2010a).
One mechanism by which innate immune cytokines such as IFN-alpha may affect monoamine metabolism and neuroendocrine function is through activation of p38 mitogen activated protein kinase (MAPK). Activation of p38 MAPK has been shown to increase the activity and expression of the serotonin transporter in vitro and in vivo (Zhu et al. 2006; Zhu et al. 2010). In addition, increased basal p38 MAPK activation in peripheral blood mononuclear cells of abused monkeys was found to correlate with decreased CSF concentrations of 5-HIAA (Sanchez et al. 2007). Cytokine-induced activation of p38 MAPK has also been shown to inhibit GR function through blocking translocation of GR from cytoplasm to nucleus (Wang et al. 2004).
To investigate the potential role of p38 MAPK in IFN-alpha-induced behavioral disturbance, in vivo p38 MAPK responses to the initial injection of IFN-alpha were examined in peripheral blood lymphocytes of patients with chronic hepatitis C virus (HCV) infection. In addition, the relationship between p38 MAPK phosphorylation (activation) and neuroendocrine (HPA axis) and immune (IL-6) responses to the initial injection of IFN-alpha as well as subsequent depression and fatigue at weeks 4 and 12 of IFN-alpha treatment were explored.
Methods and Materials
Participants
Eleven HCV-positive subjects were enrolled. Exclusion criteria included decompensated liver disease; liver disease from any cause other than HCV; unstable cardiovascular, endocrinologic, hematologic, renal or neurologic disease (as determined by physical exam and laboratory testing); and history of schizophrenia or bipolar disorder and/or diagnosis of major depression (MD) or substance abuse/dependence within 6 months of study entry (determined by Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders—Fourth Edition) (First et al. 1997). Subjects were required to be off all psychotropic medications and prescription medications known to affect the immune system for at least 4 weeks prior to study entry. Complete blood counts were obtained at baseline, and all subjects had white blood cell counts and percentages of lymphocytes, monocytes, and granulocytes within normal limits. All patients received either pegylated IFN-alfa-2b (Pegintron, Schering Plough, Kenilworth, NJ; n=5, 1.5μg/kg) or pegylated IFN-alfa-2a (Pegasys, Roche-Genentech, San Francisco, CA; n=6, 180mg) administered subcutaneously weekly, plus oral ribavirin (800–1400mg) daily. The subjects represent a subgroup of patients reported on previously (Raison et al. 2009; Raison et al. 2010a; Raison et al. 2010b).
Study Design
Subjects were admitted to the Emory Clinical Interactions Unit the evening prior to blood sampling. Blood was collected into EDTA-coated tubes hourly from 9AM–9PM on 2 separate days via an indwelling catheter inserted at 8AM. Blood was centrifuged at 1000×g for 10 min at 4°C, and plasma was removed and frozen at −80°C until assay. For flow cytometry, 200μl of whole blood was removed prior to plasma isolation. Pegylated IFN-alpha was administered subcutaneously after the 10AM blood draw on the second day of blood sampling. Ribavirin was not administered on the day of the first IFN-alpha injection, and was initiated the day after completion of blood sampling. Subsequent administration of IFN-alpha and ribavirin was supervised by the patients’ treating physician. Behavioral assessments were conducted on the first day of blood sampling and at follow-up visits on weeks 4 and 12. Depression was evaluated using the 10-item, clinician-administered Montgomery-Asberg Depression Rating Scale (MADRS)(Montgomery and Asberg 1979), and fatigue was assessed using the self-report, 20-item Multidimensional Fatigue Inventory (MFI)(Smets et al. 1995).
p38 Protocol
Fresh blood was lysed of red blood cells, and the resultant cell pellet was washed, fixed in 2% paraformaldehyde, and permeabilized with 90% methanol at 4 °C for 30min. Cells were incubated 1 hour at room temperature with mouse anti-phosphorylated p38 (p-p38) MAPK (T180/Y182) phycoerythrin-conjugated monoclonal antibody clone 36 (BD Biosciences, San Diego, CA), washed and resuspended in 2% paraformaldehyde for flow cytometry assessment (FACSCalibur or LSRII, BD Biosciences)(Sanchez et al. 2007). Data was analyzed using FlowJo software (Tree Star Inc., Ashland, OR). Clone 36 and similar monoclonal antibodies to p38 MAPK (T180/Y182) are commonly used in flow cytometry and Western blot analysis to measure p-p38 (Rius et al. 2010; Zampieri et al. 2007). In vitro studies were conducted to demonstrate antibody specificity and the impact of IFN-alpha on intracellular p38 protein and p38 phosphorylation in specific lymphocyte subsets (Supplementary Materials).
Hormone and Cytokine Assays
Commercially available immunoradiometric assay and radioimmunoassay kits were used for assessment of plasma adrenocorticotropic hormone (ACTH; ALPCO Diagnostics, Salem, NH and Nichols Institute Diagnostics, San Juan Capistrano, CA, when available) and cortisol (DiaSorin, Stillwater, MN), respectively. Intra- and inter-assay coefficients of variation respectively were 2.8 and 5.7% (ALPCO) or 4.5 and 6.3% (Nichols) for ACTH and 8.5 and 12.7% for cortisol. Concentrations of IL-6 were measured in duplicate by high sensitivity quantitative enzyme-linked immunosorbent assays according to manufacturer’s specifications (R&D Systems, Minneapolis, MN). Inter- and intra-assay variability for IL-6 was reliably <12%.
Statistical Analysis
Differences between groups were assessed using t tests or Fisher’s Exact Test for categorical variables. One- and two-way repeated measures analysis of variance on log-transformed data were used to assess change in lymphocyte intracellular p-p38 across time in all subjects and in subjects with a MADRS ≥15 versus <15. A MADRS score of 15 has been used as a cut-off score for clinically significant depressive symptoms (Kearns et al. 1982; Potter et al. 2004). Post-hoc comparisons between means of interest were performed using Fisher’s Least Significant Difference test. Delta maximum p-p38 MAPK (delta max p-p38) was calculated as the highest percentage of cells staining positive for intracellular p-p38 following IFN-alpha injection minus the 9AM baseline value. Pearson correlation coefficients were calculated to evaluate associations between delta max p-p38 and depression and fatigue during IFN-alpha treatment as well as the acute response of ACTH, cortisol and IL-6 to the initial IFN-alpha injection. For non-normally-distributed data, Spearman correlation coefficients were employed. Where indicated, partial correlation coefficients were determined to control for relevant clinical covariates. All tests of significance were two-tailed with alpha <0.05.
Results
In the sample as a whole (n=11), IFN-alpha injection significantly increased the percentage of lymphocytes positive for intracellular p-p38 MAPK compared to the 9AM baseline (F=4.5, df=6,59, p<0.001), with peak increases being observed 5 hours after IFN-alpha injection (Supplementary Materials, Fig. S1). No changes in p-p38 were observed over the diurnal cycle absent of IFN-alpha administration (data not shown). In vitro studies of IFN-alpha-treated peripheral blood mononuclear cells revealed the majority of lymphocytes positive for p-p38 were CD4+ T cells (Supplementary Materials, Fig. S2). Moreover, in vitro administration of IFN-alpha was not associated with an overall increase in p38 protein as measured by Western blot (Supplementary Materials, Fig. S2).
Six of 11 patients experienced significant depressive symptoms (MADRS score ≥15) at some point during the first 12 weeks of IFN-alpha treatment. No significant differences were found between patients with MADRS scores ≥15 versus those with MADRS scores <15 in age [48.0(SD=4.05) versus 46.8(SD=7.9), p=0.75], body mass index (BMI) [27.4(SD=6.0) versus 30.2(SD=5.2), p=0.44] or race, sex, history of MD, history of substance abuse, or type of IFN-alpha (Fisher Exact tests, all p>0.50).
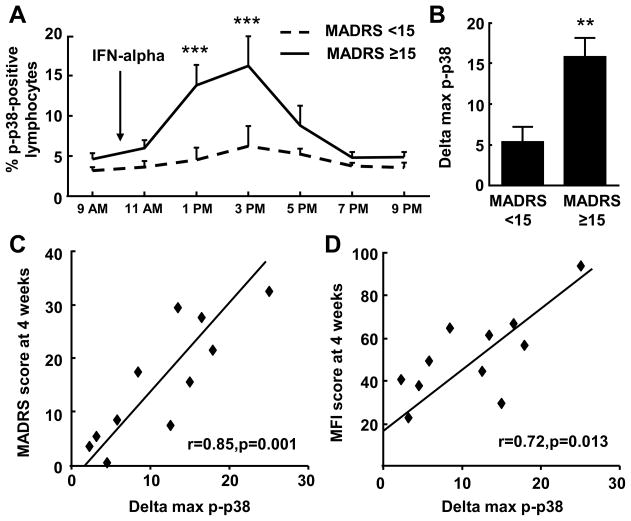
Patients who exhibited a MADRS score ≥15 during the first 12 weeks of IFN-alpha treatment demonstrated a significantly greater percent of cells positive for intracellular p-p38 MAPK after IFN-alpha injection than did patients whose MADRS scores were consistently <15 (time by treatment interaction: F=2.4, df=6,54, p<0.05). Post hoc analyses revealed significant differences between groups at 3 and 5 hours post-IFN-alpha injection (Fig. 1A). The mean maximal change in percent of lymphocytes positive for intracellular p-p38 MAPK (peak response minus 9AM baseline: delta max p-p38) was also significantly higher in patients with MADRS scores ≥15 versus those <15 (t=3.2, df=9, p<0.05)(Fig. 1B). Delta max p-p38 was positively correlated with MADRS scores at week 4 (r=0.85, df=9, p=0.001)(Fig. 1C) and 12 (r=0.70, df=9, p=0.018) of IFN-alpha therapy. Similar correlations were found between delta max p-p38 and MFI scores at week 4 (r=0.72, df=9, p=0.013)(Fig. 1D) and 12 (r=0.74, df=9, p=0.009). Of note, although cut-off scores have not been established for the MFI, individuals with MFI scores above the median (>50) during the first 12 weeks of IFN-alpha treatment also exhibited a significantly higher percentage of lymphocytes positive for p-p38 at 3 hours following IFN-alpha administration (F=3.1, df=6,54, p<0.05). All correlations between delta max p-p38 and MADRS and MFI remained significant after controlling for age, sex, BMI, history of MD and type of INF-alpha.
Fig. 1. Increased p-p38 MAPK in response to the initial IFN-alpha injection is associated with depression and fatigue during the first 12 weeks of IFN-alpha.
The mean increase in percentage of lymphocytes positive for phosphorylated (p)-p38 MAPK at 3 and 5 hours following the initial IFN-alpha injection was significantly higher in patients with MADRS scores ≥15 during the first 12 weeks of IFN-alpha treatment (A). The mean change from the 9AM baseline to the maximal percentage of lymphocytes positive for p38 MAPK (delta max p-p38) was also significantly higher in subjects with MADRS scores ≥15 versus those with scores <15 during IFN-alpha treatment (B). Delta max p-p38 was positively correlated with MADRS scores (C) and MFI scores (D) at week 4 of IFN-alpha treatment. IFN=interferon, MADRS=Montgomery Asberg Depression Rating Scale, max=maximal, MFI=Multidimensional Fatigue Inventory, p-p38=phosphorylated-p38. Data are summarized as mean+/−SE, **-p<0.01, ***-p<0.001
To further understand the relationship between p38 MAPK activation and development of behavioral symptoms during IFN-alpha treatment, correlations between delta max p-p38 and baseline MADRS and HPA axis responses to the first injection of IFN-alpha were explored. Both baseline depression scores and ACTH and cortisol responses to the initial injection of IFN-alpha have been found to predict development of behavioral changes during IFN-alpha treatment (Capuron et al. 2003; Lotrich et al. 2007). Consistent with previous reports, MADRS at baseline significantly correlated with depressive symptoms at week 4 (r=0.88, df=9, p=0.000) and 12 (r=0.85, df=9, p=0.002), and delta max ACTH and delta max cortisol following the initial injection of IFN-alpha were positively correlated with MADRS scores at week 4 (ACTH: r=0.69, df=9, p=0.019, cortisol: r=0.81, df=9, p=0.003) but not at week 12.
Delta max p-p38 MAPK positively correlated with MADRS scores at baseline (r=0.82, df=9, p=0.002) (Fig. 2A) and delta max cortisol responses (r=0.91, df=9, p=0.000) (Fig. 2B). Both correlations remained significant after controlling for age, sex, BMI, history of MD and type of INF-alpha. A similar relationship was observed for ACTH (r=0.75, df=9, p=0.008), however this relationship was not significant after controlling for clinical covariates noted above (r=0.73, df=4, p=0.102). No significant relationship was found between delta max p-p38 and delta max IL-6 (r=0.53, df=9, p=0.096), and as previously reported in patients receiving IFN-alpha for cancer (Capuron et al. 2003), delta max IL-6 did not correlate with MADRS scores at week 4 (r=0.16, df=9, p=0.649) or 12 (r=−0.38, df=9, p=0.247).
Fig. 2. The response of p-p38 MAPK to the initial IFN-alpha injection positively correlated with MADRS scores at baseline and the cortisol response to the first IFN-alpha injection.
Delta max phosphorylated (p)-p38 MAPK was positively correlated with the MADRS scores at baseline (A), and with the delta max cortisol response to the initial injection of IFN-alpha (B). IFN=interferon, MADRS=Montgomery Asberg Depression Rating Scale, max=maximal, p-p38=phosphorylated-p38.
Given the relationship between baseline MADRS scores and MADRS scores at weeks 4 and 12, correlations between delta max p-p38 and development of depressive symptoms during IFN-alpha treatment were repeated using delta MADRS scores at week 4 and 12 (thus controlling for baseline). Delta max p-p38 was positively correlated with delta MADRS at week 4 (r=0.84, df=9, p=0.001) and week 12 (r=0.67, df=9, p=0.025) of IFN-alpha treatment. These relationships remained significant after controlling for clinical covariates noted above.
Discussion
Activation of p38 as manifested by percent of lymphocytes positive for intracellular p-p38 following the initial injection of IFN-alpha was associated with depression and fatigue during IFN-alpha treatment. In addition, activation of p-38 was significantly correlated with previously reported predictors of IFN-alpha-induced depressive symptoms including baseline depression scores and the acute HPA axis response to the first IFN-alpha injection. These data suggest that sensitivity of p38 MAPK signaling pathways to immune stimulation in vivo may reflect an immunologic vulnerability to IFN-alpha-induced depressive symptoms. Moreover, these data are consistent with a potential impact of p38 pathways on behavior through effects on the serotonin transporter (Zhu et al. 2006; Zhu et al. 2010) and the GR (Wang et al. 2004), which may contribute to previously described effects of IFN-alpha on CSF monoamine metabolites and HPA axis function (Raison et al. 2009; Raison et al. 2010a).
Studies have demonstrated that the initial neuroendocrine response to IFN-alpha administration can predict subsequent behavioral changes. For example, HPA axis responses following the first IFN-alpha injection have been shown to predict depressive symptom severity during the first several weeks of IFN-alpha treatment in patients with cancer (Capuron et al. 2003). The present study has replicated these results in a population of subjects with chronic hepatitis C and has extended these findings to include an inflammatory signaling pathway, p38 MAPK, which not only correlated with subsequent behavioral responses but also correlated with baseline neuroendocrine and behavioral parameters known to predict IFN-alpha-induced behavioral change. The addition of p38 MAPK responses to other known predictors of IFN-alpha-induced behavioral alterations suggests that sensitization of immune responses may contribute to a pattern of vulnerability to cytokine effects on behavior. Although exaggerated immune and neuroendocrine responses may represent a genetic predisposition, such sensitized responses may also reflect the ability of environmental factors such as stress to prime inflammatory as well as neuroendocrine responses that are then revealed under conditions of immune challenge. Indeed, studies in laboratory animals have shown that exposure to stress can increase the inflammatory response to a subsequent immune stimulus (Gibb et al. 2008; Johnson et al. 2002). The correlation of baseline depression scores and p-p38 after the first IFN-alpha injection in the current study may reflect such a relationship between exposure to stress (as manifested by subclinical depressive symptoms) and a heightened inflammatory response to immune challenge.
Sensitization of p38 signaling pathways may be manifested by increased baseline biomarkers of inflammation previously found to predict depressive symptoms during IFN-alpha treatment. For example, baseline plasma concentrations of IL-6 and its soluble receptor have been found to be associated with depression during IFN-alpha therapy (Friebe et al. 2007; Prather et al. 2009; Wichers et al. 2006). Nevertheless, the relationship between these baseline immune measures and p-p38 has yet to be established.
Several strengths and weaknesses of the study warrant consideration. Time series data after a standardized immune stimulus allowed for a well-controlled assessment of responsiveness of p38 signaling pathways. Moreover, the longitudinal design provided comprehensive behavioral assessments during the first 3 months of IFN-alpha treatment in the absence of psychotropic medications. Nevertheless, the sample size was small, limiting generalizability of results and the ability to conduct mediational analysis. In addition, no assessments of activated p38 during IFN-alpha therapy or viral response were available. Furthermore, we only examined one inflammatory signaling pathway, and it is well-known that IFN-alpha influences numerous signal transduction pathways that may have influenced behavioral changes. The p38 pathway was chosen based on an established role in neuroendocrine and monoamine regulation (Sanchez et al. 2007; Wang et al. 2004; Zhu et al. 2006; Zhu et al. 2010), thus increasing its potential relevance to IFN-alpha-induced behavioral change. Finally, although p38 activation was measured in the periphery, IFN-alpha (and other cytokines induced by IFN-alpha) can access brain (Raison et al. 2009) and affect inflammatory processes in the CNS. Indeed, both IFN-alpha (as demonstrated herein) and IL-6 (Heinrich et al. 2003) can activate p38 signaling pathways, and both of these cytokines have been shown to be increased in brain during IFN-alpha treatment.
Taken together, increased sensitivity of p38 MAPK signaling pathways may represent a vulnerability to IFN-alpha-induced depression. Thus, p38 pathways may serve as a therapeutic target for reversing neuropsychiatric consequences of chronic exposure to innate immune cytokines.
Supplementary Material
Acknowledgments
This study was supported in part by grants from the National Institutes of Health to CLR (K23 MH064619, R01 MH070553) and AHM (K05 MH069124, R01 HL073921, MHR01MH075102, T32 MH020018) as well as the Emory Center for AIDS Research (P30 AI050409) and the Flow Cytometry Core Facility of the Emory University School of Medicine. In addition, the study was supported by PHS Grant UL1 RR025008 from the Clinical and Translational Science Award program and PHS Grant M01 RR0039 from the General Clinical Research Center program, National Institutes of Health, National Center for Research Resources.
Footnotes
Conflict of Interest Statement
All authors declare that there are no conflicts of interest, and all financial disclosures are listed for each author: Charles L. Raison serves as a consultant for Pamlab LLC and Biolex Therapeutics; Andrew H. Miller has served as a consultant for Abbott Laboratories, AstraZeneca, GlaxoSmithKline, Lundbeck Research USA, F. Hoffmann-La Roche Ltd., Schering-Plough Research Institute and Wyeth/Pfizer Inc. and has received research support from Centocor Inc., GlaxoSmithKline, and Schering-Plough Research Institute; Jennifer C. Felger, Oyetunde Alagbe, Thaddeus W. W. Pace, Bobbi J Woolwine, and Fang Hu have nothing to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bull SJ, Huezo-Diaz P, Binder EB, Cubells JF, Ranjith G, Maddock C, Miyazaki C, Alexander N, Hotopf M, Cleare AJ, et al. Functional polymorphisms in the interleukin-6 and serotonin transporter genes, and depression and fatigue induced by interferon-alpha and ribavirin treatment. Mol Psychiatry. 2009;14(12):1095–1104. doi: 10.1038/mp.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Raison CL, Musselman DL, Lawson DH, Nemeroff CB, Miller AH. Association of exaggerated HPA axis response to the initial injection of interferon-alpha with development of depression during interferon-alpha therapy. Am J Psychiatry. 2003;160(7):1342–1345. doi: 10.1176/appi.ajp.160.7.1342. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV. Washington DC: American Psychiatric Press; 1997. [Google Scholar]
- Friebe A, Schwarz MJ, Schmid-Wendtner M, Volkenandt M, Schmidt F, Horn M, Janssen G, Schaefer M. Pretreatment levels of sTNF-R1 and sIL-6R are associated with a higher vulnerability for IFN-alpha-induced depressive symptoms in patients with malignant melanoma. J Immunother. 2007;30(3):333–337. doi: 10.1097/01.cji.0000211346.19330.c9. [DOI] [PubMed] [Google Scholar]
- Gibb J, Hayley S, Gandhi R, Poulter MO, Anisman H. Synergistic and additive actions of a psychosocial stressor and endotoxin challenge: Circulating and brain cytokines, plasma corticosterone and behavioral changes in mice. Brain Behav Immun. 2008;22(4):573–589. doi: 10.1016/j.bbi.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374(Pt 1):1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F, Pace TW, Miller AH. Interferon-alpha inhibits glucocorticoid receptor-mediated gene transcription via STAT5 activation in mouse HT22 cells. Brain Behav Immun. 2009;23(4):455–463. doi: 10.1016/j.bbi.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, O’Connor KA, Deak T, Stark M, Watkins LR, Maier SF. Prior stressor exposure sensitizes LPS-induced cytokine production. Brain Behav Immun. 2002;16(4):461–476. doi: 10.1006/brbi.2001.0638. [DOI] [PubMed] [Google Scholar]
- Kearns NP, Cruickshank CA, McGuigan KJ, Riley SA, Shaw SP, Snaith RP. A comparison of depression rating scales. Br J Psychiatry. 1982;141:45–49. doi: 10.1192/bjp.141.1.45. [DOI] [PubMed] [Google Scholar]
- Lotrich FE, Ferrell RE, Rabinovitz M, Pollock BG. Risk for depression during interferon-alpha treatment is affected by the serotonin transporter polymorphism. Biol Psychiatry. 2009;65(4):344–348. doi: 10.1016/j.biopsych.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotrich FE, Rabinovitz M, Gironda P, Pollock BG. Depression following pegylated interferon-alpha: characteristics and vulnerability. J Psychosom Res. 2007;63(2):131–135. doi: 10.1016/j.jpsychores.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Potter GG, Kittinger JD, Wagner HR, Steffens DC, Krishnan KR. Prefrontal neuropsychological predictors of treatment remission in late-life depression. Neuropsychopharmacology. 2004;29(12):2266–2271. doi: 10.1038/sj.npp.1300551. [DOI] [PubMed] [Google Scholar]
- Prather AA, Rabinovitz M, Pollock BG, Lotrich FE. Cytokine-induced depression during IFN-alpha treatment: the role of IL-6 and sleep quality. Brain Behav Immun. 2009;23(8):1109–1116. doi: 10.1016/j.bbi.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Borisov AS, Majer M, Drake DF, Pagnoni G, Woolwine BJ, Vogt GJ, Massung B, Miller AH. Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biol Psychiatry. 2009;65(4):296–303. doi: 10.1016/j.biopsych.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Borisov AS, Woolwine BJ, Massung B, Vogt G, Miller AH. Interferon-alpha effects on diurnal hypothalamic-pituitary-adrenal axis activity: relationship with proinflammatory cytokines and behavior. Mol Psychiatry. 2010a;15(5):535–547. doi: 10.1038/mp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Rye DB, Woolwine BJ, Vogt GJ, Bautista BM, Spivey JR, Miller AH. Chronic Interferon-Alpha Administration Disrupts Sleep Continuity and Depth in Patients with Hepatitis C: Association with Fatigue, Motor Slowing, and Increased Evening Cortisol. Biol Psychiatry. 2010b doi: 10.1016/j.biopsych.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rius C, Abu-Taha M, Hermenegildo C, Piqueras L, Cerda-Nicolas JM, Issekutz AC, Estan L, Cortijo J, Morcillo EJ, Orallo F, et al. Trans- but not cis-resveratrol impairs angiotensin-II-mediated vascular inflammation through inhibition of NF-kappaB activation and peroxisome proliferator-activated receptor-gamma upregulation. J Immunol. 2010;185(6):3718–3727. doi: 10.4049/jimmunol.1001043. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Alagbe O, Felger JC, Zhang J, Graff AE, Grand AP, Maestripieri D, Miller AH. Activated p38 MAPK is associated with decreased CSF 5-HIAA and increased maternal rejection during infancy in rhesus monkeys. Mol Psychiatry. 2007;12(10):895–897. doi: 10.1038/sj.mp.4002025. [DOI] [PubMed] [Google Scholar]
- Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39(3):315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- Wang X, Wu H, Miller AH. Interleukin 1alpha (IL-1alpha) induced activation of p38 mitogen-activated protein kinase inhibits glucocorticoid receptor function. Mol Psychiatry. 2004;9(1):65–75. doi: 10.1038/sj.mp.4001339. [DOI] [PubMed] [Google Scholar]
- Wichers MC, Kenis G, Leue C, Koek G, Robaeys G, Maes M. Baseline immune activation as a risk factor for the onset of depression during interferon-alpha treatment. Biol Psychiatry. 2006;60(1):77–79. doi: 10.1016/j.biopsych.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Zampieri CA, Fortin JF, Nolan GP, Nabel GJ. The ERK mitogen-activated protein kinase pathway contributes to Ebola virus glycoprotein-induced cytotoxicity. J Virol. 2007;81(3):1230–1240. doi: 10.1128/JVI.01586-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CB, Blakely RD, Hewlett WA. The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology. 2006;31(10):2121–2131. doi: 10.1038/sj.npp.1301029. [DOI] [PubMed] [Google Scholar]
- Zhu CB, Lindler KM, Owens AW, Daws LC, Blakely RD, Hewlett WA. Interleukin-1 receptor activation by systemic lipopolysaccharide induces behavioral despair linked to MAPK regulation of CNS serotonin transporters. Neuropsychopharmacology. 2010;35(13):2510–2520. doi: 10.1038/npp.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.