Abstract
Background
Proliferating-cell nuclear antigen (PCNA) plays an important role in DNA replication and repair. The expression and potential utility of this marker in prostatic neoplasia is uncertain. With the development of this new caPCNA selective antibody, we explored the potential utility of this marker in prostate cancer.
Methods
Using a traditional primary Fab2′ rabbit anti-caPCNA antibody-HRP conjugated secondary anti-Fab2′ antibody format, the expression of the caPCNA was analyzed in prostate tissue from 89 radical prostatectomy specimens. The caPCNA expression was correlated with clinicopathologic characteristics.
Results
The fraction of cells staining positively with caPCNA antibody in prostatic adenocarcinoma (mean, 23%) was significantly higher than that in benign prostatic epithelium (mean, 2%; p < 0.001) or high-grade prostatic intraepithelial neoplasia (PIN) (mean, 6%; p < 0.05). Moreover, the intensity of caPCNA expression in prostatic adenocarcinoma (mean, 2.9) was significantly higher than that in benign prostatic tissue (mean, 0.7; p < 0.001) or high-grade PIN (mean, 2.0; p < 0.001). Benign prostatic epithelium showed only minimal or negative reactivity. There was significant correlation between the percentage of caPCNA expression and primary Gleason grade (p = 0.01), and with Gleason score (p = 0.02). Adenocarcinomas with positive vascular invasion had a significantly higher percentage of cells staining with caPCNA antibody (p < 0.0001) and a higher intensity of caPCNA expression (p = 0.04).
Conclusions
Our data indicate that increased expression of the cancer-associated isoform of PCNA is common in prostatic adenocarcinoma and its precursor and may be a useful biomarker.
Keywords: Prostate, biomarkers, proliferating-cell nuclear antigen (PCNA), Gleason grading, neoplasia, high-grade prostatic intraepithelial neoplasia (PIN), targeted therapy, progression, carcinogenesis
INTRODUCTION
Prostate cancer is one of the most common cancers in the world, and is the second leading cause of cancer death in American men. In 2009, an estimated 192,000 prostate cancer cases were diagnosed, and 27,000 deaths were expected.(1) The pathogenesis of prostate cancer is not fully understood. Both genetic and environmental factors have been implicated. The carcinogenesis of prostate cancer is thought to be a multistep process, during which accumulation of multiple genetic changes confer a selective growth advantage. It is well recognized that activation of protooncogenes and inactivation of tumor suppressor genes are involved in the development and progression of prostate cancer.
One potential mechanism leading to the accumulation of genetic alterations involves the cellular DNA replication process becoming error-prone. An increase in the error frequency of the cellular DNA synthetic machinery was shown to lead to an accumulation of mutations during the DNA replication process both in vitro2 and in vivo(2, 3) analyses. Our observations suggested to us that altered DNA replication components may promote genetic mutations in the malignant cells through the development of an error-prone DNA replication apparatus.(4)
Proliferating cell nuclear antigen (PCNA) is at the very heart of many essential cellular processes, such as DNA replication, repair of DNA damage, chromatin structure maintenance, chromosome segregation, and cell-cycle progression.(5) PCNA has been identified as a cofactor of DNA polymerase-δ and its expression occurs during the late G1 and S phase of the cell cycle. Overexpression of PCNA has been reported in various types of cancer.(6-9) A recent study by Bechtel et al demonstrated that one PCNA isoform is present in nonmalignant breast cells, whereas two PCNA isoforms are present in breast cancer cells, with one different from the isoform in nonmalignant breast tissue.(10) Variation of PCNA results from posttranslational modification.(5) Currently, commercially available antibodies recognizing PCNA react with both PCNA isoforms and do not differentiate between PCNA isoforms in malignant cells and nonmalignant cells. Our group has successfully developed a unique polyclonal antibody that can detect the cancer-associated isoform (caPCNA).(11)
With the development of this new caPCNA selective antibody, we examined the expression of caPCNA in prostatic adenocarcinoma, high-grade prostatic intraepithelial neoplasia (PIN), and adjacent benign prostatic epithelium using immunohistochemistry, and explored the potential utility of this marker in prostate cancer.
MATERIALS AND METHODS
Patients
Radical prostatectomy specimens (n = 89) containing invasive prostatic adenocarcinoma with adjacent high-grade PIN and normal prostate epithelium were obtained from the surgical pathology files of Indiana University Hospital from 1990 to 1996. These cases were selected to represent the full spectrum of Gleason grade and pathological stages. The patients ranged in age from 41 to 80 years (mean, 63 years). Grading of the primary tumor from radical prostatectomy specimens was performed according to the Gleason system. The Gleason scores ranged from 4 to 10. Pathological staging was performed according to the 1997 American Joint Committee on Cancer Tumor, Lymph Nodes, and Metastasis Staging System. The final pathological stages included T2a (5 patients), T2b (34 patients), T3a (30 patients), and T3b (20 patients). Five patients had lymph node metastases at the time of surgery. This research was approved by the Indiana University Institutional Review Board.
Preparation of caPCNA Antibody
The details of the antibody preparation process were described previously.(11) Rabbit polyclonal antibodies were prepared by a commercial vendor, (Zymed, San Francisco, CA), to a synthesized peptide fragment of PCNA coupled to keyhole limpet hemacyanin (KLH) through four cysteines residues added to the amino-terminal portion of the peptide. One hundred micrograms of the KLH conjugated to a peptide fragment of PCNA contained within amino acids 123–140 of the protein was resuspended in complete Freund adjuvant, and injected s.c. into multiple sites in two female New Zealand white rabbits. The rabbits were rested for one month before boosting the animals with a second 100-μg dose of the KLH-coupled antigen in incomplete adjuvant. The antibody titer to the antigen was determined by ELISA ≈10–14 days after immunization, and, after an additional 14-day rest period, the animals received another boost of KLH-coupled antigen. Twelve days later, 25 ml of antisera was collected from each rabbit and stored at −20°C. The antisera were dialyzed against two changes of 20 mM PBS, pH 7.0, and loaded onto a protein G Sepharose column preequilibrated with the PBS. The binding capacity of the gel was 19 mg of rabbit IgG per ml of packed gel bed. The column was washed with 10 column volumes of PBS and eluted with 10 volumes of 0.1 M glycine buffer, pH 3.0. One-milliliter fractions eluting from the column were collected at a flow rate of 1–2 ml/per min into 0.25 ml of 0.25M Tris·HCl, pH 8.0. The concentration of protein in fractions containing the protein peak eluting from the column was determined by Bradford assay, and these fractions were combined and dialyzed against PBS containing 10 mM NaN3 before being stored at 4°C until used in various analyses.
Immunohistochemistry Study
The details of the preparation process were described previously.(11) Serial 5 μm-thick sections prepared from formalin-fixed, paraffin-embedded prostate tissue were used for the study. Tissue blocks containing the maximum amount of tumor and highest Gleason score were selected for each case. One representative slide from each case was analyzed. Slides were deparaffinized in xylene (three changes) and hydrated with graded alcohols and distilled water. Antigen retrieval was performed in citrate buffer (pH 6.0) by using a microwave oven for 10 min and subsequent cooling for 20 min, followed by blocking of endogenous peroxidase activity with Peroxo-block (Zymed), and, after rinsing the slides in PBS, the slides were incubated with the Fab2′ fragment of caPCNAab (dilution: 1:400) for 1 hour. The antigen–antibody reaction was visualized by the avidin-biotin-peroxidase (Zymed Picture Plus kit: HRP/Fab polymer conjugate) with diaminobenzidine (DABplus; DAKO, Carpenteria, CA) as the chromogen. These slides were counterstained with hematoxylin (Vector Laboratories) and then cleared in alcohol and xylene. The slides were mounted with Histomount (Zymed) and visualized. Substitution of primary antibody by PBS or isotype control antibody served as the negative controls.
PCNA staining was evaluated in benign epithelium, high-grade PIN, and adenocarcinoma as previously described(12-14). Microscopic fields with the highest degree of immunoreactivity were chosen for analysis. At least 1000 cells were analyzed for each component. The number of positively staining cells was estimated, and the intensity of staining was scored on a scale of 0 to 3+ (0, no staining; 1, weak staining; 2, moderate staining; and 3, strong staining).
Statistical Analysis
The mean percentages of immunoreactive cells in benign epithelium, high-grade PIN, and adenocarcinoma were compared using Kruskal Wallis and Wilcoxon paired signed rank tests. The intensities of staining for PCNA in benign epithelium, high-grade PIN, and adenocarcinoma were compared using Cochran-Mantel-Haenszel tests for correlated ordered categorical data. Correlation between the percentage of cells staining or the intensity of staining of PCNA and pertinent clinicopathologic characteristics was tested using Kruskal Wallis and Wilcoxon paired signed rank tests, including age, Gleason grade, pathologic T stage, presence or absence of lymph node metastasis, extraprostatic extension, status of surgical margins, vascular invasion, perineural invasion, and high-grade PIN. A p < 0.05 was considered significant, and all p’s were two-sided.
RESULTS
Eighty-nine patients were included in the study. Their characteristics are illustrated in Table 1. The patients’ ages ranged from 41 to 80 years (mean, 63 years). Pathologic stages in these cases were as follows: T2 (39 patients), and T3 (50 patients).
TABLE 1.
Patient characteristics and caPCNA expression in prostate cancer
| Patient Characteristic | No. of patients (n = 89) |
Mean percentage of cells staining with caPCNA antibody (±SD) |
Mean caPCNA antibody staining intensity (±SD) |
|---|---|---|---|
| Primary Gleason Grade | |||
| 2 | 1 | 10 | 3.0 |
| 3 | 44 | 18 ± 19 | 2.9 ± 0.5 |
| 4 | 26 | 25 ± 21 | 2.9 ± 0.6 |
| 5 | 18 | 33 ± 25 | 3.1 ± 0.5 |
|
| |||
| Secondary Gleason Grade | |||
| 2 | 3 | 18 ± 13 | 2.7 ± 0.6 |
| 3 | 34 | 19 ± 21 | 2.9 ± 0.5 |
| 4 | 38 | 25 ± 22 | 3.0 ± 0.6 |
| 5 | 14 | 28 ± 22 | 3.0 ± 0.0 |
|
| |||
| Gleason Sum | |||
| < 7 | 19 | 9 ± 7 | 2.9 ± 0.3 |
| 7 | 37 | 24 ± 22 | 2.8 ± 0.7 |
| > 7 | 33 | 30 ± 24 | 3.1 ± 0.3 |
|
| |||
| T Classification | |||
| T2a | 5 | 21 ± 17 | 2.8 ± 0.4 |
| T2b | 34 | 19 ± 23 | 2.9 ± 0.5 |
| T3a | 30 | 23 ± 21 | 3.0 ± 0.0 |
| T3b | 20 | 31 ± 20 | 3.0 ± 0.8 |
|
| |||
| Lymph Node Metastasis | |||
| Negative | 84 | 23 ± 22 | 2.9 ± 0.5 |
| Positive | 5 | 26 ± 16 | 3.0 ± 0.0 |
|
| |||
| Extraprostatic Extension | |||
| Negative | 42 | 19 ± 22 | 2.8 ± 0.7 |
| Positive | 47 | 27 ± 20 | 3.0 ± 0.3 |
|
| |||
| Surgical Margin | |||
| Negative | 38 | 23 ± 22 | 2.9 ± 0.6 |
| Positive | 51 | 23 ± 21 | 2.9 ± 0.4 |
|
| |||
| Vascular Invasion | |||
| Negative | 52 | 16 ± 20 | 2.8 ± 0.6 |
| Positive | 37 | 32 ± 20 | 3.1 ± 0.3 |
|
| |||
| Perineural Invasion | |||
| Negative | 8 | 8 ± 5 | 3.0 ± 0.0 |
| Positive | 81 | 25 ± 22 | 2.9 ± 0.5 |
|
| |||
| High-grade PIN | |||
| Negative | 6 | 14 ± 13 | 3.0 ± 0.0 |
| Positive | 83 | 24 ± 22 | 2.9 ± 0.5 |
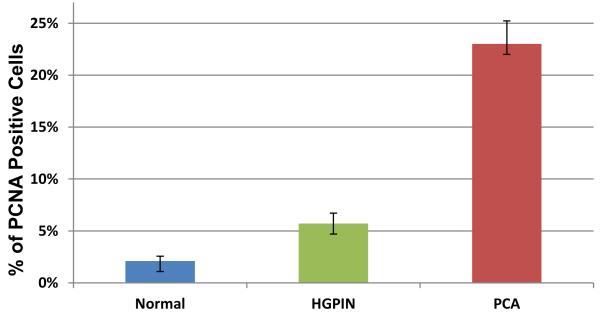
Immunohistochemically (Table 2 and Table 3), the fraction of cells staining positively with caPCNA in prostatic adenocarcinoma (mean ± SD, 23% ± 21%) was significantly higher than that in benign prostatic epithelium (mean ± SD, 2% ± 5%; p < 0.0001) or high-grade PIN (mean ± SD, 6% ± 10%; p < 0.0001) (Figures 1 and 2). Moreover, the intensity of the reaction in prostatic adenocarcinoma (mean ± SD, 2.9 ± 0.5) was significantly higher than that in benign prostatic tissue (mean± SD, 0.8 ± 1.2; p < 0.0001) or high-grade PIN (mean± SD, 2.0 ± 1.2; p < 0.0001). Benign prostatic epithelium showed only minimal or negative reactivity with the caPCNA antibody.
TABLE 2.
The percentage of cells with positive staining for caPCNA in prostate tissues in radical prostatectomy specimens.
| Tissue | Mean percentage of cells staining (±SD) |
Range (%) |
|---|---|---|
| Normal | 2.1 (±4.6) | 0-20% |
| High-grade PIN | 5.7 (±10.0) | 0-80% |
| Invasive cancer | 23.0 (±21.4) | 0-80% |
TABLE 3.
The intensity of caPCNA expression in prostate tissues in radical prostatectomy specimens.
| Tissue | Intensity of caPCNA expression | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Normal | 55 (62%) | 12 (14%) | 6 (7%) | 15 (17%) |
| High-grade PIN | 16 (20%) | 8 (10%) | 14 (18%) | 41 (52%) |
| Invasive cancer | 2 (2%) | 0 (0%) | 2(2%) | 85 (96%) |
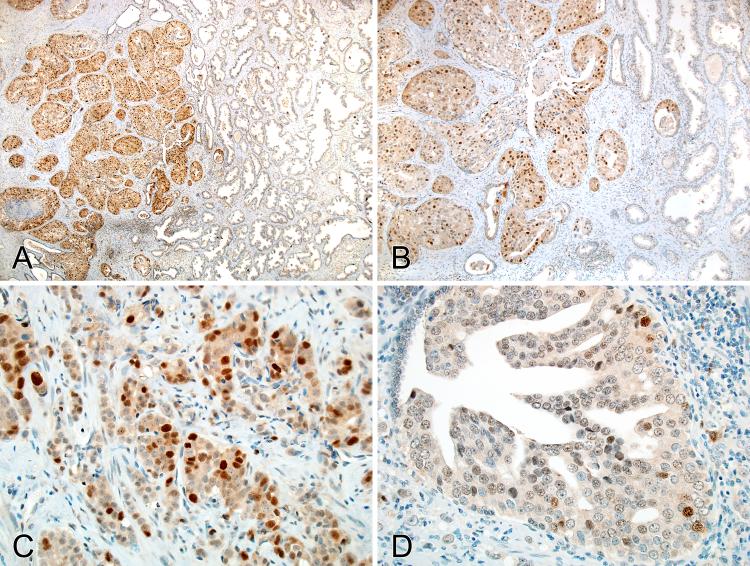
Figure 1.
caPCNA expression in the prostate. A-B, strong and intense nuclear staining in prostatic adenocarcinoma; no or minimal immunoreactivity in the adjacent normal prostatic glandular epithelium. C, strong and intense nuclear staining in prostatic adenocarcinoma. D, focal staining in high-grade prostatic intraepithelial neoplasia (PIN).
Figure 2.
Comparison of caPCNA expression (%) in the benign prostate, high-grade prostatic intraepithelial neoplasia (PIN) and prostatic adenocarcinoma.
We also assessed whether clinicopathologic parameters were related to caPCNA immunoreactivity (Table 1). There was significant correlation between the percentage of caPCNA expression and primary Gleason grade (p = 0.01), and Gleason score sum(p = 0.02). Adenocarcinomas with high Gleason scores had a significantly higher percentage of cells staining with antibody (p = 0.005). Adenocarcinomas with positive vascular invasion had a significantly higher percentage of cells staining with antibody (p < 0.0001) and a higher intensity of caPCNA expression (p = 0.04). No significant correlation was demonstrated between the percentage or intensity of caPCNA expression and other pertinent clinicopathologic features, including: patient age (p = 0.26 and 0.42), pathologic T stage (p = 0.24 and 0.42), lymph node metastasis (p = 0.53 and 0.74), extraprostatic extension (p = 0.08 and 0.06), surgical margin status (p = 0.96 and 0.81), or the presence of high-grade PIN (p = 0.34 and 0.71). In addition, there is no significant correlation between the intensity of PCNA expression and perineural invasion (p = 0.67). However, adenocarcinomas with positive perineural invasion had a significantly higher percentage of cells staining with caPCNA antibody (p = 0.04).
DISCUSSION
This is the first report that expression of caPCNA is increased during the transformation of normal prostatic epithelium to high-grade PIN to adenocarcinoma. Considering the multiple functions PCNA plays in the DNA replication and repair processes, and in maintaining chromosomal DNA integrity, we postulate that increased caPCNA expression likely contributes to the development of prostate cancer.
PCNA is one of the components of the DNA synthesome, ( a highly organized complex of proteins). It functions as a cofactor for DNA polymerase-δ, and is associated with DNA damage-repair mechanisms.(10) The PCNA gene has been located at chromosome 20p12 by in situ hybridization and has 6 exons spanning 4961 bp. PCNA is a 36 kDa protein with a ring-shaped homotrimeric structure that acts as a DNA sliding clamp.(15, 16) PCNA has no intrinsic enzyme activity. Its role in a cell depends upon its ability to mediate interaction between proteins and DNA. PCNA interacts with multiple protein partners; therefore, contributes to diverse cellular functions including DNA replication, DNA repair, cell cycle control, chromatin remodeling/epigenetic inheritance, chromatid cohesion, transcription, and other critically important functions.(17)
In some studies, PCNA has been shown to be a prognostic marker for some malignancies including prostate cancer(7, 8). Most studies examined PCNA expression using commercially available anti-PCNA antibody such as PC10, which will detect both the non-malignant cell PCNA and the caPCNA isoforms. Miyamato et al studied 60 cases of prostate cancers and observed that the PCNA labeling index was significantly higher in patients with prostate-specific antigen (PSA) over 100 ng/ml, advanced clinical stage, higher Gleason grade, or with a higher Gleason score than in those with other clinicopathologic features.(6) Taftachi et al demonstrated by immunohistochemistry that PCNA and Ki-67expression correlate significantly with progression of diseases in 114 radical prostatectomy specimens.(18) The study by Guzinska-Ustymowicz et al showed that expression of the proliferative protein may indicate the development of lymph node metastasis in colorectal cancer.(9)
Currently, data concerning caPCNA expression in the cancer is very limited. Bechtel et al(10) demonstrated that a unique form of PCNA with an acidic pI is present in malignant breast cells, both in cell cultures and tissue; and that the isoform is a result of altered posttranslational modification. Using high resolution two dimensional PAGE analyses, Naryzhny et al also showed three different PCNA isoforms that reflected the apparent acetylation status of the protein. These authors suggested that the highly acetylated acidic form and moderately acetylated main forms participated in DNA replication; whereas the more basic of these acidic isoforms was purported to be associated with the termination of DNA replication.(19) Venturi et al using two dimensional gel electrophoresis followed by Western blotting with a general PCNA antibody investigated the PCNA expression pattern of human hepatocellular carcinoma (HCC) and demonstrated that the carcinoma expresses both acidic and basic isoforms of PCNA. As with some of the breast cancer cell lines and tissue specimens examined by Bechtel et al(10), a basic PCNA isoform was also seen with some of the hepatocellular carcinoma cell lines and tissue specimens. In contrast however, Venturi et al., also reported that 10 of their 12 cases of cirrhotic liver specimens with HCC exhibited only the acidic isoforms of monomeric PCNA, while their control cirrhotic liver cells and their normal liver specimens exhibited only the acidic isoform.(20) This somewhat conflicting set of results may arise at least in part from loss of a heat -sensitive labile methylesterification21. Preliminary work from our lab suggests that methylesterification of the acidic amino acid residues on PCNA exhibit a T1/2 ~20 minutes at pH 8.5 (unpublished). Thus simple laboratory or clinical manipulations of the tissue or tissue extracts, permitting them to reside above 4 degrees for any length of time exceeding the half-life, can be sufficient to reduce the abundance of the basic isoform seen with the cell lines and anticipated to be expressed by the non-cancer tissue specimens. What is clear is that multiple isoforms of PCNA reside within cancer cells and tissues, suggesting that further investigation is needed to clarify the nature and role of PCNA isoforms in both normal, diseased, and cancer tissues. Based on the finding of Bechtel et al(10), Malkas et al9 successfully developed an antibody against the caPCNA isoform, and used the antibody to further investigate caPCNA expression in 45 cases of normal breast tissue (from reduction mammaplasty patients or disease-free patients after treatment for invasive breast cancer) and 85 cases of invasive or in situ breast cancer, and found significant expression of caPCNA, which averaged 30%.(11) In an immunohistochemistry study of 18 cases of esophageal adenocarcinoma and 30 cases of Barrett’s esophagus (14 without dysplasia, 8 with low grade dysplasia, and 8 with high-grade dysplasia), Hammoud et al(21) observed significant caPCNA staining in all of the cancer cases using the antibody developed by Malkas et al9 against caPCNA .
No prior study has investigated caPCNA isoform expression in prostate cancer. Using the caPCNA antibody developed by our institute, we analyzed caPCNA expression in 89 prostatectomy specimens. We demonstrated, immunohistochemically, that the intensity and percentage of cell staining of caPCNA were significantly higher for prostatic adenocarcinoma than for high-grade PIN or benign prostatic epithelium. Benign prostatic epithelium showed only minimal or negative staining. There was a significant correlation between the percentage of caPCNA expression and the Gleason score. These results indicate that increased caPCNA expression is common in prostatic adenocarcinoma, and may be useful in helping to distinguish prostatic adenocarcinoma from other lesions of prostatic epithelium. We also postulate that overexpression of the caPCNA isoform contributes to the carcinogenesis of the prostate. Larger studies are needed to define more clearly the significance of caPCNA expression in prostate cancer as well as other types of malignancy.
Acknowledgements
The authors wish to thank Ms. Patricia Gulley and Karla John for their expert technical assistance in performing the immunohistochemical staining of the tissues. This work was supported in part by a Synergy Grant award from the CDMRP Breast Cancer Research Program (W81XWH-07-1-0707) to RJH, and from the National Cancer Institute to LHM (RO1-CA121289).
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Hoelz DJ, Arnold RJ, Dobrolecki LE, Abdel-Aziz W, Loehrer AP, Novotny MV, Schnaper L, Hickey RJ, Malkas LH. The discovery of labile methyl esters on proliferating cell nuclear antigen by MS/MS. Proteomics. 2006;6(17):4808–16. doi: 10.1002/pmic.200600142. [DOI] [PubMed] [Google Scholar]
- 3.Yang J, Chen Z, Liu Y, Hickey RJ, Malkas LH. Altered DNA polymerase iota expression in breast cancer cells leads to a reduction in DNA replication fidelity and a higher rate of mutagenesis. Cancer Res. 2004;64(16):5597–607. doi: 10.1158/0008-5472.CAN-04-0603. [DOI] [PubMed] [Google Scholar]
- 4.Sekowski JW, Malkas LH, Schnaper L, Bechtel PE, Long BJ, Hickey RJ. Human breast cancer cells contain an error-prone DNA replication apparatus. Cancer Res. 1998;58(15):3259–63. [PubMed] [Google Scholar]
- 5.Stoimenov I, Helleday T. PCNA on the crossroad of cancer. Biochem Soc Trans. 2009;37(Pt 3):605–13. doi: 10.1042/BST0370605. [DOI] [PubMed] [Google Scholar]
- 6.Miyamoto S, Ito K, Kurokawa K, Suzuki K, Yamanaka H. Clinical validity of proliferating cell nuclear antigen as an objective marker for evaluating biologic features in patients with untreated prostate cancer. Int J Urol. 2006;13(6):767–72. doi: 10.1111/j.1442-2042.2006.01400.x. [DOI] [PubMed] [Google Scholar]
- 7.Mayer A, Takimoto M, Fritz E, Schellander G, Kofler K, Ludwig H. The prognostic significance of proliferating cell nuclear antigen, epidermal growth factor receptor, and mdr gene expression in colorectal cancer. Cancer. 1993;71(8):2454–60. doi: 10.1002/1097-0142(19930415)71:8<2454::aid-cncr2820710805>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 8.Morell-Quadreny L, Clar-Blanch F, Fenollosa-Enterna B, Perez-Bacete M, Martinez-Lorente A, Llombart-Bosch A. Proliferating cell nuclear antigen (PCNA) as a prognostic factor in renal cell carcinoma. Anticancer Res. 1998;18(1B):677–82. [PubMed] [Google Scholar]
- 9.Guzinska-Ustymowicz K, Pryczynicz A, Kemona A, Czyzewska J. Correlation between proliferation markers: PCNA, Ki-67, MCM-2 and antiapoptotic protein Bcl-2 in colorectal cancer. Anticancer Res. 2009;29(8):3049–52. [PubMed] [Google Scholar]
- 10.Bechtel PE, Hickey RJ, Schnaper L, Sekowski JW, Long BJ, Freund R, Liu N, Rodriguez-Valenzuela C, Malkas LH. A unique form of proliferating cell nuclear antigen is present in malignant breast cells. Cancer Res. 1998;58(15):3264–9. [PubMed] [Google Scholar]
- 11.Malkas LH, Herbert BS, Abdel-Aziz W, Dobrolecki LE, Liu Y, Agarwal B, Hoelz D, Badve S, Schnaper L, Arnold RJ, Mechref Y, Novotny MV, Loehrer P, Goulet RJ, Hickey RJ. A cancer-associated PCNA expressed in breast cancer has implications as a potential biomarker. Proc Natl Acad Sci U S A. 2006;103(51):19472–7. doi: 10.1073/pnas.0604614103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng L, Pan C, Zhang JT, Zhang S, Kinch MS, Li L, Baldridge LA, Wade C, Hu Z, Koch MO, Ulbright TM, Eble JN. Loss of 14-3-3sigma in prostate cancer and its precursors. Clin Cancer Res. 2004;10:3064–8. doi: 10.1158/1078-0432.ccr-03-0652. [DOI] [PubMed] [Google Scholar]
- 13.Jiang J, Neubauer BL, Graff JR, Chedid M, Thomas JE, Roehm NW, Zhang S, Eckert GJ, Koch MO, Eble JN, Cheng L. Expression of group IIA secretory phospholipase A2 is elevated in prostatic intraepithelial neoplasia and adenocarcinoma. Am J Pathol. 2002;160:667–71. doi: 10.1016/S0002-9440(10)64886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan CX, Kinch MS, Kiener PA, Langermann S, Serrero G, Sun L, Corvera J, Sweeney CJ, Li L, Zhang S, Baldridge LA, Jones TD, Koch MO, Ulbright TM, Eble JN, Cheng L. PC cell-derived growth factor expression in prostatic intraepithelial neoplasia and prostatic adenocarcinoma. Clin Cancer Res. 2004;10:1333–7. doi: 10.1158/1078-0432.ccr-1123-03. [DOI] [PubMed] [Google Scholar]
- 15.Rao VV, Schnittger S, Hansmann I. Chromosomal localization of the human proliferating cell nuclear antigen (PCNA) gene to or close to 20p12 by in situ hybridization. Cytogenet Cell Genet. 1991;56(3-4):169–70. doi: 10.1159/000133079. [DOI] [PubMed] [Google Scholar]
- 16.Travali S, Ku DH, Rizzo MG, Ottavio L, Baserga R, Calabretta B. Structure of the human gene for the proliferating cell nuclear antigen. J Biol Chem. 1989;264(13):7466–72. [PubMed] [Google Scholar]
- 17.Naryzhny SN. Proliferating cell nuclear antigen: a proteomics view. Cell Mol Life Sci. 2008;65(23):3789–808. doi: 10.1007/s00018-008-8305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taftachi R, Ayhan A, Ekici S, Ergen A, Ozen H. Proliferating-cell nuclear antigen (PCNA) as an independent prognostic marker in patients after prostatectomy: a comparison of PCNA and Ki-67. BJU Int. 2005;95(4):650–4. doi: 10.1111/j.1464-410X.2005.05356.x. [DOI] [PubMed] [Google Scholar]
- 19.Naryzhny SN, Lee H. The post-translational modifications of proliferating cell nuclear antigen: acetylation, not phosphorylation, plays an important role in the regulation of its function. J Biol Chem. 2004;279(19):20194–9. doi: 10.1074/jbc.M312850200. [DOI] [PubMed] [Google Scholar]
- 20.Venturi A, Piaz FD, Giovannini C, Gramantieri L, Chieco P, Bolondi L. Human hepatocellular carcinoma expresses specific PCNA isoforms: an in vivo and in vitro evaluation. Lab Invest. 2008;88(9):995–1007. doi: 10.1038/labinvest.2008.50. [DOI] [PubMed] [Google Scholar]
- 21.Hammoud ZT, Badve S, Saxena R, Kesler KA, Rieger K, Malkas LH, Hickey RJ. A novel biomarker for the detection of esophageal adenocarcinoma. J Thorac Cardiovasc Surg. 2007;133(1):82–7. doi: 10.1016/j.jtcvs.2006.09.011. [DOI] [PubMed] [Google Scholar]