Abstract
Objective
To determine if A2ALL (Adult-to-Adult Living Donor Liver Transplantation Cohort Study) findings are reflected in the national experience, and further define risk factors for patient mortality and graft loss in living donor liver transplantation (LDLT).
Background
A2ALL previously identified risk factors for mortality after LDLT, including early center experience, older recipient age and duration of cold ischemia.
Methods
LDLTs at the 9 A2ALL centers (n=702) and 67 non-A2ALL centers (n=1664) from 1/1/98 to 12/31/07 in the Scientific Registry of Transplant Recipients database were analyzed. Potential predictors of time to death or graft failure were tested using multivariable Cox regression, starting at time of transplant.
Results
There was no significant difference in overall mortality between A2ALL and non-A2ALL centers. Higher mortality hazard ratios (HR) were associated with donor age (HR=1.13/10 years, P<0.001), recipient age (HR=1.20/10 years, P<0.001), serum creatinine (HR=1.52 (log scale), P<0.001), diagnosis of HCC (HR=2.12, P<0.001) or HCV (HR=1.18, P=0.03), ICU (HR=2.52, P<0.001) or hospitalized (HR=1.62, P<0.001) vs home, and earlier center experience (LDLT case number ≤15, HR=1.61, P<0.001; HR=2.24, P<0.001 among A2ALL centers, HR=1.45, P=0.005 among non-A2ALL centers). Cold ischemia time (CIT) >4.5 hours was also associated with higher mortality (HR=1.79, P<0.001). Other than for center experience, comparisons of risk factor effects between A2ALL and non-A2ALL centers were not significant. Increased risk of graft failure in early experience was comparable in both groups.
Conclusions
Mortality risk factors were similar at A2ALL and non-A2ALL centers. Variables associated with graft loss were identified and showed similar trends, with some minor differences in degree of significance. These analyses demonstrate that the findings from the A2ALL consortium are relevant to other centers in the U.S. performing LDLT, and conclusions and recommendations from A2ALL may help guide clinical decision making.
Keywords: Liver transplantation, Living donor, Post-operative outcomes, Risk factors
Introduction
In response to the organ donor shortage, living donor liver transplantation (LDLT) was expanded to adult recipients, with rapid growth across the U.S. after the first reported case in 1998 (1). Limited numbers of cases were performed at most centers and approaches to the recipient and donor were so diverse across centers that it was difficult to provide reliable information on outcomes that could be generalized and used for patient education. Therefore, in 2002, the National Institutes of Health, with supplemental funding from the American Society of Transplant Surgeons and the Health Resources and Services Administration, U.S. Department of Health and Human Services, organized a consortium of nine liver transplantation centers and a data coordinating center to accrue and follow sufficient numbers of patients being considered for and undergoing right lobe LDLT to provide results from adequately powered studies. The Adult-to-Adult Living Donor Liver Transplantation Cohort Study (A2ALL) was developed with the aim of providing accurate information on the risks and benefits of adult-to-adult LDLT for both donors and recipients. Retrospective and prospective studies were initiated with a primary goal of providing information on donor and recipient outcomes over a decade from 1998 to 2008. Follow-up data collection was completed in 2009, and a subsequent renewal of A2ALL with additional centers has now been refunded by the NIH.
The first report from the nine clinical centers in the A2ALL consortium focused on the predictors of graft loss after LDLT. A learning curve was identified, with graft failure risk decreasing significantly after the first 20 adult-to-adult LDLTs in each center (2). In the initial report, additional risk factors for graft failure after LDLT were also identified, including older recipient age and duration of cold ischemia. Other variables found not to be significant included Model for End-stage Liver Disease (MELD) score, hepatitis C virus (HCV) status, and donor age.
Since graft failure is only one contributor to post-transplant mortality, it is also important to determine if the significant risk factors associated with graft failure identified by A2ALL also apply to recipient mortality at A2ALL as well as non-A2ALL centers. As A2ALL moves ahead to additional studies in its second phase, it is important to analyze the first phase of A2ALL and compare to other US centers. If the findings correlate with outcomes at other centers, then A2ALL reports can be viewed as representative of national results. Therefore, the aims of this study were to determine if the previous reported findings of the importance of center experience and other predictors of outcome in the A2ALL study are reflected in the national LDLT experience.
Experimental Procedures
Data for this study were obtained from the Scientific Registry of Transplant Recipients (SRTR) under a data use agreement. SRTR data are sourced from national transplant data voluntarily submitted by all transplant centers in the U.S. to the Organ Procurement and Transplantation Network (OPTN), and are supplemented with data from the Social Security Death Master File (SSDMF) to identify deaths not reported by transplant centers. The multiple-source follow-up or censoring date was calculated as the transplant anniversary (six-month, one-year, two-year, etc.) immediately preceding the current SRTR database snapshot date (August 1, 2008), allowing an extra three month lag to ensure completion of forms. Graft failure was defined as the date of the earlier of retransplant or death.
Comparison of A2ALL centers to non-A2ALL centers was done by analyzing LDLT recipients at nine A2ALL (n=702) and 67 non-A2ALL centers (n=1664) from 1/1/1998 to 12/31/2007. A case number was assigned for each LDLT transplant, based on the number of LDLT previously performed at that center up to that date. Cold ischemia time (CIT) was defined as time from donor cross clamp to graft removal from ice. CIT data from the A2ALL retrospective cohort study were used to augment the data available in SRTR due to substantial missing data in the latter.
Statistical analysis
Descriptive statistics included means, ranges, standard deviations (s.d.) and proportions. Potential predictors of time from LDLT to death or graft failure were tested by fitting multivariable Cox regression models, starting at time of transplant, with adjustment for center clustering using robust variances based on the sandwich estimator. Covariate effects are reported as hazard ratios (HR) with 95% confidence intervals (CI). Covariates evaluated include center type (A2ALL vs. non-A2ALL), center-specific LDLT case number, transplant year (before vs. after 12/31/2000), recipient age, recipient weight, donor age, donor weight, hepatocellular carcinoma (HCC) and HCV diagnoses, presence of ascites, pretransplant status (ICU, hospital (non-ICU), home), CIT, serum creatinine, and biological relationship (biologically related vs. non-related). A2ALL data from SRTR were augmented with CIT data from A2ALL when not available in SRTR, which was not possible for the non-A2ALL data. Because of the level of missing CIT data in SRTR, separate CIT models were fit using the subset of the cohort with complete CIT data. Statistical interactions between center type and other predictors were tested. Assessment of the association of patient and graft survival with case number was performed by estimating the HR by 5-case intervals (e.g., case 1–5, 6–10, etc.), with case number >30 as the reference category. When individual center effects were added to the models using indicator variables, variability in mortality among centers was statistically significant (P<0.001), even when limited to centers with more than 10 LDLT cases (P<0.001), thus motivating the analysis adjusted for center clustering. P-values ≤0.05 were considered to be statistically significant. All analyses were carried out using SAS 9.2 statistical software (SAS Institute Inc., Cary, NC).
Human Subjects Protection
The study was approved by the Institutional Review Boards and Privacy Boards of the University of Michigan Data Coordinating Center and each of the nine participating transplant centers.
Results
Patient characteristics
Characteristics of patients from A2ALL and non-A2ALL centers are shown in Table 1. Mean donor and recipient age were similar between A2ALL and non-A2ALL centers. A2All centers had higher mean MELD scores and a higher percentage of recipients with HCV. A2ALL centers also had a higher percentage of left lobe recipients, but this was very small in both (5% vs 4%). Overall mean creatinine was not different, but a significantly higher percentage of non-A2ALL patients had serum creatinine < 0.7 mg/dl. The non-A2ALL group also had a higher percentage of HCC and a higher percentage of ICU and hospitalized recipients. A2ALL LDLT grafts had a significantly shorter mean cold ischemia time. Because most non-A2ALL centers never exceeded 15 LDLT cases in total, the proportion of recipient case numbers ≤15 was significantly higher in this group (33% vs. 19%; P<0.001).
Table 1.
Characteristics of Patients in A2ALL and Non-A2ALL Centers
| Characteristic | A2ALL Centers (N=9) |
Non-A2ALL Centers (N=67) |
P-value* | ||||
|---|---|---|---|---|---|---|---|
| N | Range | Mean (SD) or % | N | Range | Mean (SD) or % | ||
| Recipient age | 702 | 18 – 73 | 50.1 (10.9) | 1664 | 18 – 78 | 50.6 (11.6) | 0.38 |
| Donor age | 702 | 18 – 65 | 37.1 (10) | 1663 | 16 – 73 | 37.2 (10.8) | 0.89 |
| Recipient weight† | 609 | 51 – 107 | 76.7 (17.3) | 1582 | 51 – 107 | 77.6 (17.4) | 0.26 |
| Recipient MELD score | 471 | 6–40 | 14.9 (5.7) | 1190 | 6 – 40 | 14.2 (5.3) | 0.02 |
| Serum creatinine (mg/dl) | 681 | 0.1 – 6.6 | 1.0 (0.5) | 1639 | 0.1 – 11.2 | 1.0 (0.7) | 0.26 |
| 0 to ≤0.7 | 181 | 26% | 532 | 32% | 0.004 | ||
| >0.7 to ≤0.9 | 193 | 27% | 486 | 29% | |||
| >0.9 to ≤1.1 | 140 | 20% | 257 | 15% | |||
| >1.1 | 167 | 24% | 364 | 22% | |||
| Diagnosis of HCV | 296 | 42% | 579 | 35% | 0.0007 | ||
| Diagnosis of HCC | 38 | 5% | 133 | 8% | 0.03 | ||
| Left lobe recipient (2004–2007) | 12/249 | 5% | 28/709 | 4% | 0.005 | ||
| Recipient medical condition: | <.0001 | ||||||
| Home | 624 | 89% | 1363 | 82% | |||
| Hospitalized (no ICU) | 55 | 8% | 238 | 14% | |||
| ICU | 23 | 3% | 63 | 4% | |||
| Cold ischemia time (hour)‡ | 517 | 0–12 | 1.6 (1.4) | 990 | 0 – 12.8 | 1.9 (1.7) | <0.001 |
| >4.5 hours | 19 | 4% | 59 | 6% | 0.06 | ||
| Recipient case ≤15¤ | 132 | 19% | 543 | 33% | <0.001 | ||
MELD = Model for end-stage liver disease; HCV = hepatitis C virus; HCC = hepatocellular carcinoma; ICU = intensive care unit
T-test p-values for continuous variables; chi-square test p-values for categorical variables.
The values of the 5th – 95th percentiles were reported due to outliers.
CIT values supplemented by A2ALL data, excluding ≥ 13 hours.
Case ≤15 means among the first 15 LDLT cases performed at a center, i.e., during the period of less center experience. All A2ALL centers performed at least 15 LDLTs. Most (42/67) non-A2ALL centers performed 15 or fewer LDLTs.
Transplant centers and volume
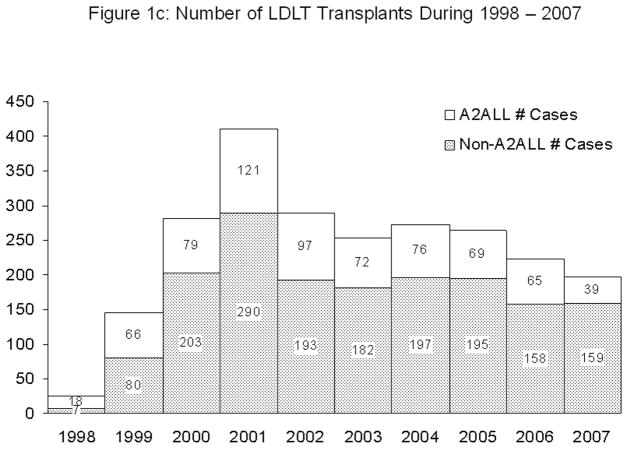
A total of 2366 LDLTs were performed during the study period, including 702 (30%) at the nine A2ALL centers and 1664 (70%) at 67 non-A2ALL centers. Two-thirds (42/67) of the non-A2ALL centers performed 15 or fewer LDLTs over the 10-year study period. Five non-A2ALL centers and three A2ALL centers each performed more than 100 LDLTs. The number of years of activity (≥1 LDLT per year) between 1998 and 2007 varied widely among non-A2ALL centers (median=4 years, range 1–10), with only 1/67 (1.5%) programs active in all 10 years. In contrast, 3/9 (30%) A2ALL centers were active during each year (median=9 years, range 7–10).
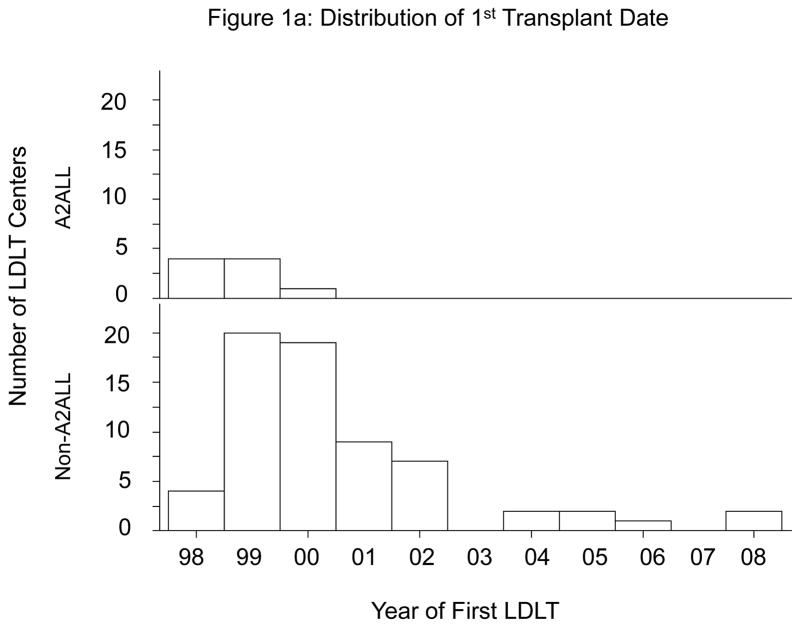
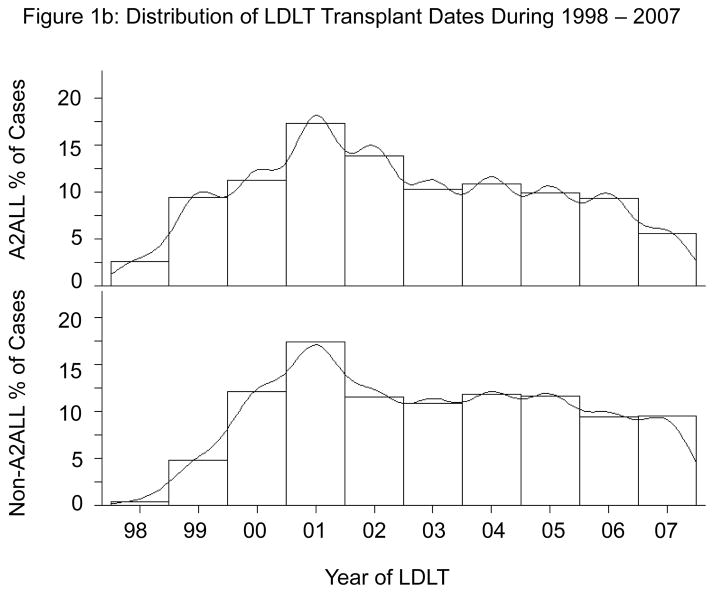
A2ALL centers were among the first to implement LDLT programs. All nine A2ALL centers performed their first LDLT by the end of 2000, whereas approximately one-third of the 67 non-A2ALL centers performed their first LDLT in 2001 or later (Figure 1a). The distribution of LDLTs over time was relatively similar between the two groups (Figure 1b). However, it is important to note that the non-A2ALL cases reflect many centers starting and stopping, whereas the A2ALL cases are from nine centers that continued their activity throughout the study period. The national trend has been toward fewer centers performing LDLT. In 2008 and 2009, only 32 and 33 (respectively) adult liver transplant programs in the U.S. performed LDLT, of which eight were A2ALL centers. A2ALL centers performed 30% and 37% of all U.S. LDLTs in those two years, respectively. The number of LDLTs performed in the U.S. peaked at 411 in 2001, and gradually declined to 198 in 2007 (Figure 1c). Although the total number of adult LDLT has continued to decline over the last 2 years in non-A2ALL centers (124 in 2008 and 106 in 2009), the A2ALL numbers have slightly increased in 2008 (n=54) and 2009 (n=62).
Figure 1.
Figure 1a. Year of first living donor liver transplant (LDLT) in A2ALL (n=9) and non-A2ALL (n=67) centers.
Figure 1b. Percent of LDLTs performed by year between 1998 and 2007 in A2ALL and non-A2ALL centers.
Figure 1c. Number of LDLTs performed by year between 1998 and 2007 in A2ALL and non-A2ALL centers.
Patient mortality
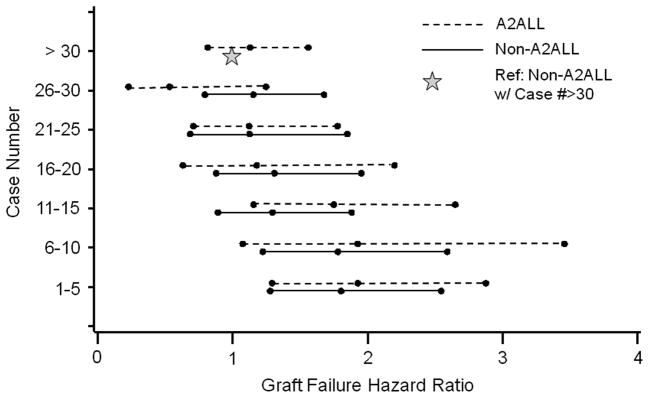
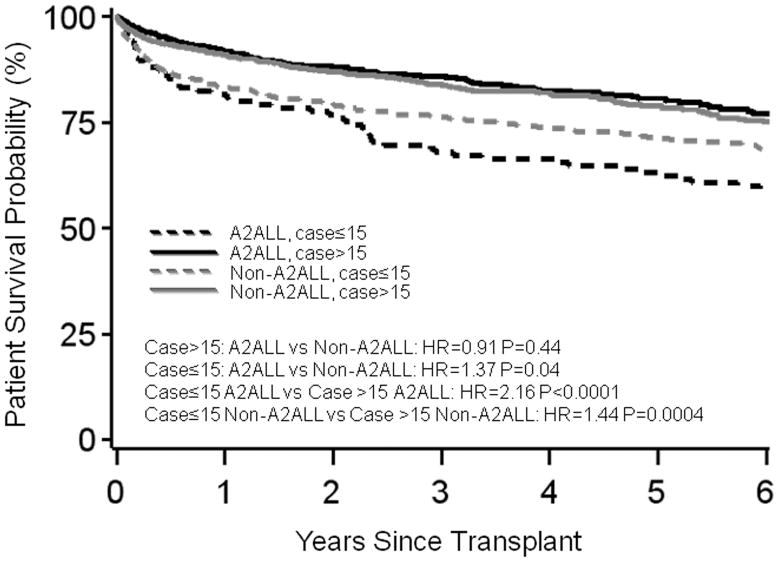
Overall adjusted post-transplant mortality risk decreased after centers gained experience. When center experience was divided into 5-case intervals, a threshold from higher to lower mortality risk was observed at case 15 at A2ALL centers, and at case 10 at non-A2ALL centers in separate models (Figure 2a). For subsequent analyses combining the A2ALL and non-A2ALL transplants, we used a threshold of case 15 to differentiate earlier center experience (cases 1 to 15) from later center experience (case 16 and higher).
Figure 2.
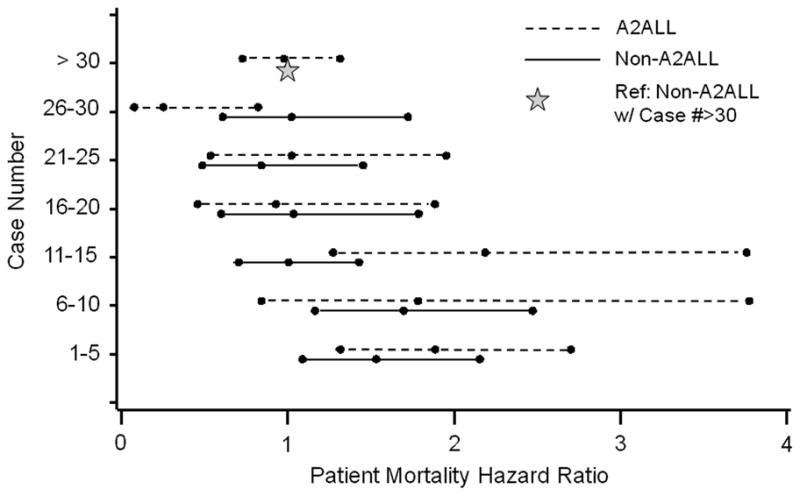
Relative risk of (a) patient mortality or (b) graft failure by center LDLT case number (Dashed line: A2ALL, Solid line: Non-A2ALL). Reference category: non-A2ALL center with case number >30. Adjusted for recipient and donor age, HCC and HCV diagnoses, creatinine, and medical condition at transplant; the graft failure model was also adjusted for recipient weight.
Compared to later experience, earlier center experience was associated with significantly higher mortality risk in both A2ALL (HR=2.24, P<0.0001) and non-A2ALL centers (HR=1.45, P=0.005) (Table 2; Figure 3a). Compared to non-A2ALL centers that went on to perform more than 15 LDLTs, mortality during the early experience was higher in both A2ALL centers (HR=1.9, P<0.001) and non-A2ALL centers that stopped by case 15 (HR=1.44, P<0.001). There was no statistical difference between A2ALL centers during early experience and non-A2ALL centers that never did more than 15 cases (HR=1.05, P=0.80).
Table 2.
Cox Regression Models for Patient Survival*
| Predictor† | A2ALL & Non-A2ALL Combined |
A2ALL Only |
Non-A2ALL Only |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Ratio | P-value | 95% Confidence Limits | Interaction w/Center Type P-value‡ | Hazard Ratio | P-value | 95% Confidence Limits | Hazard Ratio | P-value | 95% Confidence Limits | |
| LDLT case ≤15 | 1.61 | <.0001 | 1.28 – 2.02 | 0.04 | 2.24 | <.0001 | 1.71 – 2.94 | 1.45 | 0.005 | 1.12 – 1.87 |
| Donor age per 10 yr | 1.13 | 0.0002 | 1.06 – 1.20 | 0.23 | 1.22 | 0.009 | 1.05 – 1.41 | 1.10 | 0.006 | 1.03 – 1.18 |
| Recipient age per 10 yr | 1.20 | 0.0003 | 1. 09 – 1.33 | 0.11 | 1.38 | 0.001 | 1.14 – 1.66 | 1.15 | 0.006 | 1.04 – 1.27 |
| Serum creatinine (ln) | 1.52 | <.0001 | 1.26 – 1.83 | 0.48 | 1.78 | 0.001 | 1.28 – 2.46 | 1.45 | 0.0007 | 1.17 – 1.79 |
| Diagnosis of HCV | 1.18 | 0.026 | 1.02 – 1.37 | 0.57 | 1.24 | 0.20 | 0.89 – 1.72 | 1.15 | 0.09 | 0.98 – 1.35 |
| Diagnosis of HCC | 2.12 | <.0001 | 1.68 – 2.69 | 0.52 | 1.86 | 0.013 | 1.15 – 3.02 | 2.30 | <.0001 | 1.77 – 2.95 |
| Recipient medical condition: | 0.39 | |||||||||
| ICU vs home | 2.52 | <.0001 | 1.87 – 3.41 | 0.18 | 1.53 | 0.19 | 0.81 – 2.87 | 2.89 | <.0001 | 2.02 – 2.99 |
| Hospitalized (no ICU) vs home | 1.62 | <.0001 | 1.32 – 1.98 | 0.64 | 1.36 | 0.19 | 0.86 – 2.16 | 1.67 | <.0001 | 1.32 – 4.14 |
| Cold ischemia time > 4.5hours¤ | 1.79 | 0.0006 | 1.28 – 2.50 | 0.34 | 2.15 | 0.006 | 1.24 – 3.73 | 1.77 | 0.010 | 1.15 – 2.73 |
Adjusted for center clustering using robust variances based on the sandwich estimator for Cox regression.
Variables tested but not significant included recipient weight, dialysis, ascites, donor weight, donor’s biological relationship to recipient, and transplant year.
Interactions were tested one at a time in models including all main effects listed as predictors in the table above.
Results for CIT are based on separate models with a smaller sample size because of missing CIT values in 33% of cases.
Figure 3.
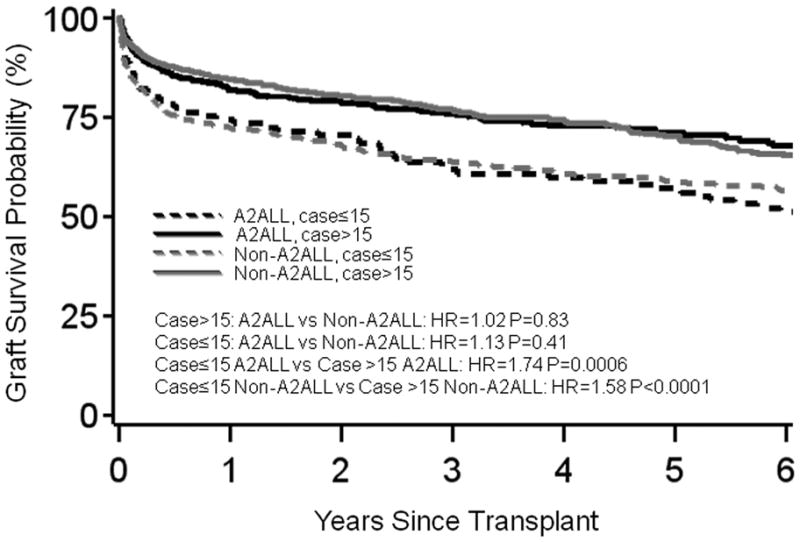
(a) Patient survival or (b) graft survival by center type and case number from an adjusted model, plotted for the mean covariate values of recipient (50) and donor age (37) at transplant, HCC (0.07) and HCV (0.37) diagnoses, creatinine (−0.09), and medical condition (ICU=0.04, hospitalized=0.12). The graft survival model was also adjusted for recipient weight (77 kg).
Additional significant predictors of mortality (both groups combined) were identified (Table 2). Older donor age (HR=1.13 per 10 years, P<0.001), older recipient age (HR=1.20 per 10 years, P<0.001), diagnosis of HCV (HR=1.18, P=0.03), diagnosis of HCC (HR=2.12, P<0.001), higher serum creatinine (HR=1.52 per loge unit increase, P<0.001), medical condition (ICU vs home: HR=2.52, P<0.001; hospitalized vs home: HR=1.62, P<0.001), and early center experience (case number ≤15 vs. >15, HR=1.61, P<0.001), were each significantly associated with higher mortality risk. In a subset with available CIT data, CIT >4.5 hours was associated with significantly higher mortality risk (HR=1.79, P<0.001), but occurred in only 4–6% of the grafts. There was no significant difference in patient mortality risk between the eras before and after 12/31/2000 after adjustment for center experience and other covariates.
In separate analyses of A2ALL centers and non-A2ALL centers, all associations were consistent in direction, although there were some differences in effect size and significance between A2ALL and non-A2ALL centers. Medical condition was a significant predictor of patient mortality in non-A2ALL centers (ICU vs home: HR=2.89, P<0.001; hospitalized vs home: HR=1.67, P<0.001), but not in A2ALL centers. We tested for differences in the association of each factor by center type (i.e., statistical interaction). The only significant interaction was with center experience, where the effect was significantly stronger at A2ALL than non-A2ALL centers, as reported above (HR=2.24 vs. HR=1.45, interaction P=0.04).
Graft failure
When center experience was divided into 5-case intervals, the threshold from higher to lower graft failure risk was identified at case 15 at A2ALL centers, and at case 10 at non-A2ALL centers (Figure 2b). As with mortality analyses, we used a threshold of case 15 to differentiate earlier from later center experience in models that included A2ALL and non-A2ALL centers.
A2ALL and non-A2ALL centers had similarly higher graft failure risk during the early experience period (combined HR=1.61, P<0.0001) compared with the experience after completing at least 15 cases (Table 3 and Figure 3b). During their early experience, non-A2ALL centers that never did more than 15 cases had significantly higher graft failure than the non-A2ALL centers with total cases > 15 (HR=1.30, P=0.002). This significant difference in early experience was not seen when non-A2ALL centers that performed ≤15 LDLT were compared with A2ALL centers during their early experience (HR=1.23, P=0.25).
Table 3.
Cox Regression Models for Graft Survival*
| Predictor† | A2ALL & Non-A2ALL Combined |
A2ALL Only |
Non-A2ALL Only |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Ratio | P-value | 95% Confidence Limits | Interaction w/Center Type P-value‡ | Hazard Ratio | P-value | 95% Confidence Limits | Hazard Ratio | P-value | 95% Confidence Limits | |
| LDLT case ≤15 | 1.61 | <0.0001 | 1.31 – 1.99 | 0.54 | 1.81 | <.0001 | 1.48 – 2.00 | 1.58 | 0.0008 | 1.21 – 2.06 |
| Donor age per 10 yr | 1.13 | 0.0002 | 1.06 – 1.21 | 0.15 | 1.22 | 0.005 | 1.06 – 1.40 | 1.10 | 0.005 | 1.03 – 1.18 |
| Recipient age per 10 yr | 1.05 | 0.26 | 0.96 – 1.16 | 0.03 | 1.26 | 0.01 | 1.05 – 1.52 | 0.99 | 0.81 | 0.92 – 1.07 |
| Recipient weight per 10 kg | 1.05 | 0.06 | 1.0 – 1.11 | 0.16 | 0.96 | 0.45 | 0.87 – 1.07 | 1.09 | 0.011 | 1.02 – 1.17 |
| Serum creatinine (ln) | 1.26 | 0.05 | 1.00 – 1.58 | 0.66 | 1.43 | 0.02 | 1.06 – 1.93 | 1.22 | 0.17 | 0.92 – 1.62 |
| Diagnosis of HCV | 1.11 | 0.23 | 0.94 – 1.31 | 0.09 | 1.41 | 0.02 | 1.05 – 1.88 | 1.01 | 0.93 | 0.84 – 1.21 |
| Diagnosis of HCC | 1.87 | <0.0001 | 1.50 – 2.33 | 0.46 | 1.64 | 0.02 | 1.09 – 2.45 | 1.99 | <0.0001 | 1.57 – 2.54 |
| Recipient medical condition: | 0.17 | |||||||||
| ICU vs home | 2.67 | <0.0001 | 2.10– 3.39 | 0.07 | 1.67 | 0.12 | 0.87 – 3.20 | 3.12 | <0.0001 | 2.37 – 4.11 |
| Hospitalized (no ICU) vs home | 1.49 | 0.0003 | 1.20 – 1.85 | 0.12 | 0.99 | 0.94 | 0.77 – 1.27 | 1.58 | 0.0002 | 1.24 – 2.02 |
| Cold ischemia time > 4.5 hours¤ | 1.49 | 0.05 | 0.99 – 2.25 | 0.03 | 3.25 | <0.0001 | 1.79 – 5.90 | 1.29 | 0.34 | 0. 77 – 2.14 |
Adjusted for center clustering using robust variances based on the sandwich estimator for Cox regression.
Variables tested but not significant included dialysis, ascites, donor weight, donor’s biological relationship to recipient, and transplant year.
Interactions were tested one at a time in models including all main effects listed as predictors in the table above.
Results for CIT are based on separate models with a smaller sample size because of missing CIT values in 33% of cases.
Significant predictors of graft failure (both groups combined) were identified (Table 3). Older donor age (HR=1.13 per 10 years, P<0.001), diagnosis of HCC (HR=1.87, P<0.001), higher serum creatinine (HR=1.26 per loge unit increase, P=0.05), medical condition (ICU vs home: HR=2.67, P<0.001; hospitalized vs home: HR=1.49, P<0.001), and early center experience (case number ≤15 vs. >15, HR=1.61, P<0.001) were associated with increased risk of graft failure. Older recipient age (P=0.26), heavier recipient weight (P=0.06), and HCV diagnosis (P=0.23) were also associated with increased graft failure, and were retained in the model for face validity, even though they were not statistically significant. In the subset with CIT data, CIT >4.5 hours (HR=1.49, P=0.05) was associated with higher graft failure risk.
In separate analyses of A2ALL centers and non-A2ALL centers with regard to factors associated with graft failure, all associations except recipient age, recipient weight, and hospitalized vs home were consistent in direction. Older recipient age (HR=1.26 per 10 years, P=0.001), serum creatinine (HR=1.43, P=0.02), and diagnosis of HCV (HR=1.41, P=0.02) were more significant predictors of graft failure in A2ALL centers than non-A2ALL centers. The effect of CIT on graft failure risk was significantly stronger in A2ALL centers (HR=3.25, P<0.0001) compared to non-A2ALL centers (HR=1.29, P=0.34). Recipient weight (HR=1.09 per 10 pounds, P=0.01) and medical condition (ICU vs home: HR=3.12, P<0.001; hospitalized vs home: HR=1.58, P<0.001) were significant predictors of graft failure in non-A2ALL centers, but not in A2ALL centers. We tested for differences by center type (i.e., statistical interaction), and found that center type only had a significant statistical interaction with recipient age (P=0.03) and CIT (P=0.03).
Discussion
As the transplant community continues to make efforts to define the most appropriate role for LDLT, it is important to identify the significant clinical risk factors associated with graft failure and recipient mortality. Within the A2ALL consortium, one of the first observations about adult-to-adult LDLT was the significant learning curve, with improved graft survival after the first 20 cases at each center (2). We have also recently described a decrease in the incidence of recipient and donor complications following a period of experience (3, 4). Similar findings have been reported in large single center reports, where patient and graft survival has improved significantly after initial center experience (5–7). Significant clinical characteristics associated with graft loss, including older recipient age and cold ischemic time were also identified. With the first phase of A2ALL completed, and the second phase beginning, it was important to determine whether the findings of the A2ALL consortium are representative of centers throughout the U.S. with respect to experience and post-transplant outcomes, specifically graft failure and patient mortality. Equally important was the goal to identify any significant similarities and differences between A2ALL and non-A2ALL centers with regard to other factors affecting outcome that might alter the applicability of A2ALL findings to the general pool of patients undergoing LDLT, and provide evidence regarding the most appropriate recipients of LDLT.
In this report we have again shown that there was an association of center experience with regard to both patient and graft outcome following LDLT in the U.S. experience. Non-A2ALL centers that never did more than 15 cases had significantly higher graft failure than those that went on to do more. After the initial 15 cases, both A2ALL and non-A2ALL centers demonstrated a significant decrease in post-transplant mortality. While the A2ALL centers had significantly higher mortality rates for their first 15 cases, it only took 10 LDLT cases for non-A2ALL centers to see their results improve, perhaps due to the fact that many non-A2ALL centers started in later years, with experienced teams moving from centers that had already performed LDLT. By comparison, most A2ALL centers maintained a stable surgery and hepatology team composition over the years.
Learning curves are often described after the introduction of a new procedure, but no new complex procedures have been introduced in the field of liver transplant in the last 10 years except for right lobe LDLT. Relationships between experience and outcome in kidney, liver, and heart transplantation have been reported (8, 9), with outcomes being better at high volume centers, and a strong relationship has been reported between higher surgeon volume and decreased morbidity and mortality with other complex surgical procedures (10, 11). Therefore, the learning curve noted here was not unexpected, since the introduction of adult-to-adult LDLT was a major technical development from deceased donor liver transplantation.
It was also equally important to determine what other clinical factors contribute to mortality after LDLT, and if they were comparable in both A2ALL and non-A2ALL centers. These findings can help centers select the most appropriate donor and recipient for the LDLT procedure for the best outcome. For all centers, we found that older recipient age, donor age, diagnosis of HCC, higher creatinine (a major component of MELD), and being hospitalized or in ICU all contributed to recipient mortality. When we explored the clinical risk factors for mortality in A2ALL and non-A2ALL centers that were available, we found that these risk factor effects were not significantly different between A2ALL and non-A2ALL centers. The separate A2ALL and non-A2ALL models gave results of consistent direction and fairly similar magnitudes.
With regard to graft failure, we also found similar risk factor estimates in the A2ALL and non-A2ALL groups. When the cohorts were combined, older donor age, diagnosis of HCC, higher serum creatinine, and location in the ICU were each associated with a higher risk of graft loss. There were some differences in the effects of individual predictors between the two groups, recipient age and CIT were the only two variables that demonstrated statistically significant differences in their effects between the A2ALL and non-A2ALL cohorts. The CIT discrepancy may be explained by the fact that we were able to supplement missing SRTR data with A2ALL data for this field, and although the missing CIT data in SRTR may have affected the coefficient estimates, we have previously reported that CIT is significantly associated with various outcomes in A2ALL (2, 3, 12, 13). It is more difficult to explain the difference in the significance of recipient age in the A2ALL group versus the non-A2ALL group. Nonetheless, each of these factors may be of clinical importance, either alone or in combination, and should be considered when making decisions regarding donor and recipient selection for LDLT.
The A2ALL multicenter consortium was formed to address outcomes in both donors and recipients with detailed prospective data collection, with the goal of generalizing these results to the national experience since detailed data collection and reporting is not feasible on a national scale. This report demonstrates that the A2ALL study results are generally representative of national outcomes. While incomplete data collection and lack of granularity in the SRTR data may contribute to some of the differences noted in this study, we have shown comparability between the groups with regard to magnitude and direction of risk factor effects.
Limitations of this study may include missing data and misclassification in the SRTR data for the covariates and outcomes investigated (14), and potential secular trends not captured in the statistical modeling, although era effects (before vs after 12/31/2000) were tested. Also, due to the limitation of variables available in the SRTR database, we were not able to demonstrate comparability of A2ALL with non-A2ALL centers for other published A2ALL results, such as donor and recipient complication, and graft size.
From these results of all U.S. centers performing LDLT, we have shown that gaining initial experience is important to improve survival after LDLT, irrespective of when a center starts an adult LDLT program. The data also demonstrate that there is a continued decline in the number of adult LDLTs performed outside of the A2ALL consortium, which may demonstrate the natural tendency of these procedures to gravitate to experienced centers.
The analyses presented here support the application of findings from the A2ALL consortium to other centers in the U.S. performing LDLT. As we embark on further studies in the second phase of A2ALL, it is the goal of the A2ALL consortium that analyses of the detailed data and the lessons learned may contribute to the future advancements on a national scale and A2ALL findings may be used to provide guidance for center performance and clinical decision-making in the field of adult-to-adult LDLT.
Acknowledgments
Funding Sources: Supported in part by the National Institutes of Health (NIDDK grant numbers U01-DK62536, U01-DK62444, U01-DK62467, U01-DK62483, U01-DK62484, U01-DK62494, U01-DK62496, U01-DK62498, U01-DK62505, U01-DK62531), the American Society of Transplant Surgeons, and the U.S. Department of Health and Human Services, Health Resources and Services Administration.
The authors would like to gratefully acknowledge Abraham Shaked, MD, PhD for concept and manuscript development, and Jay E. Everhart, MD for intellectual contributions to this project. The authors would also like to acknowledge the A2ALL Principal Investigators and their teams for their contribution to the overall study.
This study was supported by National Institute of Diabetes & Digestive & Kidney Diseases through cooperative agreements (listed below). Additional support was provided by Health Resources and Services Administration (HRSA), and the American Society of Transplant Surgeons (ASTS).
The following individuals were instrumental in the planning, conduct and/or care of patients enrolled in this study at each of the participating institutions as follows:
Columbia University Health Sciences, New York, NY (DK62483): PI: Jean C. Emond, MD; Co-PI: Robert S. Brown, Jr., MD, MPH; Study Coordinators: Rudina Odeh-Ramadan, PharmD; Scott Heese, BA
Northwestern University, Chicago, IL (DK62467): PI: Michael M.I. Abecassis, MD, MBA; Co-PI: Laura M. Kulik, MD; Study Coordinator: Patrice Al-Saden, RN, CCRC
University of Pennsylvania Health System, Philadelphia, PA (DK62494): PI: Abraham Shaked, MD, PhD; Co-PI: Kim M. Olthoff, MD; Study Coordinators: Brian Conboy, PA, MBA; Mary Shaw, RN, BBA
University of Colorado Health Sciences Center, Denver, CO (DK62536): PI: Gregory T. Everson, MD; Co-PI: Igal Kam, MD; Study Coordinators: Carlos Garcia, BS, Anastasia Krajec, RN.
University of California Los Angeles, Los Angeles, CA (DK62496): PI: Johnny C. Hong, MD; Co-PI: Ronald W. Busuttil, MD, PhD; Study Coordinator: Janet Mooney, RN, BSN
University of California San Francisco, San Francisco, CA (DK62444): PI: Chris E. Freise, MD, FACS; Co-PI: Norah A. Terrault, MD; Study Coordinator: Dulce MacLeod, RN; Vivian Tan, MD
University of Michigan Medical Center, Ann Arbor, MI (DK62498): PI: Robert M. Merion, MD; DCC Staff: Anna S.F. Lok, MD; Akinlolu O. Ojo, MD, PhD; Brenda W. Gillespie, PhD; Margaret Hill-Callahan, BS, LSW; Terese Howell, BS; Lan Tong, MS; Tempie H. Shearon, MS; Karen A. Wisniewski, MPH; Monique Lowe, BS; Abby Smith
University of North Carolina, Chapel Hill, NC (DK62505): PI: Paul H. Hayashi, MD, MPH; Study Coordinator: Tracy Russell, MA
University of Virginia (DK62484): PI: Carl L. Berg, MD; Co-PI: Timothy L. Pruett, MD; Study Coordinator: Jaye Davis, RN
Medical College of Virginia Hospitals, Virginia Commonwealth University, Richmond, VA (DK62531): PI: Robert A. Fisher, MD, FACS; Co-PI: Mitchell L. Shiffman, MD; Study
Coordinators: Andrea Lassiter, Transplant data analyst; April Ashworth, RN
National Institute of Diabetes and Digestive and Kidney Diseases, Division of Digestive Diseases and Nutrition, Bethesda, MD: James E. Everhart, MD, MPH; Leonard B. Seeff, MD; Patricia R. Robuck, PhD; Jay H. Hoofnagle, MD
Abbreviations
- A2ALL
Adult to Adult Living Donor Liver Transplantation Cohort Study
- LDLT
Living Donor Liver Transplant
Footnotes
Reprints will not be available from the authors.
1Presented in part at the American Transplant Congress, Toronto, Canada, June, 2008.
Supported in part by the National Institutes of Health (NIDDK grant numbers U01-DK62536, U01-DK62444, U01-DK62467, U01-DK62483, U01-DK62484, U01-DK62494, U01-DK62496, U01-DK62498, U01-DK62505, U01-DK62531), the American Society of Transplant Surgeons, and the U.S. Department of Health and Human Services, Health Resources and Services Administration.
3This is publication number 14 of the Adult-to-Adult Living Donor Liver Transplantation Cohort Study.
Supplemental data included here have been supplied by the Arbor Research Collaborative for Health as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
References
- 1.Wachs ME, Bak TE, Karrer FM, Everson GT, Shrestha R, Trouillot TE, Mandell MS, et al. Adult living donor liver transplantation using a right hepatic lobe. Transplantation. 1998;66:1313–1316. doi: 10.1097/00007890-199811270-00008. [DOI] [PubMed] [Google Scholar]
- 2.Olthoff KM, Merion RM, Ghobrial RM, Abecassis MM, Fair JH, Fisher RA, Freise CE, et al. Outcomes of 385 adult-to-adult living donor liver transplant recipients: a report from the A2ALL Consortium. Ann Surg. 2005;242:314–323. doi: 10.1097/01.sla.0000179646.37145.ef. discussion 323–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freise CE, Gillespie BW, Koffron AJ, Lok AS, Pruett TL, Emond JC, Fair JH, et al. Recipient morbidity after living and deceased donor liver transplantation: findings from the A2ALL Retrospective Cohort Study. Am J Transplant. 2008;8:2569–2579. doi: 10.1111/j.1600-6143.2008.02440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghobrial RM, Freise CE, Trotter JF, Tong L, Ojo AO, Fair JH, Fisher RA, et al. Donor morbidity after living donation for liver transplantation. Gastroenterology. 2008;135:468–476. doi: 10.1053/j.gastro.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lo CM, Fan ST, Liu CL, Yong BH, Wong Y, Lau GK, Lai CL, et al. Lessons learned from one hundred right lobe living donor liver transplants. Ann Surg. 2004;240:151–158. doi: 10.1097/01.sla.0000129340.05238.a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pomposelli JJ, Verbesey J, Simpson MA, Lewis WD, Gordon FD, Khettry U, Wald C, et al. Improved survival after live donor adult liver transplantation (LDALT) using right lobe grafts: program experience and lessons learned. Am J Transplant. 2006;6:589–598. doi: 10.1111/j.1600-6143.2005.01220.x. [DOI] [PubMed] [Google Scholar]
- 7.Shah SA, Levy GA, Greig PD, Smith R, McGilvray ID, Lilly LB, Girgrah N, et al. Reduced mortality with right-lobe living donor compared to deceased-donor liver transplantation when analyzed from the time of listing. Am J Transplant. 2007;7:998–1002. doi: 10.1111/j.1600-6143.2006.01692.x. [DOI] [PubMed] [Google Scholar]
- 8.Axelrod DA, Guidinger MK, McCullough KP, Leichtman AB, Punch JD, Merion RM. Association of center volume with outcome after liver and kidney transplantation. Am J Transplant. 2004;4:920–927. doi: 10.1111/j.1600-6143.2004.00462.x. [DOI] [PubMed] [Google Scholar]
- 9.Weiss ES, Meguid RA, Patel ND, Russell SD, Shah AS, Baumgartner WA, Conte JV. Increased Mortality at Low-Volume Orthotopic Heart Transplantation Centers: Should Current Standards Change? The Annals of Thoracic Surgery. 2008;86:1250–1260. doi: 10.1016/j.athoracsur.2008.06.071. [DOI] [PubMed] [Google Scholar]
- 10.Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137:511–520. doi: 10.7326/0003-4819-137-6-200209170-00012. [DOI] [PubMed] [Google Scholar]
- 11.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–2127. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 12.Berg CL, Gillespie BW, Merion RM, Brown RS, Jr, Abecassis MM, Trotter JF, Fisher RA, et al. Improvement in survival associated with adult-to-adult living donor liver transplantation. Gastroenterology. 2007;133:1806–1813. doi: 10.1053/j.gastro.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaked A, Ghobrial RM, Merion RM, Shearon TH, Emond JC, Fair JH, Fisher RA, et al. Incidence and severity of acute cellular rejection in recipients undergoing adult living donor or deceased donor liver transplantation. Am J Transplant. 2009;9:301–308. doi: 10.1111/j.1600-6143.2008.02487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gillespie BW, Merion RM, Ortiz-Rios E, Tong L, Shaked A, Brown RS, Ojo AO, et al. Database comparison of the adult-to-adult living donor liver transplantation cohort study (A2ALL) and the SRTR U.S. Transplant Registry. Am J Transplant. 2010;10:1621–1633. doi: 10.1111/j.1600-6143.2010.03039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]