Abstract
Objectives
We tested the hypothesis that leukotriene B4 (LTB4) mediates pancreatic inflammation in rats via activation of the Transient Receptor Potential Vanilloid 1 (TRPV1).
Methods
LTB4 or vehicle was administered to adult rats via celiac axis injection following pretreatment with the TRPV1 antagonist, capsazepine, or vehicle and the severity of subsequent pancreatitis was assessed by measuring pancreatic edema, myeloperoxidase (MPO) activity, and histological grading. In a second experiment, acute pancreatitis was induced by common pancreaticobiliary duct ligation (CPBDL). Six hours postoperatively, pancreatic tissue levels of LTB4 were determined by ELISA. Also, the effects of inhibition of LTB4 biosynthesis by pretreatment with the 5-lipoxygenase activating peptide (FLAP) inhibitor, MK-886, were determined.
Results
Celiac axis administration of LTB4 significantly increased pancreatic edema and MPO activity, and produced histological evidence of pancreatic edema, neutrophil infiltration, and necrosis. Capsazepine pretreatment significantly reduced all inflammatory parameters in LTB4-induced pancreatitis. Pancreatic tissue levels of LTB4 were significantly elevated in rats that underwent CPBDL compared to control rats. MK-886 pretreatment significantly inhibited pancreatic edema, histological damage, and pancreatic MPO concentrations.
Conclusions
Common pancreaticobiliary duct obstruction causes an increase in pancreatic LTB4 concentrations that in turn mediates activation of TRPV1 resulting in acute pancreatitis.
Introduction
Much evidence has been presented that the ion channel receptor Transient Receptor Potential Vanilloid 1 (TRPV1) mediates inflammation in several animal models of acute pancreatitis.1, 2 TRPV1 is a ligand- and heat-gated cation channel expressed primarily by small diameter primary sensory neurons that innervate the pancreas as well as other organs and tissues.3 TRPV1 is directly activated by heat, protons, some lipoxygenase products, the plant vanilloid, capsaicin, and the plant neurotoxin, resiniferatoxin (RTX).4-6 When activated, TRPV1 depolarizes primary sensory neurons resulting in neurotransmitter release centrally in the spinal cord and peripherally in the stimulated tissue. These neurons express a variety of neurotransmitters including proinflammatory neuropeptides such as the tachykinin, substance P (SP). Early evidence that TRPV1 is involved in pancreatic inflammation came from demonstrations that capsaicin causes vasodilation and plasma extravasation, two features of neurogenic inflammation, in the rat7 and mouse8 pancreas and that this effect was mediated by SP release.9, 10 It was also shown that genetic deletion of the SP neurokinin-1 receptor (NK-1) inhibits secretagogue-induced pancreatitis in mice.11
Further evidence for an important role for TRPV1 in pancreatitis came from a study demonstrating that neonatal administration of capsaicin in rats, a procedure that permanently destroys TRPV1-expressing primary sensory nerves, ameliorates both secretagogue-induced pancreatitis12 and duct obstruction-induced pancreatitis.13 Additional support for this conclusion was provided by the finding that pharmacological administration of the TRPV1 antagonist drug, capsazepine, significantly reduced secretagogue-induced pancreatitis in rats.14, 15 It was subsequently shown in rats that capsazepine pretreatment reduces nociception in acute pancreatitis as well.16
TRPV1-expressing primary sensory neurons that innervate the pancreas are known to pass through the celiac ganglion. Therefore, if these nerves play a role in pancreatic inflammation, destruction of the celiac ganglion should protect the pancreas from damage and such a procedure may have potential for treating human pancreatitis. We were able to demonstrate that surgical excision of the celiac ganglion or local intoxication of the ganglion by local application of resiniferatoxin (RTX) reduces the severity of secretagogue-induced pancreatitis.17 Additional evidence for an important role of TRPV1 in acute pancreatitis came from the demonstration that pancreatic ductal injection of the contrast solution used in human endoscopic retrograde cholangiopancreatography (ERCP) caused a pH-dependent acute pancreatitis similar to that seen in human patients undergoing ERCP.18 Contrast solutions at acidic pHs caused TRPV1-dependent increases in pancreatic inflammation and inclusion of RTX in the contrast solution protected the pancreas.
Since both secretagogue-induced and duct obstruction-induced pancreatitis appear to be mediated at least in part by TRPV113, this suggests that both of these inflammatory treatments may activate TRPV1 by the same mechanism. It is unlikely that TRPV1 is activated by heat, low pH, or plant compounds like capsaicin or RTX in inflammatory diseases. Instead, endogenous agonist ligands of TRPV1 that may be released or produced in response to inflammatory stimuli have been thought to be likely candidates as mediators of inflammatory disease states. Known endogenous agonist ligands of TRPV1 include the endocannabinoids, anandamide and 2-arachidonoyl glycerol (2-AG)19, lipoxygenase products such as leukotriene B4 (LTB4)6, 2-arachidonyl glyceryl ether (noladin ether)20, and N-arachidonoyl-dopamine.21 Among these, LTB4 is an especially attractive candidate as an endogenous ligand of TRPV1 in inflammatory states because LTB4 has been shown to be an important proinflammatory agent in human inflammatory diseases22-25 and in animal models of inflammation.26-30 LTB4 is made from arachidonic acid in cells and the rate-limiting step in this biosynthesis is the activity of the enzyme, 5-lipoxygenase (5-LO), which requires the helper protein, 5-LO activating protein (FLAP) for efficient catalysis.31 Support for the concept that LTB4 mediates the effects of inflammatory agents on TRPV1 activation comes from a study demonstrating that acute pancreatitis caused by intraductal administration of 5% sodium taurocholate in the rat was accompanied by increased pancreatic levels of LTB4.32 In addition, genetic deletion of 5-LO in mice resulted in resistance to secretagogue-induced pancreatitis.33 Thus, it is reasonable to test the hypothesis that endogenous LTB4 may be an important activator of TRPV1 in experimental acute pancreatitis.
In the present study we show that exogenous LTB4 causes a pancreatitis in rats closely resembling duct-obstruction-induced inflammation and that pharmacologic antagonism of TRPV1 inhibits both LTB4- and duct obstruction-induced pancreatitis. In addition, we show that pharmacologic inhibition of FLAP, the activator of 5-LO, inhibits duct obstruction-induced pancreatic inflammation.
Materials and Methods
These studies were approved by the Duke University Institutional Animal Care and Use Committee.
Materials
Leukotriene B4 was purchased from BIOMOL (Plymouth Meeting, PA). Capsazepine was purchased from Sigma (St. Louis, MO). MK-886 was purchased from Tocris (Ellisville, MO). LTB4 was dissolved in 25% ethanol/75% phosphate buffered saline (PBS) for intraarterial (ia) injection. Capsazepine was dissolved in DMSO to give a stock solution of 0.1 M, then further diluted (1:10) in saline containing 10% Tween 80/10% ethanol for subcutaneous (sc) injection. MK-886 was suspended in 2% Ethanol/2% Tween 80/96% sterile 0.9% NaCl for intraperitoneal (ip) injection.
Surgery
Male Sprague-Dawley rats (150-200 g) were purchased from Charles River Laboratories (Wilmington, MA), housed in climate-controlled rooms with a 12:12 hour light-dark cycle, and given access to food and water ad libitum. In the first experiment, one group of rats was pretreated with either vehicle (controls) or capsazepine at a dose of 100 μmol/kg sc 30 min before surgery. They were then anesthetized with ketamine and xylazine and the abdomen was opened via midline laparotomy. The common pancreaticobiliary duct was ligated within 1 mm of the duodenal wall. Control rats underwent ligation of the bile duct at the hilum of the liver. The abdomen was closed in two layers. A second group of rats was also pretreated with either vehicle (controls) or capsazepine at a dose of 100 μmol/kg sc 30 min before surgery and were then anesthetized with ketamine and xylazine, the abdomen was opened via midline laparotomy, and the celiac artery was identified and injected with either vehicle or LTB4 at a dose of 15 μg/kg body weight using a 27 g needle. The abdomen was closed in two layers. Three hours postoperatively both groups of rats were killed and their pancreata were quickly removed, weighed to determine edema, and divided for histological grading and for measurement of tissue myeloperoxidase (MPO) activity.
In the second experiment, rats were anesthetized with ketamine and xylazine and common pancreaticobiliary duct ligation (CPBDL) was performed as described above. Control rats underwent ligation of the bile duct at the hilum of the liver. Six hours later the rats were killed and the entire pancreas was removed and frozen on dry ice for later LTB4 assay as described below.
In the third experiment, rats were pretreated with either vehicle (controls) or MK-886 at a dose of 10 mg/kg ip one hour before surgery. The rats were anesthetized and CPBDL was performed as described above. Control rats underwent laparotomy with manipulation of the common pancreaticobiliary duct. The abdomen was closed in two layers. Four hours after surgery, the animals were killed, and the pancreas was quickly removed, weighed, and divided for histological grading and for measurement of tissue myeloperoxidase (MPO) activity.
Myeloperoxidase (MPO) Activity
A portion of the heads of the harvested pancreata were immediately frozen on dry ice and then stored at −80°C until assay. We measured the tissue activity of myeloperoxidase, an enzyme produced by neutrophils and used as a marker of inflammation associated with neutrophil infiltration, as previously described using the substrate tetramethylbenzidine.34, 35
Pancreatic MPO activity was expressed as units per mg total protein and normalized to control values.
Histopathology
Portions of the pancreata were fixed overnight in phosphate-buffered 10% formalin. The tissue was then embedded in paraffin, sectioned at 5 μm, stained with hematoxylin and eosin, and coded for examination by two blinded investigators unaware of the experimental design. The severity of pancreatitis was graded using modified scoring criteria previously described.36 The results were expressed in increments of 0.5 as a score of 0 to 3 for the histological parameters of edema and neutrophil infiltration and as a score of 0 to 7 for tissue necrosis. Total histological score is the mean of the combined scores for edema, neutrophil infiltration, and necrosis from both investigators.
LTB4 Measurement
Pancreatic LTB4 levels were measured by LTB4 enzyme immunoassay (EIA) kits purchased from Cayman Chemical (Ann Arbor, MI). Briefly, samples of pancreas were collected after various treatments in 5 volumes of ice-cold 0.1 M phosphate buffer, pH 7.4, containing 1 mM EDTA and 10 μM indomethacin and homogenized for 15 sec on ice using a Tekmar Tissumizer (Tekmar, Cincinnati, OH) at a 50% power setting. Before homogenization, 10,000 cpm of 3 H-LTB4 (120-240 Ci/mmol; Perkin-Elmer Life Sciences, Boston, MA) were added to the buffer for later assessment of LTB4 recovery. After homogenization, 2 volumes of ice-cold ethanol were added to each extract and the extracts were then incubated on ice for 5 min to precipitate proteins. After centrifugation at 3000 x gmax to remove the precipitated proteins, the ethanol in the supernatants was removed by vacuum centrifugation. The pH of the extracts was adjusted to ~4.0 by addition of 1 M sodium acetate (pH 4.0). The resulting precipitate was removed by centrifugation and the supernatant was loaded onto C-18 solid phase extraction cartridges (Cayman Chemical, Ann Arbor, MI) previously washed with methanol and distilled water, washed with distilled water followed by hexane, and then eluted at unit gravity with 5 ml of 99% ethyl acetate:1% methanol. The samples were then evaporated to dryness by vacuum centrifugation, reconstituted in LTB4 EIA buffer, and assayed according to the instructions of the kit manufacturer.
Statistical Analysis
Results are expressed as mean ± SEM. Mean differences between 2 groups were examined by the Student t test and mean differences among several groups by one-way ANOVA with the Dunnett’s or Tukey-Kramer post tests, using GraphPad InStat version 3.05 for Windows (GraphPad Software, San Diego, CA). P values < 0.05 were considered significant.
Results
To determine if direct administration of LTB4 to the pancreas caused inflammation similar to that caused by CPBDL, LTB4 was injected into the celiac artery for direct delivery to the pancreas and pancreatic inflammation was evaluated by histological examination, edema, and tissue MPO content. The histopathology caused by LTB4 included edema, neutrophil infiltration, and acinar cell necrosis (Fig. 1) and closely resembled the damage observed after CPBDL (Fig. 2). When the individual histopathology scores were analyzed, the increased edema caused by LTB4 was not statistically significant but neutrophil infiltration and acinar cell necrosis were significantly increased (Table 1). Capsazepine pretreatment inhibited the LTB4-induced pancreatic edema, neutrophil infiltration, and acinar cell necrosis but the inhibition of edema was not statistically significant (Table 1).
Figure 1.
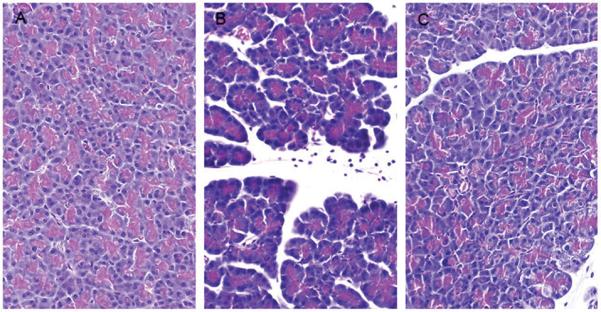
Representative photomicrographs of hematoxylin and eosin-stained pancreatic sections taken from control rats (left panel), rats treated with LTB4 injected into the celiac artery alone (middle panel), and rats pretreated with capsazepine before close arterial injection of LTB4 (right panel).
Figure 2.
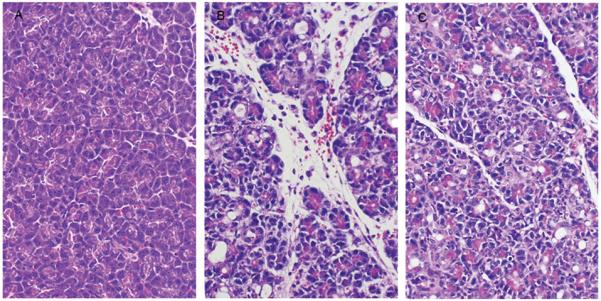
Representative photomicrographs of hematoxylin and eosin-stained pancreatic sections taken from control rats (left panel), rats subjected to common pancreaticobiliary duct ligation (CPBDL; middle panel), and rats pretreated with capsazepine before CPBDL (right panel).
Table 1.
The effects of treatment with LTB4 alone and LTB4 after pretreatment with capsazepine (CPZ) on pancreatic histopathology scores (mean ± SEM; N = 5)
| Edema | Neutrophils | Acinar Necrosis | |
|---|---|---|---|
| Control | 1.10 ± 0.29 | 0 ± 0 | 0 ± 0 |
| LTB4 | 1.70 ± 0.20 | 1.40 ± 0.24* | 2.80 ± 0.86* |
| LTB4 + CPZ | 0.80 ± 0.12 | 0.30 ± 0.20** | 0.20 ± 0.20** |
P < 0.01 vs. Control
P < 0.01 vs. LTB4
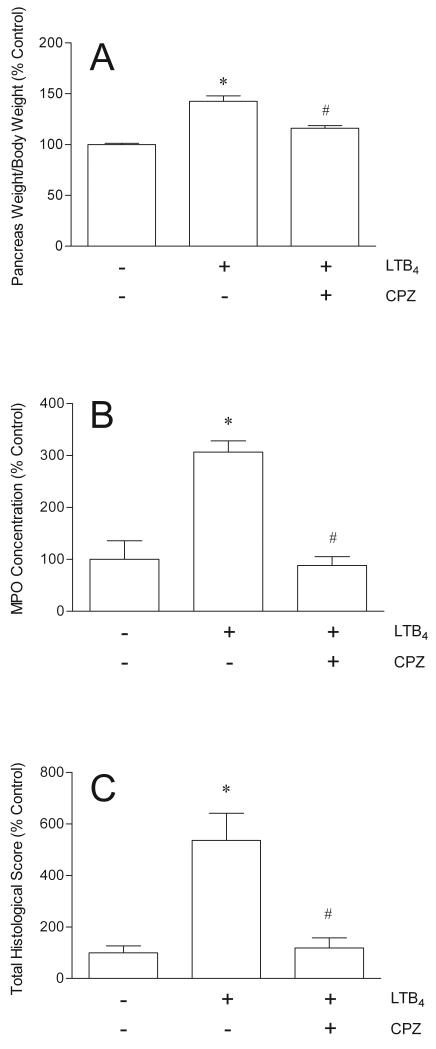
Close arterial administration of LTB4 directly into the pancreas via the celiac artery caused statistically significant pancreatic inflammation assessed by glandular edema, tissue MPO activity, and histopathology (Fig. 3). When the rats were pretreated for 30 minutes with the TRPV1 antagonist, capsazepine, before injection of LTB4, the LTB4-induced increases in pancreatic edema, MPO, and structural damage were inhibited significantly (Fig. 3). Capsazepine inhibited LTB4-induced pancreatic edema by 62%, pancreatic MPO levels by 106%, and pancreatic structural damage by 96%.
Figure 3.
The effects of LTB4 (15 μg/kg ia) alone and LTB4 after pretreatment with capsazepine (CPZ, 100 μmol/kg sc) on pancreatic edema (A), pancreatic MPO concentrations (B), and pancreatic histopathology (C) (N = 5). Pancreatic edema is expressed as the ratio of pancreatic wet weight to body weight and normalized to % Control. Pancreatic MPO concentration is expressed as units/mg pancreatic protein and normalized to % Control. Administration of capsazepine alone had no effect. *P < 0.001 versus Control (−/−); #P < 0.001 versus LTB4 alone.
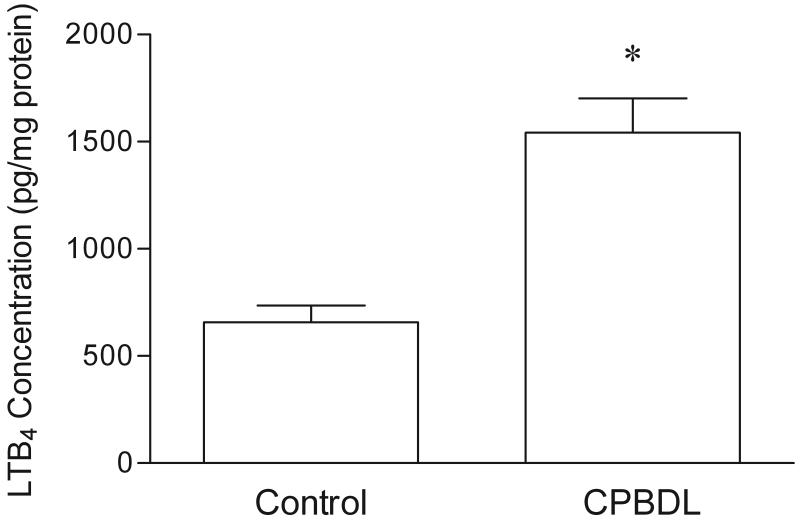
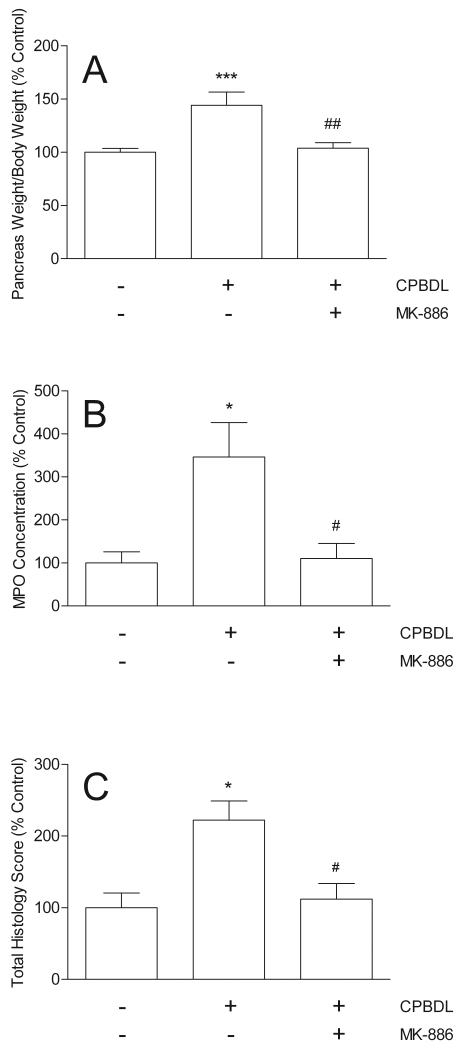
To determine if CPBDL stimulates pancreatic LTB4 synthesis, the concentration of LTB4 in pancreatic extracts was measured before and 6 hours after CPBDL. CPBDL stimulated a highly significant increase in pancreatic LTB4 content compared to controls (Fig. 4). If the CPBDL-induced increase in pancreatic LTB4 concentration mediates pancreatic damage in this model, then inhibition of LTB4 biosynthesis should reduce the various indices of pancreatitis observed. We tested this hypothesis by pretreating the rats with the pharmacologic agent, MK-886, which inhibits FLAP, a protein required for 5-LO activity.31 We found that MK-886 significantly inhibited CPBDL-induced pancreatic edema by 92%, MPO concentration by 97%, and pancreatic structural damage by 93% (Fig. 5).
Figure 4.
The concentrations of LTB4 in the pancreases of control and common pancreatic-bile duct ligated (CPBDL) rats (N = 7). *P < 0.0005 vs. Control.
Figure 5.
The effects of common pancreatic-bile duct ligation (CPBDL) alone and after pretreatment with MK-886 (10 mg/kg ip) on pancreatic edema (A; N = 6-13), pancreatic MPO concentrations (B; N = 7-9), and pancreatic histopathology (C; N = 9-10). Pancreatic edema is expressed as the ratio of pancreatic wet weight to body weight and normalized to % Control. Pancreatic MPO concentration is expressed as units/mg pancreatic protein and normalized to % Control. Administration of MK-886 alone had no effect. *P < 0.001 versus Control (−/−); #P < 0.001 versus CPBDL alone.
Discussion
LTB4 has been shown to be proinflammatory in multiple animal and human inflammatory disease states. LTB4 is elevated in inflamed intestinal tissue in animals26-28 and humans.23, 24 Inhibition of the rate-limiting enzyme in LTB4 biosynthesis, 5-lipoxygenase (5-LO), reduces tissue levels of LTB4 and inhibits enteritis in animals27, 29, 30, 37 and humans.22, 25 LTB4 causes inflammation via a potent neutrophil chemotactic action and also causes aggregation and degranulation of neutrophils and increases vascular permeability.22, 38 Two G protein-coupled receptors specific for LTB4, BLT1 and BLT2, have been described and shown to mediate some of its proinflammatory actions.38 However, LTB4 receptor antagonists have had mixed success in tests of their ability to inhibit experimental inflammation in animal models39, 40 and have not found a place in the medical management of inflammatory diseases in humans.
It has recently been shown that LTB4 can activate TRPV1 receptors as well as its own cognate BLT1/BLT2 receptors.6 TRPV1 receptors are nonspecific cation channels expressed by certain subtypes of primary sensory neurons for which the plant vanilloid, capsaicin, is an agonist.3 Activation of TRPV1 in turn causes depolarization of the sensory neuron and subsequent release of the neurotransmitters expressed by the neuron.41 One neurotransmitter released from this class of sensory neurons in response to TRPV1 stimulation is substance P (SP). Substance P has several proinflammatory actions that are similar to those exhibited by LTB4 including increasing vascular permeability and vasodilatation.41 This raises the possibility that the proinflammatory actions of LTB4 may not be mediated by BLT1 or BLT2 receptors, but instead may be a function of LTB4-stimulated TRPV1 activation resulting in SP release.
We present multiple lines of evidence here that LTB4 mediates a portion of CPBDL-induced pancreatic inflammation via activation of TRPV1. Injection of LTB4 into the celiac artery supplying blood directly into the pancreas caused increases in pancreatic edema, tissue MPO concentrations, and histological damage of similar magnitude to those seen after CPBDL. Most significantly, pretreatment of the animals with the TRPV1 antagonist, capsazepine, significantly inhibited all indices of inflammation caused by LTB4. Capsazepine has been reported to have nonspecific actions on calcium channels42 and nicotinic cholinergic receptors43 in addition to its antagonism of TRPV144, but capsazepine was used here at the dose that we previously demonstrated antagonizes TRPV1 specifically.14
LTB4 is biosynthesized from arachidonic acid by the action of cytosolic phospholipase A2, 5-LO in conjunction with FLAP, and leukotriene A4 (LTA4) hydrolase.31 5-LO activity is FLAP- and calcium-dependent and is considered to be the key regulator of LTB4 biosynthesis because it initiates leukotriene synthesis. If LTB4 mediates the inflammatory effects of CPBDL in the pancreas, then CPBDL should cause local generation of LTB4 in pancreatic tissue. We tested this hypothesis in two ways. First, we measured CPBDL-induced tissue LTB4 levels by specific enzyme immunoassay and found that CPBDL stimulates a several-fold increase in tissue LTB4 concentrations. Second, we reasoned that if LTB4 mediates CPBDL-induced pancreatitis in this model, then pretreatment with MK-886, a drug that inhibits 5-LO by blocking the activity of 5-lipoxygenase activating peptide (FLAP), should also inhibit CPBDL-induced inflammation. MK-886 pretreatment significantly inhibited CPBDL-induced pancreatic edema, MPO activity, and structural damage thus demonstrating that CPBDL results in the production of LTB4. MK-886 has been shown to inhibit peroxisome proliferator-activated receptor α (PPARα) as well as FLAP45, but PPARα inhibition cannot explain the present findings because activation of PPARα has been shown to reduce pancreatic injury in experimental pancreatitis46; a drug inhibiting PPARα such as MK-886 would thus be expected to enhance pancreatitis instead of reducing it as occurred here.
Support for the concept that LTB4 activates TRPV1 in pancreatic inflammation comes from a study in another model in which acute pancreatitis caused by intraductal administration of 5% sodium taurocholate in the rat was accompanied by increased pancreatic levels of LTB4.32 In addition, genetic deletion of 5-LO in mice resulted in resistance to secretagogue-induced pancreatitis.33 Genetic deletion of 5-LO alone does not prove specifically that LTB4 is involved because 5-LO is also involved in the biosynthesis of other active metabolites such as leukotriene C4, leukotriene D4, and leukotriene E 474, which do not activate TRPV1.
Products of lipoxygenases other than LTB4 have also been shown to be TRPV1 agonists. In particular, 12-(S)-HPETE, 15-(S)-HPETE, 5-(S)-HETE, and 15-(S)-HETE have significant TRPV1 agonist activity.6 It is possible that one or more of these eicosanoids also mediates the inflammatory activity of CPBDL in the rat pancreas. However, the present data show that the inflammatory effects of LTB4 are very similar to those of pancreatic duct obstruction and that inhibition of the rate-limiting enzyme in LTB4 biosynthesis, 5-LO, also inhibits pancreatic duct obstruction-induced pancreatitis in this model. Coupled with the previously demonstrated proinflammatory role of LTB4 in both animal and human intestinal inflammatory diseases30, these results suggest that LTB4 may be the most important endogenous lipoxygenase product activating TRPV1-mediated pancreatitis. Final resolution of this question awaits future studies involving direct measurement of tissue levels of the various TRPV1-activating eicosanoids in this and other pancreatitis models.
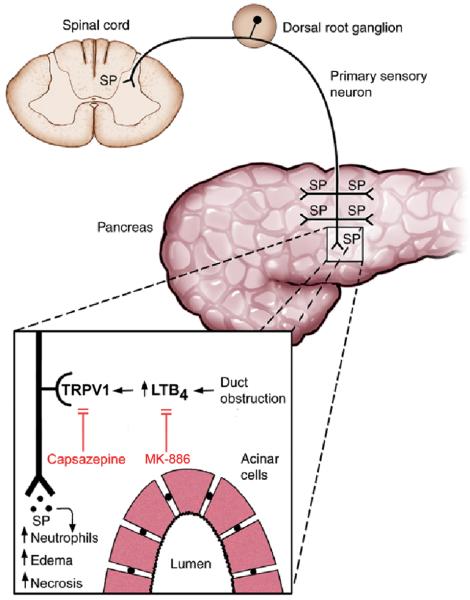
In summary, we have presented evidence that the inflammatory effects of duct obstruction in the pancreas may be mediated by generation of LTB4 and subsequent LTB4 activation of TRPV1 in primary sensory neurons (Fig. 7). This pathophysiological mechanism provides a conceptual basis for the potential treatment of pancreatic inflammatory diseases by inhibition of this pathway.
Figure 7.
A model of the proposed mechanism of common pancreatic-bile duct ligation on pancreatic inflammation. Duct obstruction causes the release of LTB4 locally within the pancreas. LTB4stimulates TRPV1 expressed in the plasma membrane of primary sensory nerves innervating the parenchyma of the gland. When stimulated, TRPV1 depolarizes the neurons resulting in the propagation of orthograde action potentials causing the release of proinflammatory neurotransmiters such as substance P (SP) in the pancreas to cause damage and retrograde action potentials causing the release of nociceptive neurotransmitters in the spinal cord to cause pain. The TRPV1 antagonist, capsazepine, blocks LTB4 stimulation of TRPV1. MK-886 reduces LTB4 levels by inhibiting 5-lipoxygenase activating peptide (FLAP). Both capsazepine and MK-886 ameliorate duct obstruction-induced pancreatitis. For simplicity and clarity, other mechanisms that may be involved in these processes such as protease activated receptor-2 (PAR2) activation of TRPV1 and co-release of calcitonin gene-related peptide (CGRP) with substance P have been omitted.
Figure 6.
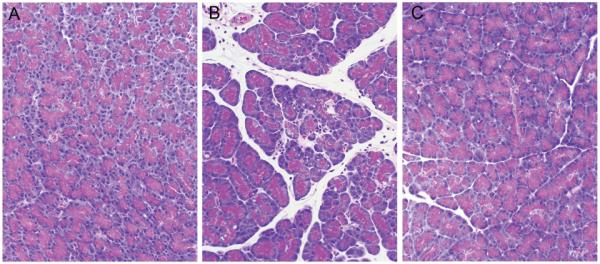
Representative photomicrographs of hematoxylin and eosin-stained pancreatic sections taken from control rats (left panel), rats treated with common pancreatic-bile duct ligation alone (middle panel), and rats pretreated with MK-886 before common pancreatic-bile duct ligation (right panel).
Acknowledgments
Grant Support: National Institutes of Health Grant DK-064213 (to R. A. L.)
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Steven R. Vigna, Depts. of Cell Biology & Medicine, Duke University Medical Center, Durham, NC.
Rafiq A. Shahid, Dept. of Medicine, Duke University Medical Center, Durham, NC.
Jaimie D. Nathan, Dept. of Surgery, Duke University Medical Center, Durham, NC.
Douglas C. McVey, Dept. of Medicine, Duke University Medical Center, Durham, NC.
Rodger A. Liddle, Dept. of Medicine, Duke University Medical Center, Durham, NC.
References
- 1.Liddle RA, Nathan JD. Neurogenic inflammation and pancreatitis. Pancreatology. 2004;4:551–559. doi: 10.1159/000082180. [DOI] [PubMed] [Google Scholar]
- 2.Liddle RA. The role of Transient Receptor Potential Vanilloid 1 (TRPV1) channels in pancreatitis. Biochim Biophys Acta. 2007 Aug;1772:869–878. doi: 10.1016/j.bbadis.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caterina MJ, Schumacher MA, Tominaga M, et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 4.Szallasi A, DiMarzo V. New perspectives on enigmatic vanilloid receptors. Trends Neurosci. 2000;23:491. doi: 10.1016/s0166-2236(00)01630-1. [DOI] [PubMed] [Google Scholar]
- 5.Caterina MJ, Julius D. The vanilloid receptor: A molecular gateway to the pain pathway. Ann Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- 6.Hwang SW, Cho H, Kwak J, et al. Direct activation of capsaicin receptors by products of lipoxygenases: Endogenous capsaicin-like substances. Proc. Natl Acad Sci U.S.A. 2000;97:6155. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saria A, Lundberg JM, Skofitsch G, et al. Vascular protein linkage in various tissue induced by substance P, capsaicin, bradykinin, serotonin, histamine and by antigen challenge. Naun Schmied Arch Pharmacol. 1983;324:212–218. doi: 10.1007/BF00503897. [DOI] [PubMed] [Google Scholar]
- 8.Figini M, Emanueli C, Grady EF, et al. Substance P and bradykinin stimulate plasma extravasation in the mouse gastrointestinal tract and pancreas. Am J Physiol. 1997;272:G785–G793. doi: 10.1152/ajpgi.1997.272.4.G785. [DOI] [PubMed] [Google Scholar]
- 9.Maa J, Grady EF, Kim EH, et al. NK-1 receptor desensitization and neutral endopeptidase terminate SP-induced pancreatic plasma extravasation. Am J Physiol Gastrointest Liver Physiol. 2000;279:G726–G732. doi: 10.1152/ajpgi.2000.279.4.G726. [DOI] [PubMed] [Google Scholar]
- 10.Grady EF, Yoshimi SK, Maa J, et al. Substance P mediates inflammatory oedema in acute pancreatitis via activation of the neurokinin-1 receptor in rats and mice. Br J Pharmacol. 2000;130:505–512. doi: 10.1038/sj.bjp.0703343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhatia M, Saluja AK, Hofbauer B, et al. Role of substance P and the neurokinin 1 receptor in acute pancreatitis and pancreatitis-associated lung injury. Proc Natl Acad Sci U.S.A. 1998;95:4760–4765. doi: 10.1073/pnas.95.8.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nathan JD, Peng RY, McVey DC, et al. Neonatal capsaicin administration ameliorates secretagogue-induced pancreatitis in rats. Surg Forum. 2001;52:23–25. [Google Scholar]
- 13.Nathan JD, Peng RY, Wang Y, et al. Primary sensory neurons - A common final pathway for inflammation in experimental pancreatitis in rats. Am J Physiol. 2002;283:G938–G946. doi: 10.1152/ajpgi.00105.2002. [DOI] [PubMed] [Google Scholar]
- 14.Nathan JD, Patel AA, McVey DC, et al. Capsaicin vanilloid receptor-1 mediates substance P release in experimental pancreatitis. Am J Physiol. 2001;281:G1322–G1328. doi: 10.1152/ajpgi.2001.281.5.G1322. [DOI] [PubMed] [Google Scholar]
- 15.Hutter MM, Wick EC, Day AL, et al. Transient receptor potential vanilloid (TRPV-1) promotes neurogenic inflammation in the pancreas via activation of the neurokinin-1 receptor (NK-1R) Pancreas. 2005;30:260–265. doi: 10.1097/01.mpa.0000153616.63384.24. [DOI] [PubMed] [Google Scholar]
- 16.Wick EC, Hoge SG, Grahn SW, et al. Transient receptor potential vanilloid 1, calcitonin gene-related peptide, and substance P mediate nociception in acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2006;290:G959–G969. doi: 10.1152/ajpgi.00154.2005. [DOI] [PubMed] [Google Scholar]
- 17.Noble MD, Romac J, Wang Y, et al. Local disruption of the celiac ganglion inhibits substance P release and ameliorates caerulein-induced pancreatitis in rats. Am J Physiol Gastrointest Liver Physiol. 2006;291:G128–G134. doi: 10.1152/ajpgi.00442.2005. [DOI] [PubMed] [Google Scholar]
- 18.Noble MD, Romac J, Vigna SR, et al. A pH-sensitive, neurogenic pathway mediates disease severity in a model of post-ERCP pancreatitis. Gut. 2008;57:1566–1571. doi: 10.1136/gut.2008.148551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zygmunt PM, Petersson J, Andersson DA, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- 20.Hanus L, Abu-Lafi S, Fride E, et al. 2-Arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB 1 receptor. Proc Natl Acad Sci U.S.A. 2002;98:3662–3665. doi: 10.1073/pnas.061029898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang SM, Bisogno T, Trevisani M, et al. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc Natl Acad Sci U.S.A. 2002;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stenson WF. Role of eicosanoids as mediators of inflammation in inflammatory bowel disease. Scand J Gastroenterol. 1990;172(Suppl):13–18. doi: 10.3109/00365529009091903. [DOI] [PubMed] [Google Scholar]
- 23.Sharon P, Stenson WF. Enhanced synthesis of leukotriene B4 by colonic mucosa in inflammatory bowel disease. Gastroenterology. 1984;86:453–460. [PubMed] [Google Scholar]
- 24.Lobos EA, Sharon P, Stenson WF. Chemotactic activity in inflammatory bowel disease. Role of leukotriene B4. Dig Dis Sci. 1987;32:1380–1388. doi: 10.1007/BF01296664. [DOI] [PubMed] [Google Scholar]
- 25.Stenson WF, Lobos E. Sulfasalazine inhibits the synthesis of chemotactic lipids by neutrophils. J Clin Invest. 1982;69:494–497. doi: 10.1172/JCI110474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharon P, Stenson WF. Metabolism of arachidonic acid in acetic acid colitis in rats. Similarity to human inflammatory bowel disease. Gastroenterology. 1985;88:55–63. doi: 10.1016/s0016-5085(85)80132-3. [DOI] [PubMed] [Google Scholar]
- 27.Wallace JL, MacNaughton WK, Morris GP, et al. Inhibition of leukotriene synthesis markedly accelerates healing in a rat model of inflammatory bowel disease. Gastroenterology. 1989;96:29–36. doi: 10.1016/0016-5085(89)90760-9. [DOI] [PubMed] [Google Scholar]
- 28.Zipser RD, Nast CC, Lee M, et al. In vivo production of leukotriene B4 and leukotriene C4 in rabbit colitis. Relationship to inflammation. Gastroenterology. 1987;92:33–39. doi: 10.1016/0016-5085(87)90836-5. [DOI] [PubMed] [Google Scholar]
- 29.Wallace JL, Keenan CM. An orally active inhibitor of leukotriene synthesis accelerates healing in a rat model of colitis. Am J Physiol. 1990;258:G527–G534. doi: 10.1152/ajpgi.1990.258.4.G527. [DOI] [PubMed] [Google Scholar]
- 30.McVey DC, Vigna SR. The role of leukotriene B4 in Clostridium difficile toxin A-induced ileitis in rats. Gastroenterology. 2005;125:1306–1316. doi: 10.1053/j.gastro.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 31.Peters-Golden M, Brock TG. 5-lipoxygenase and FLAP. Prostaglandins Leukot Essent Fatty Acids. 2003;69:99–109. doi: 10.1016/s0952-3278(03)00070-x. [DOI] [PubMed] [Google Scholar]
- 32.Folch E, Closa D, Prats N, et al. Leukotriene generation and neutrophil infiltration after experimental acute pancreatitis. Inflammation. 1998;22:83–93. doi: 10.1023/a:1022399824880. [DOI] [PubMed] [Google Scholar]
- 33.Cuzzocrea S, Rossi A, Serraino I, et al. 5-lipoxygenase knockout mice exhibit a resistance to acute pancreatitis induced by cerulein. Immunology. 2003;110:120–130. doi: 10.1046/j.1365-2567.2003.01715.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Bradley PP, Priebat DA, Christensen RD, et al. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 35.Dawra R, Ku YS, Sharif R, et al. An improved method for extracting myeloperoxidase and determining its activity in the pancreas and lungs during pancreatitis. Pancreas. 2008;37:62–68. doi: 10.1097/MPA.0b013e3181607761. [DOI] [PubMed] [Google Scholar]
- 36.Spormann H, Sokolowski A, Letko G. Effect of temporary ischemia upon development and histological patterns of acute pancreatitis in the rat. Pathol Res Pract. 1989;184:507–513. doi: 10.1016/S0344-0338(89)80143-8. [DOI] [PubMed] [Google Scholar]
- 37.Zingarelli B, Squadrito F, Graziani P, et al. Effects of zileuton, a new 5-lipoxygenase inhibitor, in experimentally induced colitis in rats. Agents Actions. 1993;39:150–156. doi: 10.1007/BF01998968. [DOI] [PubMed] [Google Scholar]
- 38.Toda A, Yokomizo T, Shimizu T. Leukotriene B4 receptors. Prostaglandins and Other Lipid Mediators. 2002;68-69:575–585. doi: 10.1016/s0090-6980(02)00056-4. [DOI] [PubMed] [Google Scholar]
- 39.Fretland DJ, Anglin CP, Widomski D, et al. Pharmacological activity of the second generation leukotriene B 4 receptor antagonist, SC-53228: Effects on acute colonic inflammation and hepatic function in rodents. Inflammation. 1995;19:503–515. doi: 10.1007/BF01539131. [DOI] [PubMed] [Google Scholar]
- 40.Ten Hove T, Drillenburg P, Wijnholds J, et al. Differential susceptibility of multidrug resistance protein-1 deficient mice to DSS and TNBS-induced colitis. Dig Dis Sci. 2002;47:2056–2063. doi: 10.1023/a:1019629013945. [DOI] [PubMed] [Google Scholar]
- 41.Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: Involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988;24:739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- 42.Docherty RJ, Yeats JC, Piper AS. Capsazepine block of voltage-activated calcium channels in adult rat dorsal root ganglion neurones in culture. Br J Pharmacol. 1997;121:1461–1467. doi: 10.1038/sj.bjp.0701272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu L, Simon SA. Capsazepine, a vanilloid receptor antagonist, inhibits nicotinic acetylcholine receptors in rat trigeminal ganglia. Neurosci Lett. 1997;228:29–32. doi: 10.1016/s0304-3940(97)00358-3. [DOI] [PubMed] [Google Scholar]
- 44.Szallasi A, Blumberg PM. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- 45.Kehrer JP, Biswal SS, La E, et al. Inhibition of peroxisome-proliferator-activated receptor (PPAR)alpha by MK886. Biochem J. 2001;356:899–906. doi: 10.1042/0264-6021:3560899. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Genovese T, Mazzon E, Di Paola R, et al. Role of peroxisome proliferator-activated receptor-alpha in acute pancreatitis induced by cerulein. Immunology. 2006;118:559–570. doi: 10.1111/j.1365-2567.2006.02393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luo M, Lee S, Brock TG. Leukotriene synthesis by epithelial cells. Histol Histopathol. 2003;18:587–595. doi: 10.14670/HH-18.587. [DOI] [PubMed] [Google Scholar]