Abstract
MicroRNAs are endogenous small non-coding RNAs that regulate gene expression and cancer development. A rare population of hepatocellular cancer stem cells (HSCs) holds the extensive proliferative and self-renewal potential necessary to form a liver tumour. We postulated that specific transcriptional factors might regulate the expression of microRNAs and subsequently modulate the expression of gene products involved in phenotypic characteristics of HSCs. We evaluated the expression of microRNA in human HSCs by microarray profiling, and defined the target genes and functional effects of two groups of microRNA regulated by IL-6 and transcriptional factor Twist. A subset of highly chemoresistant and invasive HSCs was screened with aberrant expressions of cytokine IL-6 and Twist. We demonstrated that conserved let-7 and miR-181 family members were up-regulated in HSCs by global microarray-based microRNA profiling followed by validation with real-time polymerase chain reaction. Importantly, inhibition of let-7 increases the chemosensitivity of HSCs to sorafenib and doxorubicin whereas silencing of miR-181 led to a reduction in HSCs motility and invasion. Knocking down IL-6 and Twist in HSCs significantly reduced let-7 and miR-181 expression and subsequently inhibited chemoresistance and cell invasion. We showed that let-7 directly targets SOCS-1 and caspase-3, whereas miR-181 directly targets RASSF1A, TIMP3 as well as nemo-like kinase (NLK). In conclusion, alterations of IL-6- and Twist-regulated microRNA expression in HSCs play a part in tumour spreading and responsiveness to chemotherapy. Our results define a novel regulatory mechanism of let-7/miR-181s suggesting that let-7 and miR-181 may be molecular targets for eradication of hepatocellular malignancies.
Keywords: microRNAs (miRNAs), human hepatocellular cancer stem cells (HSCs), Interleukin-6 (IL-6), Twist, chemotherapy, invasion
Introduction
Cancer stem cells (CSCs) are key players in the progression of cancer growth [1]. Present chemotherapy modalities eliminate the bulk of tumour cells, but cannot eliminate a core of these CSCs that have a high capacity for renewal [2]. Identification of these cells is the first step in the development of novel and improved therapeutic modalities [3].
miRNAs are small non-coding RNAs that are well suited to suppress previously active programs and, thereby, provide robustness to stem cell differentiation and transformation [4–7]. miRNAs identify their targets by base pairing of the seed sequence of the miRNA with complementary binding within the target mRNA's 3′ untranslated region (UTR). This targeting is carried out in synchronization with the RNA-induced silencing complex (RISC), and often results in either degradation or translational inhibition of the targets [8]. Recent studies have demonstrated that the microRNA pathway is essential for controlling self-renewal, differentiation and transformation in normal and CSCs [9–12].
Hepatocellular cancer (HCC) is the fifth most common cause of cancer and the third leading cause of cancer death worldwide [13]. Majority of the cases (>80%) are not curable because they are diagnosed at advanced stages. Progress in the identification of CSCs in solid tumours including those in GI malignancies has prompted the strong belief that HCC is a stem cell disease [14]. Studies have demonstrated that inflammatory cytokine IL-6 alters miRNA expression in human cancer cells [15, 16]. Expression analysis of HCC tumours showed marked activation of the IL-6 pathway, suggesting that HCC may develop from IL-6-driven CSCs with disrupted TGF-β signalling. Besides IL-6, the basic helix-loop-helix transcription factor Twist, an organizer of the epithelial mesenchymal transition (EMT), is a marker for HCC. Twist has been found to be correlated with metastasis in various cancers including human HCC [17]. Several microRNAs have been demonstrated to be the key mediators in Twist-regulated tumour metastasis [18]. However, the specific roles of microRNAs in HCC stem cell (HSC) development and progression, especially characteristic changes during cancer metastasis and chemo-resistance needs to be addressed. We postulated that specific transcriptional factors regulated miRNAs may contribute to tumour spread and chemoresistance by modulating the expression of gene products involved in phenotypic characteristics of CSCs such as cell migration, invasion and response to chemotherapy.
Material and methods
Cell lines and cultures
Human hepatocellular CSCs and parental HCC cells were provided by Celprogen Inc. (San Pedro, CA, USA). Three groups of HSCs were chosen for the studies from seven sets on the basis of self-renewal and real-time polymerase chain reaction (PCR) tests for the specific markers (Fig. 1). The selected group of cells was cultured in Human Liver Cancer Stem Cell Undifferentiated Media to keep the stem cell phenotype (HSC-SR), and in Human Liver Cancer Stem Cell Differentiated Media for 10 passages for differentiated progenitor cell phenotype (HSC-DF). Malignant HCC cells (HepG2) were obtained from the American Type Culture Collection (Manassas, VA, USA) and cultured as recommended by the supplier. Normal human liver stem cells were also obtained from Celprogen Inc.
Fig 1.

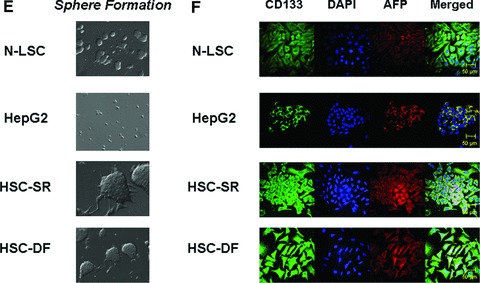
Characterization of Cancer Stem Cells from human HCC tissues. (A) Real-time analysis of Oct 4, CD 133, Nestin, telom-erase, SSEA-4, AFP and CEA mRNA expression in HCC stem cell under self-renewal condition (HSC-SR), under differentiation (HSC-DF) and HepG2 HCC cells. All the markers are significantly up-regulated in HCC cancer stem cells. *P < 0.05 when compared with HepG2 group. (B) FACS analysis of HCC stem cells on day 1 before cell sorting. Results are given as the percentage of Oct-4 and CD133+ cells in the total population. In the histograms, the red line represents staining with Oct-4 or CD133 mAb, and the green lines represent the isotype control-matched mAb. (C) Cell lysates were obtained and immunoblotting analysis was performed for Oct-4, CD133, α-fetoprotein (AFP), CK-7, CK-19 and EpiCam using specific antibodies. The blots were stripped and reprobed for β-actin as a loading control and for quantitation. (D) StemTAG™ Alkaline Phosphatase assays performed at passage 4–6 HSCs and LSCs. HSC-SR and LSCs are maintained in an undifferentiated stage on specific culture dishes, as indicated by the high AP activity, thus confirming the self-renewal within these cells. (E) Formation of tumour spheres in HCC stem cells. In 1% FCS culture media 70–90% of the HSC-SR and HSC-DF became adherent after 7 days, with a minority of floating cells forming spheres composed of three to five cells. After an additional 7 days, the floating spheres were expanded to contain 15–20 HSC-SR cells, with bright appearance and sharp edge. (F) Immunocytochemistry for CD133 and AFP was performed in Normal liver stem cells (N-LSC), HepG2 cells, hepatocellular cancer stem cells growing under self-renewal conditions (HSC-SR) and differentiation conditions (HSC-DF). An increase in CD133 expression along with the enhanced expression of AFP is observed in HSC-SR and HSC-DF cells.
miRNA array hybridization and analysis
RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA). Total RNA (5 μg) was reverse transcribed using biotin end-labelled random octamer oligonucleotide primers. Hybridization of biotin-labelled complementary DNA was performed using a custom miRNA microarray chip (ncRNA Program at Center for Targeted Therapy, M.D. Anderson Cancer Center, USA). The data from three samples for each cell type were further analysed by the BRB-ArrayTools software from National Cancer Institute.
Real-time PCR assays for mature miRNAs
microRNA was isolated as described previously [16] and the expression of specific mature miRNAs was confirmed using real-time PCR analysis using a TaqMan Human MicroRNA Assay kit (Applied Biosystems, Foster City, CA, USA).
Northern blot analysis
The Northern blot analysis was performed using MiRNA Northern Blot Assay Kit obtained from Signosis, Inc. (Sunnyvale, CA, USA).
Flow cytometry analysis and FACS sorting Oct-4 and CD133+ cells
HCC stem cells were collected and stained with PerCP-Cy5.5 and/or phycoerythrin (PE) tagged antibodies for 30 min. The staining was analysed by flow cytometry FACSCalibur (Becton Dickinson, San Jose, CA, USA). Oct-4 and CD133+ cells were sorted by DAKO cytomation (DAKO, Carpinteria, CA, USA) [19].
In vitro proliferation, migration, invasion and sphere formation assay
Commercial available kits were used for proliferation, migration, invasion and sphere formation assay in normal and malignant hepatic cells (details in Supporting Information).
Western blotting
Protein was extracted from cultured cells or homogenized tissues, and Western blot was performed as previously described [16]. Please see the Supporting Information for more details.
Xenograft model
Eight- to ten-week-old male SCID Berge mice were obtained from Charles River Laboratories (Wilmington, MA, USA) and fed food and water ad libitum. The mice were maintained in accordance with the Institutional Animal Care and Use Committee (IACUC) procedures and guidelines. HSC-SF (1000) and parental HCC cells (5 × 106 cells) were suspended in 0.25 ml of extracellular matrix gel, and the mixture was injected subcutaneously into the right and left flanks. At day 10 the tumour was visible, and then the doxorubicin treatment was started and the mice received the desired concentration of the drug intraperoteneal three times per week for 14 days. At the end of the 14 days, the tumour was dissociated into single cell suspension and assayed with Almar Blue to determine percent inhibition of the drug. For analysis of chemotherapy-induced miRNA and mRNA expression in vivo, the xenografts were excised, and tissue was homogenized. An aliquot of the lysates was used for miRNA isolation as described earlier and the remainder used for mRNA expression studies.
Statistical analysis
Data are expressed as the mean ± S.E. from at least three separate experiments performed in triplicate, unless otherwise noted. The differences between groups were analysed using a double-sided Student's t-test when only two groups were present and the null hypothesis was rejected at the 0.05 level unless otherwise specified. Please see Supporting Information for more detailed information of this section.
Results
Purification and characterization of pluripotency and self-renewal capacity in HCC stem cells
The HCC stem cells (HSCs) obtained from Celprogen Inc. were originally isolated from liver cancer tissues using Oct-4, CD133, Nestin, telomerase, SSEA-4, alkaline phosphatase, α-fetoprotein (AFP), carcino-embryonic antigen (CEA) as positive markers [3, 14, 20]. Three groups of HSCs were carefully chosen from seven sets based on the self-renewal and real-time PCR tests for the specific markers. The enhanced expression of Oct-4, CD133, Nestin, telomerase, SSEA-4, AFP and CEA were verified by real-time PCR analysis using different primer sets (Fig. 1A). HSCs were further purified by flow cytometry after two culture passages in undifferentiated medium. We directed our purification for cells expressing two of eight CSC markers provided by the company: Oct-4 and CD133. Both Oct-4 and CD133 have been shown to be expressed in liver cancer as well as embryonic stem cells. HSCs were enriched into a CD133+ fraction of Oct-4+ cells (Fig. 1B). To determine the self-renewal properties of HSCs, we utilized two approaches using three cell lines—HSCs, HepG2 HCC cells and normal human liver stem cells (N-LSC) that are isolated from adult human liver tissue using AFP, CD34 and alkaline phosphatase as specific markers [21]. In the first approach, we performed western blotting, all of which showed that both HSCs growing under self-renewal conditions (HSC-SR) and differentiation conditions (HSC-DF) had substantially higher levels of the representative markers of self-renewal than the HepG2, which includes CK-19 and newly discovered malignant hepatic stemness marker EpCAM [20] (Fig. 1C and F). Alkaline phosphatase assays, performed to measure self-renewal within these cells (Fig. 1D), revealed that the levels of self-renewal in HSCs are 12 ± 3 times higher than HepG2 cells, along with the significant enhanced sphere formation ability in HSCs (Fig. 1E). Furthermore, the levels of self-renewal in HSCs under differentiation conditions were less than half of that in HSCs under self-renewal conditions.
To provide other potential mechanisms to explain the CSC effect of HCC progression, we performed real-time PCR-based superarray analysis. The Human Cancer & Estrogen Receptor Signalling PCR Array as well as Cancer Pathway Finder PCR Array were chosen because it contains 168 key genes (84 × 2), representing the 10 cancer-related biological pathways. The array groups also included genes associated with cancer cells’ invasion and response to chemotherapy. We found that 7 of 168 genes were dramatically up-regulated in HSCs related to HepG2 controls, whereas we could also detect several down-regulated genes. The two most up-regulated genes are IL-6 and Twist (Fig. 2A and B). Up-regulation of those genes was also evaluated by reverse transcriptional real-time PCR, which confirmed array data (Fig. 2C). Activated Stat3 was found in HSC cells accompanied by reduced level of TIMP-3. Interestingly, mesenchymal marker N-Cadherin (CDH2) was also overexpressed in HCC stem cells (Fig. 2D). Furthermore, the human liver cancer tissue array showed the signal intensity was strong (+++) or positive (++) in 20 of 24 HCC tissues for IL-6 and 17 of 24 HCC tissues for Twist (Fig. S1).
Fig 2.

The expressions of Twist and IL-6 are up-regulated in HCC cancer stem cells. (A) Relative gene expression profile between HSC stem cells (HSC-SR) versus HepG2 cells is shown. The expression of a panel of diverse cancer-associated genes was evaluated by real-time polymerase chain reaction (PCR) using Human Cancer & Estrogen Receptor Signalling PCR Array (#PAHS005, A) as well as Cancer Pathway Finder PCR Array (#PAHS033, B) from SABioscience Corporation (Frederick, MD, USA). Gene expression relative to glyceraldehyde-3-phosphate dehydrogenase was plotted as the Volcano Plots, depicting the relative expression levels (Log2) for selected genes in HSC-SR versus HepG2 control panels (A and B, Top). The relative expression levels and P values for each gene in the related samples were also plotted against each other in the scatter plot (A and B, Bottom). IL-6 and Twist are the most up-regulated genes among the 10 cancer signalling pathways in HCC stem cells. (C) Total RNA was extracted from N-LSC, HepG2, HSC-SR, HSC-DF cells and Twist and IL-6 mRNA expressions were assessed by quantitative real-time PCR. The relative mRNA expression was normalized to expression of N-LSC as percentage of control. *P < 0.05 when compared with mRNA expression of N-LSC and HepG2 cells. (D) The expression of phosphor-Stat3, Twist and downstream protein N-Cadherin CDH2) is up-regulated in HSCs. Western blots of cell lysates were performed and sequentially probed with antibodies against Twist, P-Stat3, CDH2 and β-actin as a loading control in hepatocytes and HCC stem cells as indicated. Representative immunoblots are shown.
miRNAs are differentially expressed in human hepatocellular cancer stem cells
We then compared miRNA expression between HCC CSCs and HepG2/N-LSCs. The pattern of miRNA expression in HCC CSCs was markedly different from that of either normal human liver stem cells or the HepG2 HCC cells (Fig. 3A). The expression of a group of miRNA was differentially increased in HSC cells. These include the human let-7 and miR-181 family members, all of which were expressed >4.0-fold in HSC-SR and HSC-DF CSCs compared with HepG2 HCC cells as well as normal liver stem cells (Fig. 3B). Aberrant expressions of let-7a, let-7b and miR-181a were verified by Northern blot (Fig. 3A) as well as real-time PCR analysis (Fig. 3B). Furthermore, silencing IL-6 and Twist in HSCs significantly reduced let-7a and miR-181a expression respectfully, but not vice versa (Fig. S2), implicated the specific link between IL-6 and let-7, as well as Twist and miR-181 in tumour stem cell behaviour.
Fig 3.

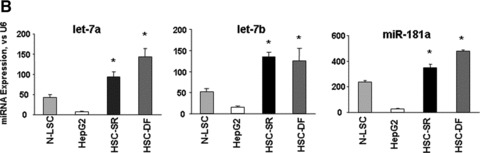
miRNA expression profiles in human HCC cancer stem cells [under self-renewal (SR) or differentiation (DF)], malignant and non-malignant hepatocytes. (A) miRNA was isolated and profiling by hybridization to miRNA-specific probes on epoxy-coated slides. Cluster analysis identifies a group of miRNA that are increased in expression in HCC cancer stem cells (HSC-SR and HSC-DF) and reduced in hepG2 cells when compared to normal human liver stem cells (Cluster 2). An enlarged view of this group, containing 11 miRNAs including members of let-7 and miR-181 family is shown. Lower middle panel illustrated representative Northern blot analysis of let-7a and miR-181a using total RNA isolated from four cell lines. This cluster of miRNAs are among the group, which increased in HSC-SR by fourfold, and P < 0.05 when compared to HepG2 cells (lower right panel). (B) The expressions of mature let-7a, let-7b and miR-181a miRNAs were validated using real-time PCR and correlated with the pattern in the miRNA array. Data represent mean ± S.E. from four separate experiments.
Resistance of HSCs cells to standard chemotherapy
HCCs are commonly resistant to treatment because the malignant cells survive chemotherapy [19]. To explore the possible role of CSC cells in the development of chemoresistance, cultured HSC-SR, HSC-DF, HepG2 and N-LSC cells were treated with sorafenib and doxorubicin and the IC50 was analysed by MTS assay. Doxorubicin has been considered to be the most effective single agent and the multi-kinase inhibitor sorafenib has recently been approved for the therapy of advanced HCC [22]. Sorafenib acts by inhibition of the RAF/MEK/ERK pathway and down-regulation of MCL-1, leading to a disruption of survival signals in HCC cells [22, 23]. As shown in Figure 4A–D, IC50s of HSCs in two chemotherapeutic reagents tested are significantly higher than HepG2 HCC cells as well as normal liver stem cells, suggesting that HCC stem cells have greater chemoresistance in vitro. In addition, HSC xenograft tumours in nude mice are more resistant to doxorubicin treatment than the control HCC xenografts (Fig. 4E). Enhanced CD133 and let-7a expressions are observed in doxorubicin-treated HSC xenograft tumours when compare to controls (Fig. 4F and G), further implicated a functional link between CD133/let-7a and tumour stem cell phenotypes.
Fig 4.

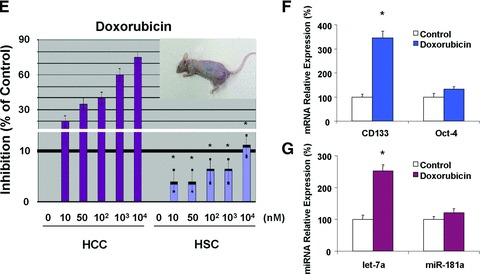
HCC stem cells are resistant to conventional chemotherapy. (A, C) After serum starvation of cultured cells overnight, sorafenib and doxorubicin were added at various concentrations (10−3–10−10 M) and cell viability was assessed after 72 hrs. IC50 graph of high-content image analysis of human hepatocytes and HCC stem cells treated by sorafenib and doxorubicin was illustrated. Quantitative data of MTS assay in the sorafenib treated cells were calculated for IC50 with XLfit software, which is marked in the middle of the box. (B, D) IC50 of sorafenib and doxorubicin in hepato-cytes and HCC stem cells is illustrated in bar graph. HCC stem cells under either self-renewal (HSC-SR) or differentiation (HSC-DF) are more resistance to sorafenib and doxorubicin than HepG2 cells and normal liver stem cells. IC50 results are expressed as the mean ± S.E. of eight different experiments. (E) Groups of five SCID Berge mice were selected 8–10 weeks old per treatment group. The human HSC-SRs (1000) as well as parental HCC cells (5 × 106) were injected subcutaneously and allowed to engraft for 10 days. At day 10 the tumour was visible, and then the doxorubicin treatment was started and the mice received the desired concentration of the drug intraper-oteneal three times per week for 14 days. At the end of the 14 days the tumour was dissociated into single cell suspension and assayed with Almar Blue to determine percent inhibition of the drug. (F, G) Differential expression of CD133 and let-7a in vivo following treatment with doxorubicin by real-time PCR is shown. Data represent percentage change in expression in doxo-cibicin-treated tumours compared with controls. *P < 0.05 relative to controls.
To identify further the cell subpopulations that contribute to HCC chemoresistance, we studied the chemosensitivity of Oct-4+CD133+ and Oct-4−CD133− tumour stem cell subpopulations. Oct-4+CD133+ cells showed much higher expression of IL-6 than Oct-4−CD133− HSCs (Fig. 5A). Because chemotherapy-induced cell killing is generally attributed to mitotic cell death, soft agar clonogenic assays to assess the replicative competence of sorafenib- and doxorubicin-treated subpopulations were performed. Although no significant difference was observed between the two subpopulations in the absence of two reagents, Oct-4+CD133+ cells isolated from HSCs cells were more resistant to sorafenib and doxorubicin treatment than were corresponding Oct-4−CD133− cells (Fig. 5B and C). To determine the role of IL-6 signalling in mediating chemoresistance of HCC subpopulation, purified Oct-4+CD133+ cells were infected with retroviral siRNA against IL-6 and allowed to grow in Matrigel for 1 week in the presence or absence of sorafenib and doxorubicin. As expected, transduction of the cells with interfering siRNA significantly reduced IL-6 expression (Fig. 5A) and, importantly, diminished chemoresistant colonies (Fig. 5B and C). These results indicate that IL-6 signalling was required for protection of Oct-4+CD133+ HCC stem cells from chemotherapeutics-induced cytotoxicity.
Fig 5.
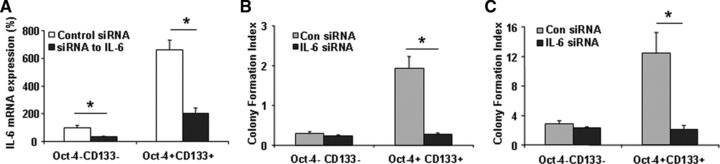
IL-6 is involved in HCC stem cells survival. (A) Total RNA was extracted and IL-6 expression was assessed by quantitative real-time PCR. *P < 0.05 when compared with Control siRNA group. (B, C) Oct-4+CD133+ and Oct-4−CD133− HCC stem cells were seeded in 96-well plates (10,000 per well) in specific medium with 20% FBS with sorafenib (B) and doxorubicin (C, 10 μM). Cells were incubated for 7 days in a humidified incubator at 37°C, after which the total number of colonies was labelled by Calcein AM (Molecular Probes, Eugene, OR, USA) and quantified fluorometrically. Colony survival rate of Oct-4+CD133+ and Oct-4−CD133− HCC stem cells were shown after 7-day exposure to sorafenib and doxorubicin (10 μM) in soft agar. The surrogate cancer stem cell assay has demonstrated that silencing IL-6 significantly reduced transformed HCC cancer stem cell survival rate.
Let-7a and let-7b contributes to survival signalling by IL-6 in HSCs
We next evaluated the effect of let-7a and let-7b inhibition on the response to chemotherapy in vitro. The effects of the anti-let-7a and anti-let-7b were assessed by cotransfecting with the pRL-Tk let-7a and let-7b firefly luciferase constructs that contains two let-7a and let-7b sites in the 3′-UTR of the firefly luciferase reporter. The significant increases in luciferase activity confirmed the efficacy of anti-let-7a and let-7b under the conditions used for our studies (Fig. 6A and B). Meanwhile, introduction of siRNA to IL-6 in HSC cells also up-regulates the luciferase activity, suggested IL-6 directly enhanced let-7a and let-7b expressions (Fig. 6C). Cytotoxicity in response to diverse agents such as sorafenib and doxorubicin was enhanced by pre-incubation with antisense inhibitors to let-7a and let-7b. IC50s to all two reagents have significantly reduced in response to chemotherapy in HSC cells pre-incubated with anti-let-7a when compared with a control inhibitor (Fig. 6D and E). Similarly, an increase in activated caspase-3 expression, the predicted target gene of let-7 family members, was noted in response to anti-let-7a in Western blots from sorafenib-treated HSC cells. Furthermore, anti-let-7a treatment in HCC CSCs has successfully increased SOCS1 expression, another predicted target gene of let-7a, and subsequently reduced Stat3 activity (Fig. 6F). Thus, inhibition of let-7a and let-7b increases chemotherapy-induced apoptosis in HCC CSCs, which may be involved in enhanced expression of Caspase-3 as well as SOCS1-regulated Stat3 pathway.
Fig 6.
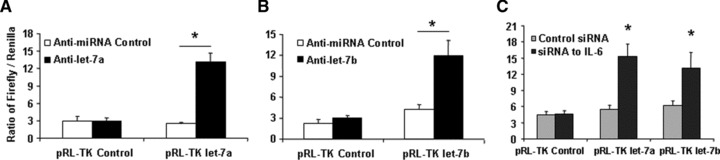

MicroRNA let-7 is involved in the HCC stem cell resistance to chemotherapy. (A, B) To validate the efficacy of the anti-let-7 inhibitor, HCC stem cells plated (2 × 106 cells/well) in six-well plates were transfected with 1 μg of pRL-TK let-7a or let-7b (firefly luciferase construct), 1 μg of pRL-TK (Renilla luciferase construct), and either anti-let-7a (A), anti-let-7b (B) or control inhibitor. Luciferase assays were performed 48 hrs after transfection. The anti-let-7a (anti-let-7b) inhibitor directly blocked the effect of endogenous let-7a (let-7b) on the luciferase reporter. (C) siRNA to IL-6 or control was trans-fected with 1 μg of pRL-TK let-7a or let-7b (firefly luciferase construct), 1 μg of pRL-TK (Renilla luciferase construct). Dual-luciferase assay has demonstrated that silencing IL-6 significantly increased the miRNA expressions of let-7 family. (D, E) Human hepatocytes and HCC stem cells were seeded in a 96-well plate and incubated with different concentrations of sorafenib. The MTS assays were performed 72 hrs after treatment. The data were expressed as percentage of control group. The IC50 values were calculated with Excel XLFit program and marked in the middle of the box. (D) Illustrates the detailed IC50 analysis in different cell lines with anti-let-7a treatment whereas E exemplified IC50 results expressed as the mean ± S.E. of eight different experiments. Silencing of let-7a significantly sensitizes HCC cancer stem cells to sorafenib treatment. (F, G) The expression of SOCS1 and downstream kinase is regulated by let-7a. Western blots of cell lysates were performed and sequentially probed with antibodies against SOCS1, Caspase-3, phospho and total Stat3, and tubulin as a loading control in HepG2 or HSC-SR cells transfected with let-7a mimics or inhibitors with related controls. Representative immunoblots (F) and quantitative data (G, mean ± S.E.) from four separate blots are shown.
Oct-4+CD133+ HSCs demonstrate enhanced migration and invasion in vitro
It has been suggested that tumour invasion and metastasis may be mediated by the CSC population [43, 44]. We observed human HCC cells and stem cells by light microscopy to examine invasion of the Matrigel. Movements of malignant cells in Matrigel were associated with formation of peripheral ruffles. Compared to HepG2 and N-LSC cells, HSCs had more cells with membrane ruffles, and cells were more invasive in the Matrigel (Fig. 7A and B). In vitro assays were used to compare sorted subpopulations from the perspective of differences in cell migration, and invasion (Fig. 7C and D). Oct-4+CD133+ cells were observed to be significantly more migratory towards serum (Fig. 6C), and significantly more invasive through ECM gel (Fig. 7D) than respective Oct-4−CD133− cell subsets (P < 0.001).
Fig 7.
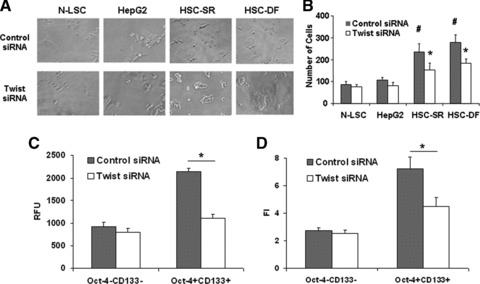
Twist is involved in cell motility and invasion potentials if HCC stem cells. (A) Light microscopy was used to document morphologic differences between the Twist and control siRNA treated N-LSC, HepG2 and HSCs. HSCs displayed an aggressive phenotype, which became elongated, and forming the leading edge pseudopodia. Silencing Twist in HSCs significantly reduced the percentage of cells with invasive phenotypes. (B) Matrigel invasion assay showing the invading capability of normal and malignant liver stem cells with and without Twist silencing. (C, D) Cell migration and invasion was assessed in Oct-4+CD133+ and Oct-4−CD133− cells with or without Twist silencing. Cell migration was expressed as arbitrary fluorescence units. Cell invasion was assessed in 96-well plates pre-coated with extracellular matrix. RFU is the absolute florescence intensity value and FI is the florescence intensity after normalization with the standard curve. The mean and standard error from four separate experiments are illustrated. #P < 0.05 when compare with HepG2 and N-LSC groups. *P < 0.05 when compared with siRNA controls.
To determine the effect of Twist overexpression was because of a direct effect on tumour invasion or alternatively was because of an increase in CSCs, we tested the ability of Oct-4+CD133+ cells transfected with siRNA to Twist and Oct-4−CD133− populations of transfected wild-type Twist plasmid in HSC cells to invade matrigel. The Oct-4+CD133+ subpopulations were considerably more invasive than Oct-4−CD133− populations. Furthermore, silencing Twist significantly reduced the percentage of cells with invasive phenotypes (Fig. 7A and B), as well as the invasive index in Oct-4+CD133+ HSC cells (Fig. 7C) whereas overexpression of Twist resulted in 4.55 ± 0.68-fold increase in invasiveness in the Oct-4−CD133− populations.
miR-181 family members are involved in Twist-dependent effects in HSCs invasion
Twist overexpression has been demonstrated to correlate with hepatocellular carcinoma metastasis through different mechanisms [24, 25]. Twist transcriptionally regulated gene expression through the promoter region containing an E-Box (a promoter element known to be bound by Twist [25, 26]). Interestingly, several binding E-boxes element (CATCTG) known to be essential for Twist transcriptional activity were found in the promoter regions of miR-181 (Fig. 8A). Transfection of siRNA to Twist in HSC cells significantly reduced miR-181a expression by real-time PCR binding assay (Fig. 8B). To further evaluate the contribution of miR-181 to oncogenic effects of HSCs, we assessed the impact of miR-181 inhibition on HSC cell in vitro. Anti-miR-181 inhibitions have significantly reduced HSC cell invasion ability by 0.6 ± 0.1-fold. Moreover, the specific miRNA–protein binding assay has demonstrated that Twist–miR-181 binding activity was significantly enhanced in HCC stem cells (Fig. 8C and D). In combination, these studies define an important role for miR-181 as a mediator of the oncogenic effects of Twist on CSC invasion in human HCC.
Fig 8.
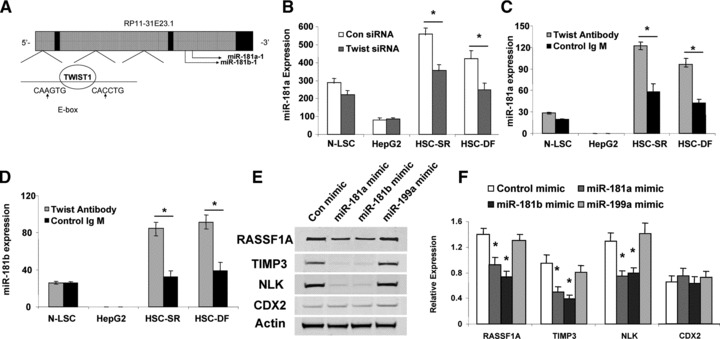
Regulation of miR-181 expression by Twist in HCC stem cells. (A) The schematic diagrams of miR-181 genes showing the Twist1 binding e-boxes are located in 5′-promoter regions of miR-181a-1/miR-181b-1. (B) Real-time PCR confirmed reduced expression of mature miR-181a in HSC-SR and HSC-DF cells in Twist silencing group when compared with expression in their respective controls. (C, D) Total RNAs were isolated using Magna RIP™ RNA-Binding Protein Immunoprecipitation Kit and real-time PCR assay was carried out using Taqman MicroRNA Assay kit. Increased miR-181a and miR-181b binding with Twist were observed in HCC cancer stem cells under self-renewal or differentiation condition. *P < 0.05 when compared with respective controls. (E, F) Overexpression of miR-181a and miR-181b inhibited RASSF1A, TIMP3 and NLK protein expressions in HSCs. Representative immunoblots (E) and quantitative data (F, mean ± S.E.) from four separate blots are shown. *P < 0.05 relative to expression in controls.
Identification of the target proteins for let-7 and miR-181
Based on 3′-UTR sequence analysis and prediction algorithms, several proteins can be potentially targeted by let-7 family members. Interestingly, SOCS1 and Caspase-3, two well-characterized genes involved in cancer cell survival, were identified as two such putative targets of almost all the members of let-7 family using the TargetScan (http://targetscan.org/) and miRBase (http://microrna.sanger.ac.uk/) databases. SOCS1 and Caspase-3 (CASP3) are both significantly down-regulated in HCC stem cells (Fig. 1C). To verify that SOCS1 and CASP3 targets of translational regulation by let-7 in HSCs, we performed studies using luciferase reporter constructs containing the let-7 recognition sequence (Fig. 9A, top) from the 3′-UTR of SOCS1 and CASP3 inserted downstream of the luciferase gene. Transfection with let-7a and let-7b mimics decreased reporter activity in HSCs, whereas miR-181a mimic failed to show any effects. However, when these studies were repeated with reporter constructs containing random mutations in the recognition sequence, the effects of reporter deactivation by let-7a and let-7b mimics were abolished (Fig. 9A, bottom). Moreover, the increases in SOCS1 and CASP3 expressions occurred after 3 days in cells incubated with anti-let-7a inhibitor (Fig. 6F). Concomitant with enhanced CASP3 expression, there was a reduction of cell survival (Fig. 6D and E). Taken together, these findings indicate that SOCS1 and CASP3 are the biologically relevant targets of let-7a and let-7b.
Fig 9.


Targeting of SOCS1 and CASP3 3′-UTR by let-7, as well as RASSF1A, TIMP3 and NLK by miR-181a. Firefly luciferase activity was normalized to Renilla luciferase activity for each sample. Top: The sequences of the mutated target sites of SOCS1, CASP3, RASSF1A, TIMP3 and NLK with mutations to disrupt base pairing between the specific binding sites and microRNAs are also displayed. Bottom: The decreases in relative firefly luciferase with pMIR-WT-luc compared with the pMIR- MUT-luc constructs in let-7a and let-7b overexpressed cells (A), as well as miR-181a overexpressed cells (B) confirms that the SOCS1 and CASP3 (RASSF1A, TIMP3 and NLK) complementary sequence in the 3′-UTR and the genes are the direct targets of modulation by let-7a and let-7b (miR-181a). The data represent the mean and standard errors from four independent transfections. *P < 0.05 relative to respective controls.
Recent studies have shown that TIMP-3 and NLK may be directly targeted by miR-181 [20, 27]. miR-181 is also predicted to target tumour suppressor gene RASSF1A. Thus, we postulated that alterations in miR-181 expression could represent a mechanism by which Twist modulates RASSF1A, TIMP-3 and NLK expression. Studies were performed to evaluate and experimentally validate RASSF1A, TIMP-3 and NLK as the bona fide targets of translational regulation by miR-181a. First, we verified that the RASSF1A, TIMP-3 and NLK 3′-UTR is the targets for miR-181s using luciferase reporter constructs containing the shared recognition sequence from the 3′-UTR of RASSF1A, TIMP-3 and NLK inserted downstream of the luciferase gene (Fig. 9B, top). Transfection with mimics of miR-181a significantly modulated reporter activity in HCC stem cells (Fig. 9B, bottom). Next, the studies were repeated with random mutations in the shared recognition sequence, which resulted in abolition of the reporter activation by miR-181a mimics (Fig. 9B, bottom). Thereafter, we assessed whether ectopic expression of individual miR-181a and miR-181b sequences induces down-regulation of endogenous RASSF1A, TIMP-3 and NLK protein expression. Transfection of HSCs with either miR-181a or miR-181b decreased RASSF1A, TIMP-3 and NLK expressions after 3 days (Fig. 8E). Meanwhile, transfection with a mimic to miR-199a, a miRNA that is regulated in a Twist-dependent manner but which does not have complementary sequences to the RASSF1A, TIMP-3 and NLK 3′-UTR, did not significantly alter the expression of RASSF1A, TIMP-3 and NLK with a relative expression of 1.06 ± 0.23-fold, 0.91 ± 0.21-fold and 1.02 ± 0.15-fold of controls, respectively. In summary, these data confirm that RASSF1A, TIMP-3 and NLK are the biologically relevant targets of miR-181a.
Discussion
Hepatocellular carcinoma is a complex genetic disease caused by the accumulation of mutations that lead to the dysregulation of gene expression and to uncontrolled cell proliferation [28]. miRNAs can contribute to hepatic oncogenesis, functioning as tumour suppressors (e.g. miR-122a and miR-125a), or as oncogenes (e.g. miR-21, the miR221 and miR-143). Consequently, events activating or inactivating miRNAs are now viewed as cooperating with abnormal protein-coding genes in human hepatic tumourigenesis [29]. Our comprehensive investigation of IL-6 and Twist-dependent miRNAs as both potential identification markers and therapeutic agents in HCC stem cells has ultimately filled important gaps in our knowledge of the mechanisms of CSCs in hepatocellular malignancies and may perhaps address critical barriers to progress in the treatment of highly chemoresistant and metastatic tumours in HCC patients.
Expression analysis of HCC tumours showed marked activation of IL-6 pathway, suggesting that HCC may develop from IL-6-driven CSCs with disrupted TGF-β signalling [30]. Suppression of IL-6 signalling through the generation of mouse knockouts with a positive IL-6 regulator, inter-α-trypsin inhibitor heavy chain 4, resulted in a reduction of HCC in elf+/− mice [30], thereby demonstrating a link between two major signalling pathways in the development of cancer and suggesting a possible therapeutic target. Other studies have found overexpression of IL-6 in chemotherapy-resistant breast, lung, leukaemic and colonic cancer cells [31]. Because IL-6 act as an anti-apoptotic ligand during malignant transformation, and is overexpressed in liver CSCs, this could be the reason why liver CSCs are resistant to chemo- and radiation therapy resistance. In addition, Twist, an organizer of EMT and an HCC marker, has been found to regulate the expression of specific miRNA cluster [18, 26]. The identification of alterations in transcriptional-regulated miRNA expression in hepatocellular CSCs is likely to be essential to understand the mechanisms and role of altered gene expression and their mediated ‘stemness’ in human malignancies.
Different from the normal pluripotent state, initiation of oncogenic cellular differentiation is associated with aberrant expression of miRNA populations [32, 33]. Multiplicity (one miRNA targeting more than one gene) and cooperation (one gene targeted by several miRNAs) have been demonstrated recently in normal and tumour stemness. There is increasing evidence for autoregulatory interactions involving let-7 and miR-181 in the signalling pathways that control stem cell maintenance and differentiation. Inhibition of the let-7 family has been demonstrated to promote de-differentiation of somatic cells to induced pluripotent stem cells [10]. In ES cells, the pluripotency factor Lin-28, a target gene for let-7, acts as a specific inhibitor of let-7 nuclear and cytoplasmic processing [34–37]. Lin-28 interaction with the Let-7 precursor loop mediates regulated microRNA processing. Within the biogenesis pathway, Dicer was recently shown to be another let-7 target [38], a mechanism proposed to account for the regulation of more than 1000 proteins upon let-7 overexpression in cancer cells [39]. Therefore, aberrant expressions of let-7 family in CSCs may promote the malignant transformation and creation of oncogenic phenotypes. We now report that Socs1 and Caspase-3, two let-7 target genes specific to hepatocellular CSCs, encodes a negative inhibitor of the miRNA pathway. Our results show that Socs1 physically interacts with essential components of the Jak-Stat3 effector complex, the confirmed downstream of IL-6 dependent signalling pathway. Focusing on let-7 family members, we show that Socs1 and Caspase-3 are the direct targets that mediate the survival of CSCs.
miR-181s are components of the molecular circuitry that controls vertebrate development. The roles of miR-181 in cellular differentiation show a discrepancy with evidence as both positive and negative regulators of this phenotype, but their mechanisms remain unclear. Enhanced miR-181 levels are accompanied by various organ differentiation [40, 41], whereas miR-181 expression slowly declines after the specific organ terminal differentiation [41]. Meanwhile, the roles of miR-181 in human carcinogenesis have been explored recently. Similar to the cellular differentiation process, miR-181s have been demonstrated to function both as oncogenes and tumour suppressors depending on the origin of tissues and cells. Accordingly, miR-181 overexpression has been observed in breast [42], colon tumours [43] and pancreatic cancer [44]. The down-regulation of this miRNA has also been noticed in gliomas [45] and aggressive CLL [46]. Furthermore, the aberrant expression of miR-181b was also seen in patients with NASH [47] and HCCs [20], demonstrating that this miRNA has important role in liver injury and cancer development. In our study, we found that miR-181a and miR-181b are up-regulated in hepatocellular CSC populations, implying that miR-181 functions in maintaining an undifferentiated state of CSCs. Meanwhile, miR-181s are the critical mediators of Twist-regulated CSC invasion, which may be through repressing its target TIMP3. Recurrence and metastasis are associated commonly with a poor prognosis, and therefore targeting these mechanisms may lead to more effective treatment for HCC patients. CSCs are also postulated to be involved not only in carcinogenesis but also in tumour invasion and formation of metastases. There is considerable interest in therapy specifically targeting key signalling pathways involved in cancer stemness such as the Wnt/β-catenin pathway, which is regulated by NLK, the confirmed target gene of miR-181a in our study.
In summary, we have demonstrated a subset of highly chemoresistant and invasive hepatocellular CSCs with aberrant expressions of cytokine IL-6 and transcription factor Twist, as well as their regulated let-7 and miR-181 family members. Notably, inhibition of IL-6/let-7 increases the chemosensitivity of HSCs to sorafenib and doxorubincin whereas silencing of Twist/miR-181 led to a reduction in HSCs motility and invasion. Moreover, we have proved that let-7 could directly target SOCS-1 and Caspase-3, whereas miR-181 could directly target RASSF1A, TIMP3 as well NLK (Fig. 10). Therefore, the modulation of aberrantly expressed miRNA in HSCs may be a useful strategy to limit CSC differentiation, invasion or improve responses to cytotoxic therapies. Furthermore, the identification of specific miRNA gene targets that are involved in tumour cell stemness such as SOCS1, Caspase-3, RASSF1A, NLK, TIMP3; elucidation of the mechanisms by which they are regulated; and their functional effects will provide potential targets to reduce chemoresistance and metastasis. Knowledge of specific processes that are regulated in a miRNA manner and identification of critical targets for individual miRNA in HCC stem cells will yield useful information and novel insights into the mechanisms of tumourigenesis, chemoresistance as well as metastasis in HCC and other cancers.
Fig 10.
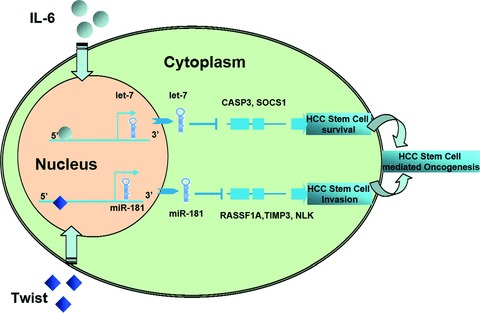
IL-6/Twist promotes hepatocellular cancer stem cell survival/invasion by activation of miRNA-dependent signalling pathways.
Acknowledgments
This study was supported by NIH grants (NIH RO1-DK054811) and the Veterans Affairs (VA) Merit Award to G.A. (NIH R01-DK081442), S.G. (NIH K01-DK078532), S.D.
Conflict of interest
The authors confirm that there are no conflicts of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Aberrant expression of IL-6 and Twist in human HCC tumour sections.
IL-6 and Twist specifically regulated let-7 and miR-181 expressions in HCC stem cells.
References
- 1.Adams JM, Strasser A. Is tumor growth sustained by rare cancer stem cells or dominant clones? Cancer Res. 2008;68:4018–21. doi: 10.1158/0008-5472.CAN-07-6334. [DOI] [PubMed] [Google Scholar]
- 2.Alvero AB, Chen R, Fu HH, et al. Molecular phenotyping of human ovarian cancer stem cells unravels the mechanisms for repair and chemoresistance. Cell Cycle. 2009;8:158–66. doi: 10.4161/cc.8.1.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mishra L, Banker T, Murray J, et al. Liver stem cells and hepatocellular carcinoma. Hepatology. 2009;49:318–29. doi: 10.1002/hep.22704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosa A, Brivanlou AH. microRNAs in early vertebrate development. Cell Cycle. 2009;8:3513–20. doi: 10.4161/cc.8.21.9847. [DOI] [PubMed] [Google Scholar]
- 5.Sartipy P, Olsson B, Hyllner J, Synnergren J. Regulation of ‘stemness’ and stem cell differentiation by microRNAs. IDrugs. 2009;12:492–6. [PubMed] [Google Scholar]
- 6.Kashyap V, Rezende NC, Scotland KB, et al. Regulation of stem cell pluripotency and differentiation involves a mutual regulatory circuit of the NANOG, OCT4, and SOX2 pluripotency transcription factors with polycomb repressive complexes and stem cell microRNAs. Stem Cells Dev. 2009;18:1093–108. doi: 10.1089/scd.2009.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hornstein E, Shomron N. Canalization of development by microRNAs. Nat Genet. 2006;38:S20–4. doi: 10.1038/ng1803. [DOI] [PubMed] [Google Scholar]
- 8.Chang TC, Yu D, Lee YS, et al. Widespread microRNA repression by Myc contributes to tumorigenesis. Nat Genet. 2008;40:43–50. doi: 10.1038/ng.2007.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao C, Sun G, Li S, et al. MicroRNA let-7b regulates neural stem cell proliferation and differentiation by targeting nuclear receptor TLX signaling. Proc Natl Acad Sci USA. 2010;107:1876–81. doi: 10.1073/pnas.0908750107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melton C, Judson RL, Blelloch R. Opposing microRNA families regulate self-renewal in mouse embryonic stem cells. Nature. 2010;463:621–6. doi: 10.1038/nature08725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wellner U, Schubert J, Burk UC, et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol. 2009;11:1487–95. doi: 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- 12.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Block TM, Mehta AS, Fimmel CJ, Jordan R. Molecular viral oncology of hepatocellular carcinoma. Oncogene. 2003;22:5093–107. doi: 10.1038/sj.onc.1206557. [DOI] [PubMed] [Google Scholar]
- 14.Sell S, Leffert HL. Liver cancer stem cells. J Clin Oncol. 2008;26:2800–5. doi: 10.1200/JCO.2007.15.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meng F, Wehbe-Janek H, Henson R, et al. Epigenetic regulation of microRNA-370 by interleukin-6 in malignant human cholangiocytes. Oncogene. 2008;27:378–86. doi: 10.1038/sj.onc.1210648. [DOI] [PubMed] [Google Scholar]
- 16.Meng F, Henson R, Wehbe-Janek H, et al. The MicroRNA let-7a modulates interleukin-6-dependent STAT-3 survival signaling in malignant human cholangiocytes. J Biol Chem. 2007;282:8256–64. doi: 10.1074/jbc.M607712200. [DOI] [PubMed] [Google Scholar]
- 17.Cheng GZ, Zhang W, Wang LH. Regulation of cancer cell survival, migration, and invasion by Twist: AKT2 comes to interplay. Cancer Res. 2008;68:957–60. doi: 10.1158/0008-5472.CAN-07-5067. [DOI] [PubMed] [Google Scholar]
- 18.Katoh Y, Katoh M. Hedgehog signaling, epithelial-to-mesenchymal transition and miRNA (review) Int J Mol Med. 2008;22:271–5. [PubMed] [Google Scholar]
- 19.Ma S, Lee TK, Zheng BJ, et al. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene. 2008;27:1749–58. doi: 10.1038/sj.onc.1210811. [DOI] [PubMed] [Google Scholar]
- 20.Ji J, Yamashita T, Budhu A, et al. Identification of microRNA-181 by genome-wide screening as a critical player in EpCAM-positive hepatic cancer stem cells. Hepatology. 2009;50:472–80. doi: 10.1002/hep.22989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sancho-Bru P, Najimi M, Caruso M, et al. Stem and progenitor cells for liver repopulation: can we standardise the process from bench to bedside? Gut. 2009;58:594–603. doi: 10.1136/gut.2008.171116. [DOI] [PubMed] [Google Scholar]
- 22.Palmer DH. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:2498. ; author reply 9. [PubMed] [Google Scholar]
- 23.Liu L, Cao Y, Chen C, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851–8. doi: 10.1158/0008-5472.CAN-06-1377. [DOI] [PubMed] [Google Scholar]
- 24.Matsuo N, Shiraha H, Fujikawa T, et al. Twist expression promotes migration and invasion in hepatocellular carcinoma. BMC Cancer. 2009;9:240. doi: 10.1186/1471-2407-9-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee TK, Poon RT, Yuen AP, et al. Twist overexpression correlates with hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clin Cancer Res. 2006;12:5369–76. doi: 10.1158/1078-0432.CCR-05-2722. [DOI] [PubMed] [Google Scholar]
- 26.Lee YB, Bantounas I, Lee DY, et al. Twist-1 regulates the miR-199a/214 cluster during development. Nucleic Acids Res. 2009;37:123–8. doi: 10.1093/nar/gkn920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang B, Hsu SH, Majumder S, et al. TGFbeta-mediated upregulation of hepatic miR-181b promotes hepatocarcinogenesis by targeting TIMP3. Oncogene. 2010;29:1787–97. doi: 10.1038/onc.2009.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pietrangelo A, Oude Elferink R, Prieto J, Bacon BR. Genetics in liver diseases. J Hepatol. 2007;46:1143–8. doi: 10.1016/j.jhep.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 29.Calin GA, Liu CG, Ferracin M, et al. Ultraconserved regions encoding ncRNAs are altered in human leukemias and carcinomas. Cancer Cell. 2007;12:215–29. doi: 10.1016/j.ccr.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 30.Tang Y, Kitisin K, Jogunoori W, et al. Progenitor/stem cells give rise to liver cancer due to aberrant TGF-beta and IL-6 signaling. Proc Natl Acad Sci USA. 2008;105:2445–50. doi: 10.1073/pnas.0705395105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Logan RM, Stringer AM, Bowen JM, et al. The role of pro-inflammatory cytokines in cancer treatment-induced alimentary tract mucositis: pathobiology, animal models and cytotoxic drugs. Cancer Treat Rev. 2007;33:448–60. doi: 10.1016/j.ctrv.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Sempere LF, Freemantle S, Pitha-Rowe I, et al. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol. 2004;5:R13. doi: 10.1186/gb-2004-5-3-r13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Houbaviy HB, Murray MF, Sharp PA. Embryonic stem cell-specific MicroRNAs. Dev Cell. 2003;5:351–8. doi: 10.1016/s1534-5807(03)00227-2. [DOI] [PubMed] [Google Scholar]
- 34.Rybak A, Fuchs H, Smirnova L, et al. A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nat Cell Biol. 2008;10:987–93. doi: 10.1038/ncb1759. [DOI] [PubMed] [Google Scholar]
- 35.Newman MA, Thomson JM, Hammond SM. Lin-28 interaction with the Let-7 precursor loop mediates regulated microRNA processing. RNA. 2008;14:1539–49. doi: 10.1261/rna.1155108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Viswanathan SR, Daley GQ, Gregory RI. Selective blockade of microRNA processing by Lin28. Science. 2008;320:97–100. doi: 10.1126/science.1154040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heo I, Joo C, Cho J, et al. Lin28 mediates the terminal uridylation of let-7 precursor MicroRNA. Mol Cell. 2008;32:276–84. doi: 10.1016/j.molcel.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 38.Tokumaru S, Suzuki M, Yamada H, et al. let-7 regulates Dicer expression and constitutes a negative feedback loop. Carcinogenesis. 2008;29:2073–7. doi: 10.1093/carcin/bgn187. [DOI] [PubMed] [Google Scholar]
- 39.Selbach M, Schwanhausser B, Thierfelder N, et al. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455:58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- 40.Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–8. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 41.Choong ML, Yang HH, McNiece I. MicroRNA expression profiling during human cord blood-derived CD34 cell erythropoiesis. Exp Hematol. 2007;35:551–64. doi: 10.1016/j.exphem.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Yan LX, Huang XF, Shao Q, et al. MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. RNA. 2008;14:2348–60. doi: 10.1261/rna.1034808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakajima G, Hayashi K, Xi Y, et al. Non-coding MicroRNAs hsa-let-7g and hsa-miR-181b are associated with chemoresponse to S-1 in colon cancer. Cancer Genom Proteom. 2006;3:317–24. [PMC free article] [PubMed] [Google Scholar]
- 44.Lee EJ, Gusev Y, Jiang J, et al. Expression profiling identifies microRNA signature in pancreatic cancer. Int J Cancer. 2007;120:1046–54. doi: 10.1002/ijc.22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi L, Cheng Z, Zhang J, et al. hsa-mir-181a and hsa-mir-181b function as tumor suppressors in human glioma cells. Brain Res. 2008;1236:185–93. doi: 10.1016/j.brainres.2008.07.085. [DOI] [PubMed] [Google Scholar]
- 46.Calin GA, Pekarsky Y, Croce CM. The role of microRNA and other non-coding RNA in the pathogenesis of chronic lymphocytic leukemia. Best Pract Res Clin Haematol. 2007;20:425–37. doi: 10.1016/j.beha.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 47.Cheung O, Puri P, Eicken C, et al. Nonalcoholic steatohepatitis is associated with altered hepatic MicroRNA expression. Hepatology. 2008;48:1810–20. doi: 10.1002/hep.22569. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Aberrant expression of IL-6 and Twist in human HCC tumour sections.
IL-6 and Twist specifically regulated let-7 and miR-181 expressions in HCC stem cells.


