Abstract
Outside pregnancy, acute caffeine consumption is associated with insulin resistance. We investigated if during pregnancy plasma concentrations of caffeine and its metabolite, paraxanthine, were associated with insulin resistance. Caffeine, paraxanthine, glucose and insulin were measured and insulin resistance estimated by homeostasis model assessment (HOMA) in banked samples from 251 fasting subjects at mean gestational age of 20.3 ± 2.0 weeks. Analysis of covariance and adjusted logistic regression were performed. Most (96.4%) women had caffeine and/or paraxanthine present. Caffeine concentrations in the upper two quartiles (> 266 ng/ml) were associated with 3-fold higher odds of having higher insulin resistance estimated by log HOMA ≥ 75th percentile (3rd quartile OR, 3.02; 95% CI: 1.21 – 7.54 and 4th quartile OR, 2.95; 95% CI: 1.19 – 7.31). Paraxanthine concentrations in the upper quartile (> 392 ng/ml) were also associated with 3-fold higher odds of having higher insulin resistance (OR, 3.04; 95% CI: 1.28 – 7.25). Adjusted mean HOMA in the 1st caffeine to paraxanthine ratio quartile was 1.5 ± 2.2 versus 1.3 ± 2.3 in the 4th quartile (P < .01). Both high caffeine and paraxanthine concentrations were associated with insulin resistance, but slow versus fast metabolism did not make an important difference.
Keywords: caffeine, insulin resistance, paraxanthine
INTRODUCTION
Eight-seven percent of persons in the United States consume caffeine, with coffee being the primary source (71%).1 More than 75% of pregnant women drink caffeine containing beverages.2 In the non-pregnant population, caffeine intake is associated with acute impaired glucose tolerance secondary to increased insulin resistance.3–7 Evidence supports a caffeine stimulated catecholamine mediated pathway, with epinephrine antagonizing the effects of insulin.8–10 The insulin resistance that occurs after acute caffeine ingestion may be exaggerated in pre-diabetic non-pregnant adults. 11 Other studies have also demonstrated increased insulin resistance after caffeine administration in obese and diabetic adults.3,4 However, epidemiologic studies in non-pregnant adults have demonstrated a decreased risk for type 2 diabetes with long term coffee consumption.12
Pregnancy is a state of mild insulin resistance, and it is important to know if caffeine is associated with greater insulin resistance since insulin resistance is associated with adverse pregnancy outcomes such as gestational diabetes, hypertensive disease and accelerated fetal growth.13–15 One study in the third trimester of pregnancy found that caffeine ingestion before a 2 hour glucose tolerance test decreased the insulin sensitivity index by 18% in women with gestational diabetes mellitus (GDM), but not in normal controls.16 Another study found a decreased risk of GDM in women who drank lower amounts of caffeinated coffee compared to non-coffee drinkers, but the timing was before pregnancy.17 Neither of these studies took caffeine metabolism into account and metabolism is an important consideration since it slows with advancing gestation.18 In addition, the major pathway for caffeine metabolism is by the P-450 cytochrome CYP1A2 and polymorphisms cause differential metabolism of caffeine.19 Thus, the effects of caffeine in pregnancy may depend upon its metabolism rate, which has been approximated by the ratio of caffeine to paraxanthine, its major metabolite. For example, fast caffeine metabolizers (high paraxanthine to caffeine ratio) have been found to be at increased risk of intrauterine growth restriction whereas slow metabolizers (low paraxanthine to caffeine ratio) have larger neonates.20
Our primary objective was to investigate the relationship between plasma concentrations of caffeine and/or its metabolite, paraxanthine, and insulin resistance in pregnancy. Our second objective was to investigate whether slow metabolizers of caffeine, as estimated by the caffeine to paraxanthine ratio, are at further increased risk for insulin resistance. We hypothesized that caffeine would be associated with insulin resistance, and that slow metabolizers (high caffeine to paraxanthine ratio) would have higher insulin resistance compared to fast metabolizers. Finally, we explored the association between caffeine, paraxanthine and differences in caffeine metabolism on infant birth weight. We hypothesized that slow metabolism would be associated with higher birth weight.
METHODS
Study description
We used banked plasma and serum samples from specimens drawn at 18–21 weeks gestation from fasting subjects enrolled in the Pregnancy Exposures and Preeclampsia Prevention Study (PEPP) and stored at −80° C. PEPP is an ongoing study of preeclampsia approved by the University of Pittsburgh Institutional Review Boards. This was a prospective study of pregnant women (n = 2812) enrolled at <21 weeks gestation from 1997–2006 and followed to the post partum period. Fasting samples were collected beginning in 2002, and a total of 706 nulliparous women were invited to give a fasting sample. Of these, 260 plasma and serum samples were available. We excluded women with a history of pre-pregnancy diabetes, chronic hypertension, or those with missing data, leaving a total of 251 samples. This subset of women was about one year older (24.4 versus 23.3 years, P=.01) and slightly more likely to have a high school education (89.4% versus 85.6%, P=.01) than the other women in the longitudinal study. They did not differ, however, regarding race or gestational age at delivery.
Baseline demographic information and medical history were collected via a structured interview. Pregnancy outcomes were also recorded from the medical record as part of the original study. Neonatal outcomes assessed included birth weight and birth weight centile, adjusted for race, sex and gestational age at delivery from a reference population of over 10,000 births at Magee-Womens Hospital. Small for gestational age (SGA) was defined as birth weight < 10th centile. Gestational diabetes screening is routinely performed in our hospital between between 24 and 28 weeks gestation or earlier if indicated, and was diagnosed with a three hour glucose challenge test using Carpenter and Coustan criteria or a one hour 50 g value of ≥ 200 mg/dl.21,22 Covariates included education, maternal race, pre-pregnancy body mass index (BMI, kg/m2), gestational age at delivery and maternal age at time of plasma collection. Education was categorized as less than high school (12 years), or at least a high school level (≥12 years). Maternal race was classified as either non-black or black because of the low frequencies in the original cohort of non-white, non-black participants. The baseline characteristics of the non-white, non-black participants were comparable to white participants, and therefore we elected to combine these groups. Gestational age was determined by best obstetrical assessment, using early ultrasound where available.
Plasma samples
Insulin was measured in serum by an ELISA kit from LINCO Research. The inter-assay variation was 10.0%. Glucose was measured in serum using a colorimetric assay from Pointe Scientific, Inc. (Canton, MI) Kit G7519. The inter-assay variability was 5–12%. HOMA was calculated as described by Matthews et al. using the formula: insulin resistance = insulin/(22.5e−lnglucose).23 HOMA has been validated during pregnancy using a hyperinsulinemic clamp and shown to have good correlation in the 2nd and 3rd trimesters.24
Maternal plasma concentrations of caffeine and paraxanthine were determined by high-pressure liquid chromatography (HPLC) as described by Holland et al. with modifications.25 In brief, plasma samples were treated with an equal volume of 0.8 mol/L perchloric acid to precipitate plasma proteins (i.e. 150μl of plasma and 150μl of 0.8 mol/L perchloric acid). Samples were vortexed, centrifuged at 14,000×G for 10 minutes at room temperature and the supernatant transferred to an appropriate sample vial. Extracted plasma samples (100μl) were injected onto a prepared HPLC system. Separation of caffeine and its metabolites were performed using a Waters Xterra MS C18 Column (4.6 × 100 mm, 5μm particle size) with an appropriate Xterra Guard column (3.9 × 20 mm, 5 μm particle size). The mobile phase consisted of 15 mmol/L potassium phosphate buffer, pH 4.9 and methanol (85:15, v/v) and the analytes were eluted isocratically at a flow rate of 1ml/min and detected at 274nm wavelength. The elution time for each analyte was: 6.5 minutes for paraxanthine and 16 minutes for caffeine. Standard curves were generated using known quantities of caffeine and paraxanthine ranging from 25 to 1.56 μmol/L, and were linear for each component. All samples were measured in duplicate and the average was used for statistical analysis. The inter-assay variability was 4% for caffeine and 8% for paraxanthine.
Statistical analysis
Spearman correlation coefficients were calculated for caffeine and its metabolite, paraxanthine. The caffeine to paraxanthine ratio was calculated and the value 0 was replaced by 0.005 as described by Grosso et al. to allow a ratio to be calculated for all values.20 This imputation was only done for the ratios, not for any other analyses. Caffeine and paraxanthine concentrations were explored as both continuous and categorical variables. We analyzed the data as quartiles to investigate the possibility of a nonlinear relationship, and for results to be comparable to other studies.20 Analysis of covariance was performed to investigate the mean insulin, glucose and insulin resistance as calculated by HOMA, by quartiles of caffeine, paraxanthine, and the ratio of caffeine to paraxanthine. HOMA and insulin values were log-transformed (resulting in a normal distribution) prior to analysis. Of the covariates considered (maternal age, pre-pregnancy body mass index, maternal race, smoking, education and gestational age of sample), only maternal age and BMI significantly contributed to the final model. We previously demonstrated that self-report of smoking status approximates plasma cotinine concentrations in our population with 82% agreement and κ = 0.64.26 Therefore, we chose to use a woman’s self-report to indicate smoking status. Smoking was collected as a categorical variable in 3 levels: none, 1–10 cigarettes per day, and >10 cigarettes per day. Only 9 women smoked >10 cigarettes per day. We performed the analysis using the categorical variables as well as a binary outcome of yes/no. The significance of the results did not change when smoking was included or excluded from the model, and therefore it was not adjusted for in the final model. Results did not change significantly when potential outliers, and influential and leverage points, were dropped from the model, so they were retained. Logistic regression was performed to investigate the association between increased insulin resistance as measured by the log HOMA in the ≥ 75th percentile and increasing caffeine and paraxanthine quartiles. We also investigated the association of caffeine and paraxanthine with fasting glucose concentrations as measured in the present study in the upper categories as described in the “Hyperglycemia and adverse pregnancy outcomes” (HAPO) study by logistic regression.27 Linear regression was performed to investigate the associations between birth weight and centile, and caffeine and paraxanthine concentration. Analysis of variance and Kruskal-Wallis testing were performed to compare mean neonatal birth weight and birth weight centiles, adjusted for race, sex and gestational age at delivery, by caffeine, paraxanthine and caffeine to paraxanthine ratio quartiles. For the overall model, an α of < 0.05 was considered statistically significant. Analyses were performed with Stata (StataCorp LP, College Station, TX) version 10.0 for Windows.
RESULTS
Plasma caffeine, paraxanthine and insulin resistance
A total of 251 samples were available for analysis with a mean gestational age at sampling of 20.3 ± 2.0 weeks. Of these, 242 (96.4%) had caffeine and/or paraxanthine present in their plasma. The median caffeine concentration was 265.5 (interquartile range 92.8 – 555.4) ng/ml and median paraxanthine concentration was 171.7 (interquartile range 59.3 – 392.7) ng/ml. Black race and higher education were associated with lower caffeine concentrations (Table 1). Other demographic and neonatal characteristics did not differ by caffeine quartiles. Caffeine was highly correlated with paraxanthine (Spearman r=0.82, P<.001). The mean glucose that was measured in the analysis was 79.1 (S.D. ± 11.6 mg/dL) and median insulin was 6.9 ( interquartile range 4.6 – 12.5) μU/ml. Gestational diabetes mellitus complicated 9 (3.6%) of the pregnancies.
Table 1.
Characteristics of the women and their newborns by caffeine quartiles.
| Characteristic | Caffeine quartiles (ng/ml) | p-value for trend | |||
|---|---|---|---|---|---|
| 1st (0–93) | 2nd (94–266) | 3rd (267–555) | 4th (>555) | ||
| N=63 | N=63 | N=63 | N=62 | ||
| Maternal age (years) | 24.6 (6.1) | 24.5 (4.9) | 24.7 (5.2) | 23.3 (5.1) | 0.2 |
| BMI (kg/m2) | 24.6 (5.6) | 25.6 (6.5) | 25.4 (5.9) | 24.6 (6.2) | 0.9 |
| Sample age | 20.0 (2.0) | 19.9 (2.0) | 20.7 (1.7) | 20.7 (2.1) | 0.4 |
| Maternal Race | |||||
| Nonblack | 32 (50.8%) | 48 (76.2%) | 49 (77.8%) | 48 (77.4%) | 0.03 |
| Black | 31 (49.2%) | 15 (23.8%) | 14 (22.2%) | 14 (22.6%) | |
| Smokers | 10 (15.9%) | 15 (23.8%) | 17 (27.0%) | 15 (6.0%) | 0.2 |
| Education | |||||
| < 12 years | 3 (4.8%) | 7 (11.1%) | 4 (6.3%) | 12 (19.4%) | 0.02 |
| GA at delivery (weeks) | 38.8 (2.2) | 38.9 (2.2) | 38.5 (2.4) | 38.8 (1.8) | 0.8 |
| SGA | |||||
| ≤10th centile | 4 (6.3%) | 8 (12.7%) | 4 (6.3%) | 5 (8.1%) | 0.9 |
BMI, body mass index; GA, gestational age; SGA, small for gestational age and is adjusted for race, sex and gestational age.
Data are presented as means (SD) or numbers (percentages, where the denominator is the n for that quartile.)
Insulin resistance as measured by the mean of HOMA was higher with increasing caffeine and paraxanthine quartiles after adjusting for maternal age at sampling and pre-pregnancy BMI (Table 2). This insulin resistance was largely evidenced by higher insulin concentrations, with no significant differences in glucose concentration. There was no interaction between smoking and caffeine or paraxanthine. Caffeine concentrations in the upper two quartiles (> 266 ng/ml) were associated with a 3-fold higher odds of having higher insulin resistance as measured by the log HOMA in the ≥ 75th percentile, (3rd quartile adjusted OR, 3.02; 95% confidence interval: 1.21 – 7.54 and 4th quartile adjusted OR, 2.95; 95% confidence interval: 1.19 – 7.31, Figure). Paraxanthine concentrations in the upper quartile (> 392 ng/ml) were also associated with a 3-fold higher odds of having higher insulin resistance as measured by the log HOMA in the ≥ 75th percentile, (adjusted OR, 3.04; 95% confidence interval: 1.28 – 7.25). Neither caffeine nor paraxanthine in the 3rd or 4th quartiles were associated with glucose 95 mg/dl or more, which corresponds to the upper two HAPO fasting categories 6 and 7.27
Table 2.
Insulin resistance (HOMA), insulin and glucose by caffeine and paraxanthine quartiles.
| Outcome | Caffeine quartiles (ng/ml) | p Value | |||
|---|---|---|---|---|---|
| 1st (0–93) | 2nd (94–266) | 3rd (267–555) | 4th (>555) | ||
| HOMA | 1.2 (2.2) | 1.6 (2.5) | 1.5 (2.5) | 1.8 (2.5) | < 0.01 |
| Insulin μU/L | 6.0 (2.2) | 8.2 (2.5) | 8.2 (2.2) | 9.0 (2.2) | < 0.01 |
| Glucose mg/dL | 78.7 (10.2) | 79.5 (10.9) | 78.5 (12.4) | 79.5 (12.9) | 0.3 |
| Outcome | Paraxanthine quartiles (ng/ml) | p Value | |||
| 1st (0–59) | 2nd (60–170) | 3rd (171–392) | 4th (>392) | ||
| HOMA | 1.3 (2.2) | 1.3 (2.7) | 1.6 (2.0) | 2.0 (2.5) | < 0.01 |
| Insulin μU/L | 6.7 (2.0) | 6.7 (2.7) | 8.2 (1.8) | 10.0 (2.2) | < 0.01 |
| Glucose mg/dL | 78.0 (10.5) | 78.8 (11.1) | 79.8 (10.8) | 79.6 (13.8) | 0.2 |
| Outcome | Caffeine to paraxanthine ratio quartiles | p Value | |||
| 1st | 2nd | 3rd | 4th | ||
| HOMA | 1.5 (2.2) | 1.6 (2.7) | 1.7 (2.6) | 1.3 (2.3) | < 0.01 |
| Insulin μU/L | 7.7 (2.0) | 8.1 (2.5) | 8.8 (2.4) | 7.0 (2.1) | < 0.01 |
| Glucose mg/dL | 78.9 (11.6) | 80.1 (10.4) | 79.4 (13.7) | 77.9 (10.7) | 0.2 |
p Value is from the F-statistic in the analysis of covariance model adjusted for maternal age at sampling and pre-pregnancy BMI.
HOMA, homeostasis model assessment calculated by the formula: insulin resistance = insulin/(22.5elnglucose).
Values are presented as mean (standard deviation).
Figure.
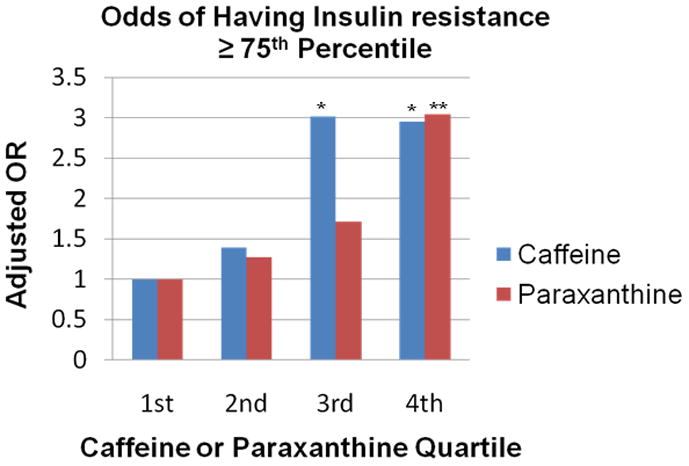
Odd ratios for log HOMA ≥ 75th percentile according to the plasma concentration of caffeine or paraxanthine adjusted for maternal age at sampling and pre-pregnancy body mass index. For caffeine, the reference category is values less than 94 ng/ml, or the first quartile. Caffeine quartiles are defined as follows: 2nd quartile, 94 – 266 ng/mL; 3rd quartile, 267 – 555 ng/ml, 4th quartile > 555 ng/ml. For paraxanthine, the reference category is values less than 60 ng/ml, or the first quartile. Paraxanthine quartile are defined as follows: 2nd quartile, 60 – 170 ng/mL; 3rd quartile, 171 – 392 ng/ml; 4th quartile > 392 ng/ml.
*P = .02 for both.
**P = .01.
HOMA and insulin concentrations were directly related to the caffeine to paraxanthine ratio with the highest HOMA values and insulin concentrations in the 2nd and 3rd quartiles of this ratio (Table 2). There were no significant differences in glucose concentration among quartiles.
Caffeine, paraxanthine and birth weight
Mean birth weight and birth weight centile were lower but not significantly in women with caffeine in the 4th quartile compared to 1st (Table 3, P=.4 for both). Findings were similar for paraxanthine (P=.5 and P=.2, respectively). Overall, caffeine concentration was not associated with birth weight (P=0.7) or birth weight centile (P=0.4), but for each 2 ng/ml increase in paraxanthine, there was a 1 g decrease in birth weight (P=0.04) and no association with birth weight centile (P=0.7). For women with low caffeine to paraxanthine ratio (fast metabolizers), the mean birth weight was 195 grams lower compared to women with high caffeine to paraxanthine ratio (slow metabolizers), but these differences were not significant (Table 3, P=.08) nor was growth evaluated as birth weight centile (52.4% vs. 58.4%, p=0.4. We calculated a sample size necessary to detect differences in infant birth weight with an α = 0.05 and β = 0.1. For the caffeine to paraxanthine ratio, a total of 326 women would be needed to detect the 195 gram difference in birth weight between the highest and lowest quartiles.
Table 3.
Birth weight and birth weight centile by caffeine, paraxanthine and caffeine to paraxanthine ratio quartiles.
| Quartile |
p Value* | p for trend | ||||
|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | |||
| Caffeine | 3301 (612) | 3327 (647) | 3257 (614) | 3266 (532) | 0.4 | 0.7 |
| 56.7 (27.4) | 52.3 (31.5) | 56.0 (29.5) | 50.3 (26.7) | 0.4 | 0.4 | |
| Paraxanthine | 3263 (703) | 3367 (471) | 3318 (657) | 3205 (540) | 0.2 | 0.5 |
| 54.8 (29.2) | 56.6 (29.1) | 56.1 (28.7) | 47.9 (28.0) | 0.3 | 0.2 | |
| Caffeine to paraxanthine ratio | 3245 (600) | 3282 (621) | 3184 (669) | 3440 (478) | 0.08 | 0.2 |
| 52.4 (29.6) | 53.8 (28.7) | 50.8 (29.0) | 58.4 (28.0) | 0.4 | 0.4 | |
p Value is from the F-statistic in the analysis of covariance model adjusted for maternal age at sampling and pre-pregnancy BMI. Birth weight centile additionally adjusted for race, sex and gestational age at delivery.
S.D., standard deviation
Birth weight centile additionally adjusted for race, sex and gestational age at delivery.
Birth weight (S.D.) – grams
Birth weight centile (S.D.) – %
DISCUSSION
Caffeine concentrations > 266 ng/ml and paraxanthine concentrations > 392 ng/ml were associated with higher insulin resistance in mid-gestation, determined by higher insulin and not glucose. Neither the highest ratios of caffeine to paraxanthine nor lowest ratios had higher insulin resistance, indicating that slow versus fast metabolism did not affect the association of caffeine with insulin resistance. There was a non-significant trend towards lower birth weight with higher caffeine and paraxanthine concentrations, an association that differed when metabolism was taken into account. Women with a high caffeine to paraxanthine ratio (slow metabolizers corresponding to the 4th quartile) tended to have neonates with a higher birth weight while women with a low caffeine to paraxanthine ratio (fast metabolizers corresponding to the 1st quartile) tended to have neonates with a lower birth weight, although the study was underpowered to detect these differences.
Our findings are similar to other studies that have consistently reported acute caffeine intake to impair glucose tolerance.3,4,7,28 Caffeine is a methylxanthine derivative that acts both peripherally and centrally, as it can cross the blood-brain barrier.5 Caffeine increases insulin secretion from pancreatic islet β-cells in vitro.29,30 In several studies where caffeine was administered before a 75-gram oral glucose tolerance test, caffeine increased serum insulin and C-peptide concentrations.3,28 However, the insulin sensitivity index was about 15% lower, and blood glucose concentrations were higher with caffeine compared with placebo treatment. The direct effects of caffeine on insulin release do not overcome the hyperglycemic response secondary to an increase in insulin resistance, which has been postulated to be catecholamine mediated.5 Since our study was in fasting subjects, it is unknown whether glucose was originally elevated after initial caffeine ingestion; however, our finding of higher insulin resistance is consistent with impaired glucose tolerance.
A strength of our study is that we measured maternal caffeine and paraxanthine concentrations which is a more accurate way of assessing caffeine effects compared to maternal recall. Caffeine content can vary widely depending upon different types of coffee, tea and sodas. However, a limitation is that since almost 2/3 of the women with caffeine in the highest quartile also had paraxanthine in the highest quartile, the effects of differential metabolism (fast versus slow) on insulin resistance may have been blunted by our use of samples collected from fasting subjects (necessitated by concomitant determination of insulin resistance). Women had to be fasting for at least 6 hours prior to collection for the study, but the fasting interval could have been longer and the timing of the last caffeine consumption and the blood sampling was unknown. Even women who were slow metabolizers could have had time to metabolize a large portion of the caffeine. However, caffeine metabolism slows in pregnancy.31,32 Outside of pregnancy, the half-life of caffeine is 2 to 4.5 hours but can be 12 hours in slow-metabolizers, while it increases to 10 hours at 17 weeks gestation, and from 11.5–18 hours by the end of pregnancy in non-smokers.18,33 Thus even in a fasting state, we expected that the slower caffeine metabolism should have allowed us to observe some effects of metabolism on insulin resistance if there was an association.
There were too few women who developed gestational diabetes in our cohort to investigate whether caffeine or paraxanthine was associated with development of GDM. However, we did not find an association with the highest two HAPO categories (fasting glucose ≥ 95 mg/dl).27 The relationship of caffeine intake and the development of gestational diabetes or effects on fetal growth, and how the different rates of metabolism could affect these outcomes will require a larger study. In addition, we do not know the extent that paraxanthine approximated caffeine intake versus being a marker for metabolism in our study. A pharmacodynamic study would better be able to study the effects of metabolism.
Similar to studies in non-pregnant adults, high concentrations of caffeine or its major metabolite, paraxanthine, were associated with insulin resistance in mid-gestation of pregnancy. Differential maternal metabolism did not appear to influence this association, but may be an important factor in the association between caffeine and neonatal birth weight. Caffeine consumption is common during pregnancy, and there already is an epidemic of women entering pregnancy with increasing insulin resistance and obesity.34 High caffeine consumption may further exacerbate the insulin resistance and potentially increase the risk of adverse maternal and neonatal outcomes such as gestational diabetes, preeclampsia, and abnormal fetal growth.
Acknowledgments
This research is supported by the Irene McLenahan Young Investigators Research Fund of the Magee-Womens Health Foundation, the Pregnancy Exposures and Preeclampsia Prevention Study Magee-Womens Hospital Clinical, NIH P01 HD030367, and the Translational Research Center funded by the University of Pittsburgh Clinical and Translational Science Award, UL1 RR024153.
Abbreviations
- GDM
gestational diabetes mellitis
- HAPO
“Hyperglycemia and adverse pregnancy outcomes” study
- HOMA
homeostasis model assessment
- SGA
small for gestational age
Footnotes
This research was presented as a poster at the Annual Society for Maternal-Fetal Medicine Meeting, Chicago, IL on February 6, 2010.
References
- 1.Frary CD, Johnson RK, Wang MQ. Food sources and intakes of caffeine in the diets of persons in the United States. J Am Diet Assoc. 2005;105:110–113. doi: 10.1016/j.jada.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 2.Eskenazi B. Caffeine--filtering the facts. N Engl J Med. 1999;341:1688–1689. doi: 10.1056/NEJM199911253412210. [DOI] [PubMed] [Google Scholar]
- 3.Robinson LE, Savani S, Battram DS, et al. Caffeine ingestion before an oral glucose tolerance test impairs blood glucose management in men with type 2 diabetes. J Nutr. 2004;134:2528–2533. doi: 10.1093/jn/134.10.2528. [DOI] [PubMed] [Google Scholar]
- 4.Petrie HJ, Chown SE, Belfie LM, et al. Caffeine ingestion increases the insulin response to an oral-glucose-tolerance test in obese men before and after weight loss. Am J Clin Nutr. 2004;80:22–28. doi: 10.1093/ajcn/80.1.22. [DOI] [PubMed] [Google Scholar]
- 5.Keijzers GB, De Galan BE, Tack CJ, Smits P. Caffeine can decrease insulin sensitivity in humans. Diabetes Care. 2002;25:364–369. doi: 10.2337/diacare.25.2.364. [DOI] [PubMed] [Google Scholar]
- 6.Lane JD, Barkauskas CE, Surwit RS, Feinglos MN. Caffeine impairs glucose metabolism in type 2 diabetes. Diabetes Care. 2004;27:2047–2048. doi: 10.2337/diacare.27.8.2047. [DOI] [PubMed] [Google Scholar]
- 7.Pizziol A, Tikhonoff V, Paleari CD, et al. Effects of caffeine on glucose tolerance: a placebo-controlled study. European journal of clinical nutrition. 1998;52:846–849. doi: 10.1038/sj.ejcn.1600657. [DOI] [PubMed] [Google Scholar]
- 8.Thong FS, Graham TE. Caffeine-induced impairment of glucose tolerance is abolished by beta-adrenergic receptor blockade in humans. J Appl Physiol. 2002;92:2347–2352. doi: 10.1152/japplphysiol.01229.2001. [DOI] [PubMed] [Google Scholar]
- 9.Thong FS, Derave W, Kiens B, et al. Caffeine-induced impairment of insulin action but not insulin signaling in human skeletal muscle is reduced by exercise. Diabetes. 2002;51:583–590. doi: 10.2337/diabetes.51.3.583. [DOI] [PubMed] [Google Scholar]
- 10.Avogaro A, Toffolo G, Valerio A, Cobelli C. Epinephrine exerts opposite effects on peripheral glucose disposal and glucose-stimulated insulin secretion. A stable label intravenous glucose tolerance test minimal model study. Diabetes. 1996;45:1373–1378. doi: 10.2337/diab.45.10.1373. [DOI] [PubMed] [Google Scholar]
- 11.Lane JD. Caffeine May Raise Insulin Resistance in Prediabetics. ObGynNews. 2007 June 1;:36. [Google Scholar]
- 12.van Dam RM, Hu FB. Coffee consumption and risk of type 2 diabetes: a systematic review. Jama. 2005;294:97–104. doi: 10.1001/jama.294.1.97. [DOI] [PubMed] [Google Scholar]
- 13.Wolf M, Sandler L, Jimenez-Kimble R, et al. Insulin resistance but not inflammation is associated with gestational hypertension. Hypertension. 2002;40:886–891. doi: 10.1161/01.hyp.0000042085.65467.9f. [DOI] [PubMed] [Google Scholar]
- 14.Wolf M, Sandler L, Munoz K, et al. First trimester insulin resistance and subsequent preeclampsia: a prospective study. The Journal of clinical endocrinology and metabolism. 2002;87:1563–1568. doi: 10.1210/jcem.87.4.8405. [DOI] [PubMed] [Google Scholar]
- 15.Hadden DR. Prediabetes and the big baby. Diabet Med. 2008;25:1–10. doi: 10.1111/j.1464-5491.2007.02331.x. [DOI] [PubMed] [Google Scholar]
- 16.Robinson LE, Spafford C, Graham TE, Smith GN. Acute caffeine ingestion and glucose tolerance in women with or without gestational diabetes mellitus. J Obstet Gynaecol Can. 2009;31:304–312. doi: 10.1016/S1701-2163(16)34147-0. [DOI] [PubMed] [Google Scholar]
- 17.Adeney KL, Williams MA, Schiff MA, Qiu C, Sorensen TK. Coffee consumption and the risk of gestational diabetes mellitus. Acta Obstet Gynecol Scand. 2007;86:161–166. doi: 10.1080/00016340600994992. [DOI] [PubMed] [Google Scholar]
- 18.Daly J. Caffeine, Coffee and Health. In: Garattini S, editor. Mechanism of action of caffeine. New York: Raven Press; 1993. pp. 97–149. [Google Scholar]
- 19.Grosso LM, Bracken MB. Caffeine metabolism, genetics, and perinatal outcomes: a review of exposure assessment considerations during pregnancy. Ann Epidemiol. 2005;15:460–466. doi: 10.1016/j.annepidem.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Grosso LM, Triche EW, Belanger K, et al. Caffeine metabolites in umbilical cord blood, cytochrome P-450 1A2 activity, and intrauterine growth restriction. Am J Epidemiol. 2006;163:1035–1041. doi: 10.1093/aje/kwj125. [DOI] [PubMed] [Google Scholar]
- 21.Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. American journal of obstetrics and gynecology. 1982;144:768–773. doi: 10.1016/0002-9378(82)90349-0. [DOI] [PubMed] [Google Scholar]
- 22.Dooley SL, Metzger BE, Cho N, Liu K. The influence of demographic and phenotypic heterogeneity on the prevalence of gestational diabetes mellitus. Int J Gynaecol Obstet. 1991;35:13–18. doi: 10.1016/0020-7292(91)90057-c. [DOI] [PubMed] [Google Scholar]
- 23.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 24.Cohen O, Epstein GS, Weisz B, Homko CJ, Sivan E. Longitudinal assessment of insulin sensitivity in pregnancy. Validation of the homeostasis model assessment. Clin Endocrinol (Oxf) 2006;64:640–644. doi: 10.1111/j.1365-2265.2006.02519.x. [DOI] [PubMed] [Google Scholar]
- 25.Holland DT, Godfredsen KA, Page T, Connor JD. Simple high-performance liquid chromatography method for the simultaneous determination of serum caffeine and paraxanthine following rapid sample preparation. J Chromatogr B Biomed Sci Appl. 1998;707:105–110. doi: 10.1016/s0378-4347(97)00590-2. [DOI] [PubMed] [Google Scholar]
- 26.Jeyabalan A, Powers RW, Durica AR, et al. Cigarette smoke exposure and angiogenic factors in pregnancy and preeclampsia. Am J Hypertens. 2008;21:943–947. doi: 10.1038/ajh.2008.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 28.Graham TE, Sathasivam P, Rowland M, et al. Caffeine ingestion elevates plasma insulin response in humans during an oral glucose tolerance test. Canadian journal of physiology and pharmacology. 2001;79:559–565. [PubMed] [Google Scholar]
- 29.Shi CL. Effects of caffeine and acetylcholine on glucose-stimulated insulin release from islet transplants in mice. Cell Transplant. 1997;6:33–37. doi: 10.1177/096368979700600107. [DOI] [PubMed] [Google Scholar]
- 30.Bruton JD, Lemmens R, Shi CL, et al. Ryanodine receptors of pancreatic beta-cells mediate a distinct context-dependent signal for insulin secretion. Faseb J. 2003;17:301–303. doi: 10.1096/fj.02-0481fje. [DOI] [PubMed] [Google Scholar]
- 31.Parsons WD, Neims AH. Effect of smoking on caffeine clearance. Clin Pharmacol Ther. 1978;24:40–45. doi: 10.1002/cpt197824140. [DOI] [PubMed] [Google Scholar]
- 32.Kalow W, Tang BK. Caffeine as a metabolic probe: exploration of the enzyme-inducing effect of cigarette smoking. Clin Pharmacol Ther. 1991;49:44–48. doi: 10.1038/clpt.1991.8. [DOI] [PubMed] [Google Scholar]
- 33.Benowitz NL. Clinical pharmacology of caffeine. Annual review of medicine. 1990;41:277–288. doi: 10.1146/annurev.me.41.020190.001425. [DOI] [PubMed] [Google Scholar]
- 34.Hedley AA, Ogden CL, Johnson CL, et al. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. Jama. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]


