Abstract
Recent case reports of viral hemorrhagic fever in Europe and the United States have raised concerns about the possibility for increased importation of filoviruses to non-endemic areas. This emerging threat is concerning because of the increase in global air travel and the rise of tourism in central and eastern Africa and the greater dispersion of military troops to areas of infectious disease outbreaks. Marburg viruses (MARV) and Ebola viruses (EBOV) have been associated with outbreaks of severe hemorrhagic fever involving high mortality (25 – 90% case fatality rates). First recognized in 1967 and 1976 respectively, subtypes of MARV and EBOV are the only known viruses of the Filoviridae family, and are among the world’s most virulent pathogens. This article focuses on information relevant for health care practitioners in travel medicine to include, the epidemiology and clinical features of filovirus infection and efforts toward development of a filovirus vaccine.
Keywords: Filovirus vaccines, imported Marburg infection
Introduction
MARV and EBOV belong to the Filoviridae family of single-stranded negative-sense RNA viruses. Filoviridae derive their name from the Latin word “filum” based on their filamentous structure. Since discovery in 1967 and 1976 respectively, MARV and EBOV have caused several outbreaks concentrated in sub-Saharan Africa but some sporadic cases have occurred elsewhere. These have been mostly related to travel, nosocomial infections and occupational contact with infected animals from the endemic regions [1, 2].
MARV and EBOV are considered re-emerging and highly infectious pathogens. Human outbreaks have been sporadic, involve high case-fatality, and are socially and economically disruptive [3]. The dramatic clinical manifestation of both MARV and EBOV, with severe hemorrhaging in most cases, has also contributed to the high publicity and fear around outbreaks and imported cases [4]. Due to their highly infectious nature and potential for use in biological weapons, MARV and EBOV are classified as Category A bioterrorism agents according to the US Centers for Disease Control and Prevention (CDC).
The genus EBOV is divided into five different species. These five species are named based on the location of recognition and include Zaire, Sudan, Ivory Coast, Bundibugyo and Reston viruses (Figure 1). The Zaire species was first recognized in 1976 in a large outbreak in Yambuku with over 300 cases and a fatality rate of 88%. Since then it has been the cause of several outbreaks with high mortality rates ranging from 50–80%. The Sudan species has been the cause of four epidemics to date; three outbreaks in Sudan and one in Uganda. The Ivory Coast species was first recognized when it was identified as a causative agent of illness in a scientist conducting an autopsy on an infected chimpanzee. The Bundibugyo species emerged in Uganda in 2007 causing an outbreak of over a hundred cases with a mortality rate of over 30%. Lastly, the Reston species was described in 1989 when it caused a lethal outbreak in macaques imported to the US from the Philippines. This species caused three more outbreaks in the USA and Europe in non-human primate (NHP) facilities. The laboratory workers taking care of the infected animals developed antibodies to this virus but did not develop any clinical illness [5, 6]. In contrast to the Ebola virus, all strains of the MARV are considered members of a single species, the Lake Victoria Marburg virus. However the species encompass different strains and these strains vary in their pathogenicity in humans. This observation is based on different mortality rates among several past outbreaks caused by MARV [1]
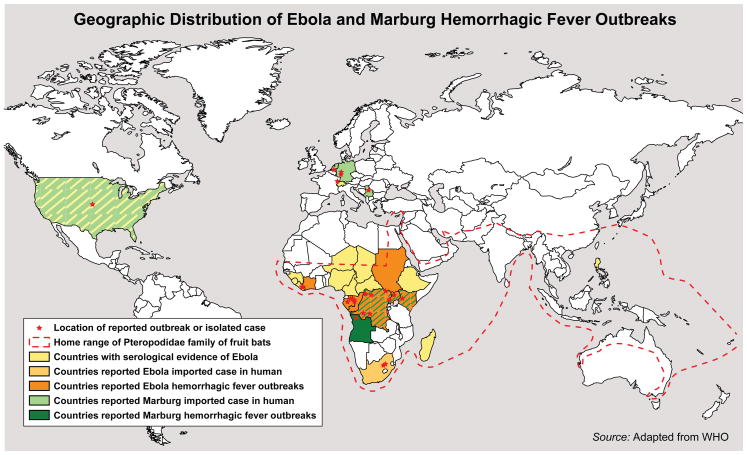
Figure 1.
The map depicting geographic distribution of Ebola and Marburg hemorrhagic fever outbreaks was adapted from the individual Ebola and Marburg maps provided by the World Health Organization and also updated to reflect most recent filovirus appearances.
The filoviruses are zoonotic viruses that are believed to be transmitted to humans from animals. In a given outbreak, attempts have been made to trace the virus from the index cases. Despite aggressive attempts, the natural reservoir remains unknown. NHPs were initially proposed as maintenance hosts for filoviruses since the initial cases of the disease were recognized in imported infected monkeys. This hypothesis does not seem plausible as susceptibility of non-human primates to fatal infection with these viruses would make them poor host reservoirs. In fact, EBOV has afflicted large populations of NHPs and this has led to a significant decline in chimpanzee and gorilla populations in Gabon and Congo [7, 8]. While the source of filoviruses in nature has not been definitively identified, increasing evidence suggests that bats may serve as reservoirs.
Human outbreaks that occurred between 2001 and 2005 in Gabon and Democratic Republic of Congo were linked to concurrent gorilla and chimpanzee outbreaks. Field collections in the region revealed Ebola specific immunoglobulin in three different bat species [9]. In September 2007, mine workers in Kitaka Cave in Uganda were diagnosed with Marburg fever. The likely source of this infection in the mine workers was thought to be due to exposure to Egyptian fruit bats. This was based on detection of MARV RNA and virus specific antibody in bat sera [10]. Further evidence to support the hypothesis that bats may in fact be harboring these deadly viruses comes from recent imported cases of Marburg infection. Both individuals contracted the infection after visiting the Python Cave in the Queen Elizabeth National Park in Uganda miles away from the Kitaka mines where the 2007 MARV outbreak had occurred [11].
Clinical manifestations of Marburg and Ebola Hemorrhagic fever
MARV and EBOV infections are similar in their clinical presentation, manifesting as hemorrhagic fever. Ebola hemorrhagic fever is a severe and often fatal disease affecting both humans and non-human primates. Most of the knowledge and clinical information comes from persons infected by the Sudan and Zaire species, which are the most virulent strains. The Bundibugyo species was observed to have a slightly lower mortality rate of approximately 30%. In contrast, the Reston species has not caused any disease in humans and only a single non-lethal case has been reported as a result of infection with the Ivory Coast species. Although both diseases are rare and thus far mostly limited to their endemic regions, they have the potential to cause geographically broader outbreaks with extremely high fatality rates [1, 5].
The incubation period of filovirus hemorrhagic fever varies from five to seven days but may exceed two weeks. Illness may come on abruptly and the early symptoms are often non-specific consisting of high fevers, chills, general malaise, headaches, myalgia and anorexia. Diarrhea, abdominal pain, cramping, nausea and vomiting may follow on days three to five and may persist for up to a week. Pharyngitis has also been observed in some patients. As many as fifty percent may develop a purple red, non-pruritic maculopapular rash on the upper trunk in the first week of their illness. Hemorrhagic manifestations that are characteristic of this disease develop during the peak of the illness. When present, bleeding most commonly occurs in the gastrointestinal tract and may manifest as melena, petechiae, conjunctival hemorrhage, hematuria, easy bruising, or intraperitoneal bleeding. Bleeding from mucous membranes and failure of venipuncture sites to clot has also been noted. These symptoms progress with time and patients develop dehydration, stupor, confusion, hypotension, multi-organ failure leading to fulminant shock and eventually death. Fatal cases tend to develop clinical signs earlier during the infection and death commonly occurs between days six and sixteen [12, 13].
Transmission
Presumably, most index cases occur due to contact with an infected animal. Transmission of the virus from person to person occurs as a result of direct contact with virus containing body fluids. Infectious body fluids include blood, vomitus, urine, feces, sweat, breast milk, saliva and respiratory secretions. Epidemiological studies have demonstrated that family members are at risk of infection if they have had contact with infected body fluids or have helped to prepare the corpse of an infected person for burial. The most common mechanism appears to be contact with virus containing material on contaminated hands of caregivers to their own mouth or eyes [14, 15]. Sexual transmission can also occur and virus has been detected in semen up to seven weeks after clinical recovery. Abstinence or use of condoms during intercourse and avoidance of breastfeeding is recommended for at least three months after recovery to prevent secondary cases [13].
Nosocomial or occupational transmission has also resulted in the spread of the infection. For instance, in the original 1976 outbreak of Ebola Zaire, usage of contaminated needles led to simultaneous Ebola infection of over one hundred patients. Another such example is spread of infection to an entire surgical team performing an exploratory laparotomy on an EBOV infected patient in Kikwit in 1995. In fact, many of the first generation cases in previous outbreaks have been health care workers who had early contact with the infected individuals. Spread of virus does not occur among health care workers when there is early recognition of cases and enforcement of appropriate infection control practices [14, 16].
Historically, outbreaks have gradually “burned out” on their own or have been controlled effectively by instituting good public health measures including isolation of sick individuals and barrier protection methods for care providers and burial services. This further leads epidemiologists to believe that transmission of these viruses requires direct contact or contact with infectious fluid as opposed to a possible aerosol route of transmission.
Pathogenesis and laboratory abnormalities
At the site of entry into the body, MARV and EBOV infect macrophages and other cells of the phagocytic system [12]. Dendritic cells and macrophages are the primary cells for viral replication. Macrophages are highly susceptible to infection and generate large numbers of viral particles and hence serve as vehicles for delivery of the virus to a variety of organs including the liver, endothelium, spleen, lymph nodes, kidney, adrenal gland, and pancreas [14]. Marked leukopenia with a left shift and atypical lymphocytes can be seen on peripheral smears of infected patients. While lymphocytes are not thought to be targets of infection by the virus, a dramatic decrease in the number of lymphocytes is thought to be a result of “bystander apoptosis” which is the death of large numbers of lymphocytes triggered by mediators released from virus infected target cells and/or secretion of viral glycoproteins (GP). Impaired secretion of pro-inflammatory cytokines and impaired stimulation of T cells has also been implicated as playing a role in this phenomenon [1, 12, 17–19].
Filovirus infections have a direct cytopathic effect resulting in damage and necrosis to many tissues, including hepatic cells. This results in elevated alanine and aspartate aminotransferase levels on blood chemistry. Hepatic transaminases usually rise rapidly by day 6–8 of infection [12, 13]. Thrombocytopenia is also routinely seen and disseminated intravascular coagulation (DIC) is an important feature of human filovirus infection. Tissue destruction of the endothelium may cause collagen exposure and tissue factor release. This has been postulated as the mechanism of DIC although some reports suggest that coagulation abnormalities may be triggered by tissue factor release directly from infected phagocytic cells [20]. The hallmark of pathogenesis is microvascular disease, impaired hemostasis and a decrease in total plasma protein reflecting a capillary leak syndrome. Renal insufficiency may occur with progression of illness probably due to a decrease in the effective circulatory volume. Severe intravascular depletion as a result of hemorrhage, metabolic abnormalities, and impaired oxygen delivery manifests as tachypnea, anuria, delirium, coma and shock.
Diagnostic tests
Diagnostic tests available for MARV and EBOV infections are research based assays and include virus isolation, detection of viral antigens in blood and body fluids using an enzyme linked immunosorbent assay (ELISA) and recognition of specific RNA sequences by reverse transcription polymerase chain reaction (RT-PCR) [21]. Confirmation of diagnosis can be done by isolating the causative agent in cell culture and visualizing viral particles using electron microscopy. These tests can only be performed at specialized high containment level laboratories such as at the CDC. If a clinical case is suspected, based on presentation and potential travel or occupational exposure, the hospital infection control services should be alerted, and the regional public health authorities as well as the national public health authorities should be contacted. Public health authorities can assist with sending blood specimens for analysis to the US CDC or other appropriate laboratories outside of the US for recognition of blood borne pathogens [22].
Treatment
There is currently no specific antiviral therapy available for Ebola or Marburg hemorrhagic fever and treatment is purely supportive. The clinical manifestations seen in filovirus hemorrhagic fever occur as a result of the host response to the viral infection and supportive care should be focused at maintenance of effective circulatory volume, blood pressure and perfusion. Fluid administration for intravascular volume resuscitation and transfusions for correction of coagulopathy and hemorrhage form the mainstay of treatment. These are all measures to sustain the patient while giving the immune system time to clear the infection and regulate the response. [22]. The common antiviral drug Ribivarin, which is used in the treatment of several other RNA viral infections, has no apparent activity against filoviruses [23].
Transfusions of blood obtained from convalescent patients were given to eight patients in a case report from the 1995 Ebola Zaire outbreak in Kikwit. This was not a controlled trial and the transfusion was administered at a varying interval of 2–15 days after onset of symptoms. Seven out of eight patients survived the infection and transfusion was thought to possibly have had a beneficial effect [24]. However, protection could not be demonstrated in EBOV infected rhesus macaques using convalescent serum raising the question that the benefit seen in the initial report was attributed to receiving better supportive medical care [25]. Purified immunoglobulin from horses immunized against EBOV with an investigational vaccine has been used for inoculation prior to EBOV challenge in mice and NHP models and has shown protection in some studies [25]. Use of this therapy as a means of post exposure treatment in the setting of accidental laboratory exposures has been approved in Russia [23]. The data on this approach is inconclusive and this approach has not been assessed in a controlled clinical trial and is not considered a recommended or standard therapy at this time. Taken together, these findings indicate that in addition to antibodies, an antigen specific T cell response is likely needed for protection.
Interferon alpha has also been utilized for treatment in cases of laboratory workers accidentally infected with filoviruses. However, it was administered as one of several components of combination therapy and as a supplement to routine supportive care. In a report by Jahrling et al, interferon alpha only slightly delayed viremia, onset of infection, and death in EBOV infected NHPs [25]. A prominent feature of filovirus hemorrhagic fever pathogenesis is activation of the coagulation cascade as a result of tissue factor release. Targeting this mechanism, rhesus macaques were treated using a recombinant nematode anticoagulant protein which is a potent inhibitor of tissue factor. Results of the study showed prolongation of survival time and attenuation of the pro-inflammatory response. However, this therapy when used in an identical experimental setting using the MARV Angola strain was less effective [26, 27].
Activated protein C has also been evaluated as an experimental post exposure treatment in a NHP study. The justification for its use stems from the clinical similarities seen in filovirus infections and severe sepsis, specifically fever, hypotension and disseminated intravascular coagulation. Serum levels of protein C have shown to be significantly reduced in EBOV infection in NHPs and this is also one of the characteristic features of sepsis. A trial of activated protein C via intravenous infusion thirty to sixty minutes after inoculation with Zaire EBOV in rhesus macaques showed an increase in survival time, lower levels of viremia and reduced activation of the coagulation cascade. Although the experimental design may not reflect a natural infection, these data indicate that activated protein C could be further evaluated as a potential treatment for filovirus infections [28]. A recombinant vesicular stomatitis virus (VSV) investigational vaccine encoding MARV surface GP’s has also been tested as post exposure treatment. When administered up to 30 minutes after virus exposure, this vaccine conferred some protection in a NHP study [29, 30].
With no effective therapy available currently, supportive care and controlling the spread of virus to others is the mainstay of treatment. Any patient with a suspicion of illness should be isolated immediately and barrier techniques should be instituted. In addition, strict precautions should be taken when handling specimens from infected patients to avoid spread to caregivers. Protective measures like gloves, gowns, face shields, masks and eye protection should be consistently used. In addition, CDC guidelines also recommend the use of respiratory precautions by using the N-95 mask or air purifying respirator. Cleaning and disinfection of articles that cannot be discarded and decontamination of surfaces and objects that the patient comes into contact with must be done to prevent spread to health care workers and family members [14].
Discovery, Outbreak History, and Imported Cases
In August 1967, simultaneous accounts of malaise, headache, fever, nausea, vomiting, and diarrhea along with other unusual symptoms were reported in Marburg and Frankfurt, Germany. Preliminary diagnoses were dysentery and typhoid fever but these were ruled out [31]. Microbiological and epidemiological investigation later showed that thirty-two individuals, in both Germany and Yugoslavia, had been exposed to the newly identified MARV after contact with the blood, organs and cell cultures of infected NHPs from Uganda [32–34]. This outbreak resulted in seven fatalities.
Almost a full decade later, in the summer of 1976, fatalities from hemorrhagic fever were reported in Zaire (now Democratic Republic of Congo, DRC) [5]. In total, 280 fatalities occurred with 318 documented human cases. Named for the river near the area of outbreak, Ebola virus infection caused an additional 151 fatalities in 284 cases in a nearly simultaneous outbreak in southern Sudan. Subsequent microbiological and epidemiological research demonstrated that the two outbreaks were independent events, caused by different strains of EBOV (Zaire and Sudan/Gulu strains respectively) [35].
EBOV reappeared two more times in Africa within the decade, causing a single human case in the DRC in 1977 and 34 human cases two years later in Sudan, with 22 fatalities. Human cases of EBOV were not identified again until the early 1990’s. It is unknown what ecological, environmental, or other factors may be attributable to the decade’s long apparent disappearance, followed by re-emergence, of EBOV infection (Table 1).
Table 1.
Filovirus Outbreak Chronology
| Year | Country | Virus | Apparent/Suspected Origin or Subtype | Human Cases | Case Fatalities |
|---|---|---|---|---|---|
| 1967 | Germany and Yugoslavia | Marburg | Uganda | 31 | 7 |
| 1975 | South Africa | Marburg | Zimbabwe | 3 | 1 |
| 1976 | Zaire (Democratic Republic of Congo (DRC) | Ebola | Ebola-Zaire | 318 | 280 |
| 1976 | Sudan | Ebola | Ebola-Sudan | 284 | 151 |
| 1976 | England | Ebola | Ebola-Sudan | 1 | 1 |
| 1977 | Zaire (DRC) | Ebola | Ebola-Zaire | 1 | 1 |
| 1979 | Sudan | Ebola | Ebola-Sudan | 34 | 22 |
| 1980 | Kenya | Marburg | Kenya | 2 | 1 |
| 1987 | Kenya | Marburg | Kenya | 1 | 1 |
| 1989–1990 | Philippines | Ebola | Ebola-Reston | 3 | 0 |
| 1990 | USA | Ebola | Ebola-Reston | 4 | 0 |
| 1994 | Gabon | Ebola | Ebola-Zaire | 52 | 31 |
| 1994 | Ivory Coast | Ebola | Ebola-Ivory Coast | 1 | 0 |
| 1995 | DRC | Ebola | Ebola-Zaire | 315 | 250 |
| 1996 | Gabon | Ebola | Ebola-Zaire | 37 | 21 |
| 1996–1997 | Gabon | Ebola | Ebola-Zaire | 60 | 45 |
| 1996 | South Africa | Ebola | Ebola-Zaire | 2 | 1 |
| 1998–2000 | DRC | Marburg | DRC | 154 | 128 |
| 2000–2001 | Uganda | Ebola | Ebola-Sudan | 425 | 224 |
| 2001–2002 | Gabon | Ebola | Ebola-Zaire | 65 | 53 |
| 2001–2002 | Republic of Congo | Ebola | Ebola-Zaire | 57 | 43 |
| 2002–2003 | Republic of Congo | Ebola | Ebola-Zaire | 143 | 129 |
| 2003 | Republic of Congo | Ebola | Ebola-Zaire | 35 | 29 |
| 2004 | Sudan | Ebola | Ebola-Sudan | 17 | 7 |
| 2004–2005 | Angola | Marburg | Angola | 252 | 227 |
| 2007 | DRC | Ebola | Ebola-Zaire | 264 | 187 |
| 2007 | Uganda | Marburg | Uganda | 2 | 2 |
| 2007–2008 | Uganda | Ebola | Ebola-Bundibugyo | 149 | 37 |
| 2008 | Netherlands | Marburg | Uganda | 1 | 1 |
| 2008 | Philippines | Ebola | Ebola-Reston | 6 | 0 |
| 2008/2009 | USA | Marburg | Uganda | 1 | 0 |
Source: CDC and WHO
In the three decades following discovery, MARV infection appeared only six times but with high fatality rates [3]. Acquisition of MARV in index cases between 1975 and 1987 all reflect relevant travel history. In 1975, a male backpacker travelling through Zimbabwe was the index case to two additional infections, in 1980 a male traveler who visited Kitum Cave in Kenya caused one subsequent infection, and in 1987 a 15-year old male traveler who had also visited Kitum Cave in Kenya succumbed to MARV with no additional cases having been detected.
An outbreak of MARV infection came in 1998 and sporadic cases persisted in the DRC causing 154 cases and 128 fatalities over two years [36]. The majority of the initial cases were male workers at a gold mine in Durba and subsequent cases were detected in neighboring villages affecting family members involved in the close care of those patients [15, 37]. A novel epidemiologic pattern was revealed in the Durba outbreak with considerable differences seen in viruses isolated from primary cases, suggesting repeated MARV introduction within the population from distinct sources [3].
Human outbreaks of EBOV between 1994 and 1997 in Gabon, Ivory Coast, and DRC, caused 467 total cases involving 348 fatalities. The large number of cases in this three-year period revealed the new Ebola subtype of E. Ivory Coast, as well as an escalation of outbreaks and outbreak potential [33, 35]. The index case for the largest outbreak in this time period occurring in Kikwit, DRC would later be linked to work in the forest near the city. The 2000–2001 Ebola virus outbreaks in Uganda consisted of over four hundred cases and the three most important risk factors associated with acquiring infection were attending funerals of those affected, having close contact with case patients as family members, and providing medical care to Ebola case patients [1]. Between 2007 and 2008 two additional outbreaks of EBOV were detected in DRC and Uganda, with identification of the Ebola subtype Bundibugyo. The outbreaks involved over four hundred cases with more than two hundred deaths.
The largest MARV outbreak on record was the 2004–2005 outbreak in Angola. Initial cases were detected within the pediatric ward of Uige provincial hospital and involved rapid spread through hospital acquired infections. In total, 252 cases were documented with 227 deaths. In September 2007, mine workers in the Kitaka Cave in Uganda were diagnosed with Marburg fever. The likely source of this infection was thought to be the cave dwelling Egyptian fruit bats. This was based on detection of Marburg virus RNA and virus specific antibody in bat sera collected by researchers [10].
The basis for the trend towards more frequently recognized filovirus outbreaks is not known, but likely involves ecological and social changes as well as improved diagnostics. This pattern suggests that filovirus infections will continue to be a public health issue in the future (Table 1). The appearance, disappearance, and subsequent more virulent reemergence of both Ebola and Marburg viruses raise alarms for health care practitioners. With an upsurge in travel to central and eastern Africa, there is also the potential for an increased number of imported cases to non-endemic areas.
Imported Case 1
On July 10, 2008, the Dutch National Institute for Public Health and the Environment released a statement announcing confirmation of MARV infection in a 41-year old woman who had recently returned from travel in Uganda [38].
Case summary [38–40]
A 41-year old Dutch female spent June 5 through June 28, 2008 touring Uganda, including visits to an empty cave on June 16 in Fort Portal and the attraction known as the Python Cave in the Maramagambo Forest, Queen Elizabeth Park on June 19. The Python cave is famous for the presence of a large number of pythons and bats and there had been reports of bats coming into contact with cave visitors [39].
On July 5th, after 3 days of chills and fever, she was referred to Elkerliek Hospital by her primary physician. Based on her recent travel history, malaria and other routine microbiological testing was performed. By July 7th, her condition worsened to include evidence of liver failure. Viral hemorrhagic fever was considered and as a safety precaution, the woman was transferred to an isolation room in Leiden University Medical Centre. Between July 7 and July 10 she developed rash, conjunctivitis, diarrhea, liver failure, kidney failure, and evidence of hemorrhaging. Over the course of these few days, plasma samples were sent to the Bernhard-Nocht-Institute for Tropical Medicine in Hamburg, Germany for filovirus antibody and RNA detection. On July 10, a positive RTPCR result for MARV, confirmed by sequence analysis of the polymerase gene, was reported. MARV presence was also confirmed by PCR by the Department of Virology at Erasmus Medical College in Rotterdam, the Netherlands. During the night between July 10 and July 11, the woman died of multi-organ system failure and cerebral edema. No secondary cases of Marburg hemorrhagic fever were reported or found by public health authorities.
Imported Case 2
On January 22, 2009, the US Centers for Disease Control and Prevention retrospectively diagnosed a case of Marburg virus infection in a US tourist who had been severely ill after vacationing in Uganda in December of 2007 [41].
Case Summary [6, 11, 41, 42]
A 44-year old American woman spent December 17 through December 31, 2007 touring Uganda, including a visit to the Python Cave in the Maramagambo Forest, Queen Elizabeth Park.
On January 4, 2008, four days after returning home to Denver from her vacation to Uganda, the female traveler experienced severe headache, chills, nausea, vomiting, and diarrhea. She also noted a loss of appetite and vaginal bleeding out of her normal cycle. Between January 6th and 7th, she consulted a physician, began treatment with anti-emetics and had laboratory testing performed which revealed an abnormally low white blood cell count (900/uL). The patient developed a rash and reported feeling that her thinking had become ‘foggy’. On January 8th, she had developed a diffuse macular rash, her illness worsened, and she was admitted to the hospital. Though afebrile upon admission, laboratory results revealed marked hepatic enzyme elevations and renal failure. While in the hospital, the patient was treated with IV fluids and empiric antibiotics. She also developed pancytopenia, coagulopathy, myositis, pancreatitis, and encephalopathy. Public health officials were involved in her evaluation and testing was negative for Yellow Fever, Dengue 1–4, Chikungunya, Malaria, West Nile virus, acute Schistosomiasis, Ebola/Marburg virus, Ricksettsial infection, Leptospirosis, HSV, and other viruses. Serum samples collected on January 14 were negative for MARV infection by virus isolation and by ELISA for anti-MARV IgM and IgG. On January 19 the woman was released from the hospital where she had a slow recovery.
In July 2008, while still recovering from persistent abdominal pain, fatigue, weakness, insomnia, and issues with memory and mental clarity, the patient heard the news story of a Dutch tourist succumbing to Marburg hemorrhagic fever following a visit to Uganda. She brought this to the attention of her physicians and requested reconsideration of her case. Samples collected from the patient during her illness were retested and a July 15, 2008 sample tested positive for anti-MARV IgG by ELISA. Nested RTPCR also confirmed MARV RNA fragments in the January 2008 sample. No secondary cases of Marburg infection were reported or found by public health authorities.
Vaccine Research
Beyond the morbidity and mortality experienced by individuals, regions affected by filovirus outbreaks suffer a negative economic impact. Imported cases of Marburg infection also highlight the risk of spread that can occur as a result of travel.
There is no approved vaccine for use to prevent EBOV or MARV infection and no effective therapy available against filoviruses. Hence, development of a safe and effective vaccine remains a goal of researchers and public health officials.
Due to theoretical safety concerns, conventional vaccine platforms such as live attenuated and killed virus vaccines are unlikely to be used in humans. Several animal models have been developed to study the pathogenesis of filovirus infection and to assess the efficacy of vaccination strategies. Some of these approaches have been evaluated in preclinical studies using guinea pigs, mice and NHP animal models. NHPs, which develop a clinical infection similar to humans, represent the most relevant models of infection. The majority of investigational platforms have focused on the use of live and replication defective virus vectors, virus like particles, and DNA vaccines expressing filovirus proteins.
Several vaccination platforms have shown varying success in small animal models. A virus like particle based vaccine elicited immune responses and showed some protection in guinea pigs [43, 44]. Recombinant vaccinia virus vectors expressing EBOV surface proteins were able to protect guinea pigs from lethal challenge [45, 46]. DNA plasmid vaccines encoding Ebola proteins have been shown to protect guinea pigs and mice against viral challenge [45, 47–49].
A chimeric human parainfluenza virus vector expressing the EBOV GP as the sole surface transmembrane envelope protein combined with internal proteins of parainfluenza virus has also been examined in animal models. Results showed that the vaccine was immunogenic and an intranasal dose showed protection against a lethal intraperitoneal challenge of guinea pig adapted EBOV [50].
While important customary models for initial evaluation in vaccine research, small animal models (mice and guinea pigs) of EBOV hemorrhagic fever may not accurately represent infection in NHPs and humans. Mice do not have the hallmark disseminated intravascular coagulation found in human and non-human primate filovirus infections. Small animal models are also easier to protect from filovirus infection than NHP models. Moreover, viremia and widespread tissue dissemination are much more apparent in NHPs and lymphocyte apoptosis is not reported to be a prominent feature of EBOV infection in mice or guinea pigs but is a consistent feature of disease in humans and NHPs [12, 46].
Multiple vaccine systems are currently being tested but only adenovirus vectors, vesicular stomatitis virus vector, and DNA vaccines combined with an adenovirus vector boost have shown efficacy against lethal EBOV challenge in NHP models of infection. A replication competent recombinant VSV vector vaccine expressing GP from three different species of EBOV was tested in non-human primates and none of the vaccinated animals succumbed to the infection. As mentioned earlier this vaccine has also been studied as an immediate post exposure vaccination strategy [51]. VSV-based EBOV vaccine has also been shown to be safe in immunocompromised simian human immunodeficiency virus (SHIV) infected NHPs and conferred partial protection against a lethal challenge in all but the most severely immunocompromised animals [52]. A combination of DNA immunization and boosting with adenovirus vectors that encode Ebola proteins (mainly GP) generated cellular and humoral immunity in cynomolgous macaques. All vaccinated animals tolerated the vaccinations and were protected and remained asymptomatic for more than six months [53].
Adenovirus vector vaccines systems are replication defective and have been examined in previous preclinical studies in NHPs as well as previous phase I clinical trials and were found to be safe and immunogenic [54, 55]. NHPs vaccinated with adenovirus expressing both Zaire EBOV GP and nucleoprotein (NP) and boosted with a second dose of the same vaccine nine weeks later and then challenged with lethal doses of Zarie EBOV were completely protected from clinical illness, and showed both humoral and cellular immune responses [55]. In a recent report, a combination or “panfilovirus” investigational adenovirus vector based vaccine that expresses Ebola surface GP from five different filoviruses including from strains of EBOV and MARV was tested. Immunization of NHPs demonstrated protection against lethal challenge with EBOV and MARV [56].
Utilizing some of the successful approaches described above, clinical trials using candidate vaccines have been conducted at the Vaccine Research Center at the National Institute of Health with follow on trials conducted at Makerere University in Kampala, Uganda. The first clinical trial testing an EBOV DNA vaccine was conducted from 2003–2004. The study enrolled twenty-seven healthy adult volunteer participants. The candidate vaccine was a multigene plasmid DNA vaccine as a mixture of three plasmids in equal concentrations that were constructed to produce three EBOV proteins: the NP from the Zaire species as well as two GP’s which are thought to be the key protective antigenic targets. Three groups of volunteers were vaccinated three times at approximately 4-week intervals by intramuscular injection of 2 mg (n = 5), 4 mg (n = 8) or 8 mg (n = 8) of the combination EBOV vaccine. The study also enrolled 6 placebo recipients. Antibodies to at least one of the three antigens were measured by ELISA in samples from all vaccine recipients. This study demonstrated that the EBOV GP encoding DNA vaccine was safe and immunogenic in humans [48].
Based on evidence from preclinical studies in which an adenovirus vector vaccine was able to protect NHPs against lethal virus challenge [55], a second Phase I clinical trial was conducted by the Vaccine Research Center evaluating a second generation version of the EBOV GP antigen. Recombinant adenovirus serotype 5 vectors encoding GP from either the Zaire or Sudan/Gulu strain were tested as a combination vaccine in a randomized, placebo controlled double blinded, dose escalation study. Thirty two volunteers were enrolled in this study. The vaccine was found to be safe and induced humoral as well as T cell responses to the Ebola glycoprotein inserts [57].
Each clinical trial assessed a more complete version of the EBOV GP, with a third generation of filovirus vaccines in trials in the USA and Uganda expressing full length, wild type EBOV or MARV GP antigens delivered in a DNA plasmid platform.
Although much headway has been made so far as demonstrated by favorable outcomes seen in both preclinical animal studies as well as human trials, more work is still needed. While the field seems settled on wild type GP as the optimal immunogenic antigen for vaccination, further research is necessary to evaluate the most ideal strategy for immunization, including platforms that will be safe and immunogenic in a broad population. Ongoing NHP and clinical research will help to guide further vaccine research and development.
Conclusion
EBOV and MARV are important viral pathogens and have the ability to cause devastating human disease, as seen in regional outbreaks and imported cases. A safe, effective and immunogenic vaccine would be a significant step forward in addressing the public health threat that filoviruses continue to pose. Preventive vaccination could potentially be used to provide protection for first responders, travelers, medical personnel, those at risk due to occupational exposures, military troops, as well as family members and caregivers. Long term immunity would be highly desirable for those that routinely come into contact with these deadly viruses [45]. A vaccine could potentially curtail the spread of these viruses and provide an added measure to contain future outbreaks. National public health officials and military organizations have indicated an interest in a filovirus vaccine. Filoviruses are Category A agents considered to have a high priority for intervention strategies. Furthermore, a vaccine would also be appealing to travelers who are at risk. Receiving vaccination before travel to endemic regions of the world could help prevent life threatening disease. An effective preventive vaccine has the potential to defend against regional outbreaks and reduce the risk of global spread of filovirus infections.
Acknowledgments
The authors thank Dr. Barney S. Graham, Dr. Ed Tramont and Michelle Barnes for their review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bray M. Epidemiology, pathogenesis and clinical manifestations of Ebola and Marburg hemorrhagic Fever. Jul 28, Uptodate 2009. [Google Scholar]
- 2.Murdoc D. Diseases potentially acquired by travel to Sub Saharan Africa. 2008. Sep 8, Uptodate. [Google Scholar]
- 3.Bray M, Murphy FA. Filovirus research: knowledge expands to meet a growing threat. J Infect Dis. 2007;196 (Suppl 2):S438–43. doi: 10.1086/520552. [DOI] [PubMed] [Google Scholar]
- 4.Isaacson M. Viral hemorrhagic fever hazards for travelers in Africa. Clin Infect Dis. 2001;33:1707–12. doi: 10.1086/322620. [DOI] [PubMed] [Google Scholar]
- 5.CDC. Ebola hemorrhagic fever: known cases and outbreaks of Ebola hemorrhagic fever in chronoligical order. 2009. Aug 26, [Google Scholar]
- 6.CDC. Outbreak Postings. 2009. Dec 23, Special Pathogens Branch. [Google Scholar]
- 7.Bermejo M, Rodriguez-Teijeiro JD, Illera G, Barroso A, Vila C, Walsh PD. Ebola outbreak killed 5000 gorillas. Science. 2006;314:1564. doi: 10.1126/science.1133105. [DOI] [PubMed] [Google Scholar]
- 8.Thacker PD. An Ebola epidemic simmers in Africa: in remote region, outbreak shows staying power. JAMA. 2003;290:317–9. doi: 10.1001/jama.290.3.317. [DOI] [PubMed] [Google Scholar]
- 9.Leroy EM, Kumulungui B, Pourrut X, et al. Fruit bats as reservoirs of Ebola virus. Nature. 2005;438:575–6. doi: 10.1038/438575a. [DOI] [PubMed] [Google Scholar]
- 10.Towner JS, Amman BR, Sealy TK, et al. Isolation of genetically diverse Marburg viruses from Egyptian fruit bats. PLoS Pathog. 2009;5:e1000536. doi: 10.1371/journal.ppat.1000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CDC. Imported case of Marburg Hemorrhagic Fever-Colorado 2008. MMWR - Morbidity & Mortality Weekly Report. 2009 December 18;:1377–81. [PubMed] [Google Scholar]
- 12.Stroher U, Feldmann H. Progress towards the treatment of Ebola haemorrhagic fever. Expert Opin Investig Drugs. 2006;15:1523–35. doi: 10.1517/13543784.15.12.1523. [DOI] [PubMed] [Google Scholar]
- 13.WHO. Marburg Hemorrhagic Fever. 2008. Jul, [Google Scholar]
- 14.Bray M. Defense against filoviruses used as biological weapons. Antiviral Res. 2003;57:53–60. doi: 10.1016/s0166-3542(02)00200-0. [DOI] [PubMed] [Google Scholar]
- 15.Dowell SF, Mukunu R, Ksiazek TG, Khan AS, Rollin PE, Peters CJ. Transmission of Ebola hemorrhagic fever: a study of risk factors in family members, Kikwit, Democratic Republic of the Congo, 1995. Commission de Lutte contre les Epidemies a Kikwit. J Infect Dis. 1999;179 (Suppl 1):S87–91. doi: 10.1086/514284. [DOI] [PubMed] [Google Scholar]
- 16.Fisher-Hoch SP. Lessons from nosocomial viral haemorrhagic fever outbreaks. Br Med Bull. 2005;73–74:123–37. doi: 10.1093/bmb/ldh054. [DOI] [PubMed] [Google Scholar]
- 17.Leroy EM, Baize S, Volchkov VE, et al. Human asymptomatic Ebola infection and strong inflammatory response. Lancet. 2000;355:2210–5. doi: 10.1016/s0140-6736(00)02405-3. [DOI] [PubMed] [Google Scholar]
- 18.Baize S, Leroy EM, Mavoungou E, Fisher-Hoch SP. Apoptosis in fatal Ebola infection. Does the virus toll the bell for immune system? Apoptosis. 2000;5:5–7. doi: 10.1023/a:1009657006550. [DOI] [PubMed] [Google Scholar]
- 19.Baize S, Leroy EM, Georges-Courbot MC, et al. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat Med. 1999;5:423–6. doi: 10.1038/7422. [DOI] [PubMed] [Google Scholar]
- 20.Schnittler HJ, Feldmann H. Viral hemorrhagic fever--a vascular disease? Thromb Haemost. 2003;89:967–72. [PubMed] [Google Scholar]
- 21.Saijo M, Niikura M, Ikegami T, Kurane I, Kurata T, Morikawa S. Laboratory diagnostic systems for Ebola and Marburg hemorrhagic fevers developed with recombinant proteins. Clin Vaccine Immunol. 2006;13:444–51. doi: 10.1128/CVI.13.4.444-451.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bray M. Diagnosis and treatment of Ebola and Marburg hemorrhagic fever. 2008. Sep 30, Uptodate. [Google Scholar]
- 23.Bray M, Paragas J. Experimental therapy of filovirus infections. Antiviral Res. 2002;54:1–17. doi: 10.1016/s0166-3542(02)00005-0. [DOI] [PubMed] [Google Scholar]
- 24.Mupapa K, Massamba M, Kibadi K, et al. Treatment of Ebola Hemorrhagic Fever with Blood Transfusions from Convalescent Patients. The Journal of Infectious Diseases. 1999;179:S18–S23. doi: 10.1086/514298. [DOI] [PubMed] [Google Scholar]
- 25.Jahrling PB, Geisbert TW, Geisbert JB, et al. Evaluation of immune globulin and recombinant interferon-alpha2b for treatment of experimental Ebola virus infections. J Infect Dis. 1999;179 (Suppl 1):S224–34. doi: 10.1086/514310. [DOI] [PubMed] [Google Scholar]
- 26.Geisbert TW, Daddario-DiCaprio KM, Geisbert JB, et al. Marburg virus Angola infection of rhesus macaques: pathogenesis and treatment with recombinant nematode anticoagulant protein c2. J Infect Dis. 2007;196 (Suppl 2):S372–81. doi: 10.1086/520608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geisbert TW, Hensley LE, Jahrling PB, et al. Treatment of Ebola virus infection with a recombinant inhibitor of factor VIIa/tissue factor: a study in rhesus monkeys. Lancet. 2003;362:1953–8. doi: 10.1016/S0140-6736(03)15012-X. [DOI] [PubMed] [Google Scholar]
- 28.Hensley LE, Stevens EL, Yan SB, et al. Recombinant human activated protein C for the postexposure treatment of Ebola hemorrhagic fever. J Infect Dis. 2007;196 (Suppl 2):S390–9. doi: 10.1086/520598. [DOI] [PubMed] [Google Scholar]
- 29.Daddario-DiCaprio KM, Geisbert TW, Stroher U, et al. Postexposure protection against Marburg haemorrhagic fever with recombinant vesicular stomatitis virus vectors in non-human primates: an efficacy assessment. Lancet. 2006;367:1399–404. doi: 10.1016/S0140-6736(06)68546-2. [DOI] [PubMed] [Google Scholar]
- 30.Geisbert TW, Daddario-DiCaprio KM, Williams KJ, et al. Recombinant vesicular stomatitis virus vector mediates postexposure protection against Sudan Ebola hemorrhagic fever in nonhuman primates. J Virol. 2008;82:5664–8. doi: 10.1128/JVI.00456-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slenczka W, Klenk HD. Forty years of marburg virus. J Infect Dis. 2007;196 (Suppl 2):S131–5. doi: 10.1086/520551. [DOI] [PubMed] [Google Scholar]
- 32.Balter M. Emerging diseases. On the trail of Ebola and Marburg viruses. Science. 2000;290:923–5. doi: 10.1126/science.290.5493.923. [DOI] [PubMed] [Google Scholar]
- 33.Borchert M, Boelaert M, Sleurs H, et al. Viewpoint: filovirus haemorrhagic fever outbreaks: much ado about nothing? Trop Med Int Health. 2000;5:318–24. doi: 10.1046/j.1365-3156.2000.00556.x. [DOI] [PubMed] [Google Scholar]
- 34.Peters CJ. Marburg and Ebola--arming ourselves against the deadly filoviruses. N Engl J Med. 2005;352:2571–3. doi: 10.1056/NEJMp058109. [DOI] [PubMed] [Google Scholar]
- 35.Pourrut X, Kumulungui B, Wittmann T, et al. The natural history of Ebola virus in Africa. Microbes Infect. 2005;7:1005–14. doi: 10.1016/j.micinf.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 36.CDC. Chronological Order. 2009. Jul 31st, Known Cases and Outbreaks of Marburg Hemorrhagic Fever. [Google Scholar]
- 37.Bausch DG, Borchert M, Grein T, et al. Risk factors for Marburg hemorrhagic fever, Democratic Republic of the Congo. Emerg Infect Dis. 2003;9:1531–7. doi: 10.3201/eid0912.030355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Environment NIfPHat. Dutch Tourist infected with Marburg Virus. 2008. Jul 10, [Google Scholar]
- 39.Timen A, Koopmans MP, Vossen AC, et al. Response to imported case of Marburg hemorrhagic fever, the Netherland. Emerg Infect Dis. 2009;15:1171–5. doi: 10.3201/eid1508.090051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.WHO. Case of Marburg Hemorrhagic fever imported into the Netherlands from Uganda. 2008. Jul 10, [Google Scholar]
- 41.Barnes M, Fuijita NK. Marburg Virus in a Wheat Ridge Colorado Hospital 2008. Bethesda: Maryland National Institutes of Health; 2009. Jun 22, [Google Scholar]
- 42.CDC. Clinician Outreach and Communication Activity Update for February 2009. 2009. Feb, [Google Scholar]
- 43.Swenson DL, Warfield KL, Negley DL, Schmaljohn A, Aman MJ, Bavari S. Virus-like particles exhibit potential as a pan-filovirus vaccine for both Ebola and Marburg viral infections. Vaccine. 2005;23:3033–42. doi: 10.1016/j.vaccine.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 44.Warfield KL, Swenson DL, Negley DL, Schmaljohn AL, Aman MJ, Bavari S. Marburg virus-like particles protect guinea pigs from lethal Marburg virus infection. Vaccine. 2004;22:3495–502. doi: 10.1016/j.vaccine.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 45.Bausch DG, Geisbert TW. Development of vaccines for Marburg hemorrhagic fever. Expert Rev Vaccines. 2007;6:57–74. doi: 10.1586/14760584.6.1.57. [DOI] [PubMed] [Google Scholar]
- 46.Geisbert TW, Jahrling PB. Towards a vaccine against Ebola virus. Expert Rev Vaccines. 2003;2:777–89. doi: 10.1586/14760584.2.6.777. [DOI] [PubMed] [Google Scholar]
- 47.Geisbert TW, Pushko P, Anderson K, Smith J, Davis KJ, Jahrling PB. Evaluation in nonhuman primates of vaccines against Ebola virus. Emerg Infect Dis. 2002;8:503–7. doi: 10.3201/eid0805.010284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin JE, Sullivan NJ, Enama ME, et al. A DNA vaccine for Ebola virus is safe and immunogenic in a phase I clinical trial. Clin Vaccine Immunol. 2006;13:1267–77. doi: 10.1128/CVI.00162-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sullivan N, Yang ZY, Nabel GJ. Ebola virus pathogenesis: implications for vaccines and therapies. J Virol. 2003;77:9733–7. doi: 10.1128/JVI.77.18.9733-9737.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bukreyev A, Marzi A, Feldmann F, et al. Chimeric human parainfluenza virus bearing the Ebola virus glycoprotein as the sole surface protein is immunogenic and highly protective against Ebola virus challenge. Virology. 2009;383:348–61. doi: 10.1016/j.virol.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Geisbert TW, Geisbert JB, Leung A, et al. Single-injection vaccine protects nonhuman primates against infection with marburg virus and three species of ebola virus. J Virol. 2009;83:7296–304. doi: 10.1128/JVI.00561-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Geisbert T, Daddario KM, Lewis MG, et al. Vesicular Stomatitis Virus-Based Ebola Vaccine is Well Tolerated and Protects Immunocompromised Nonhuman Primates. PLoS Med. 2008:4. doi: 10.1371/journal.ppat.1000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sullivan NJ, Sanchez A, Rollin PE, Yang ZY, Nabel GJ. Development of a preventive vaccine for Ebola virus infection in primates. Nature. 2000;408:605–9. doi: 10.1038/35046108. [DOI] [PubMed] [Google Scholar]
- 54.Graham B. AIDS Vaccine 2005. Montreal, Canada: 2005. Safety and immunogenicity of a multiclade HIV-1 recombinant adenovirus vacine boost in prior recipients of a multiclade HIV-1 DNA vaccine (paper #15(827P)) [Google Scholar]
- 55.Sullivan NJ, Geisbert TW, Geisbert JB, et al. Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates. Nature. 2003;424:681–4. doi: 10.1038/nature01876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Swenson DL, Wang D, Luo M, et al. Vaccine to confer to nonhuman primates complete protection against multistrain Ebola and Marburg virus infections. Clin Vaccine Immunol. 2008;15:460–7. doi: 10.1128/CVI.00431-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ledgerwood JE, Costner P, Desai N, Holman L, Enama ME, Yamshchikov G, Mulangu S, Hu Z, Andrews CA, Sheets RA, Koup RA, Roederer M, Bailer R, Mascola JR, Sullivan NJ, Nabel GJ, Graham BS the VRC 205 Study Team. A Replication Defective Recombinant Ad5 Vaccine Expressing Ebola GP Is Safe and Immunogenic. Healthy Adults. doi: 10.1016/j.vaccine.2010.10.037. Submitted. [DOI] [PubMed] [Google Scholar]