Abstract
Rate enhancements for the reduction of dioxygen by a MnII complex were observed in the presence of redox inactive Group 2 metal ions. The rate changes correlated with an increase in the Lewis acidity of the Group 2 metal ions. These studies led to the isolation of heterobimetallic complexes that contain MnIII-(μ-OH)-MII cores (MII = CaII, BaII), in which the hydroxo oxygen atom is derived from O2. This type of core structure has relevance to the oxygen evolving complexes within photosystem II.
Metal ions that function as Lewis acids are known to have a major influence on a variety of chemical transformations.1 Often they are used in combination with redox-active transition metal complexes to promote a variety of reactions involving the transfer of electrons.2 This is typified in metalloproteins, such as the copper-zinc superoxide dismutases, in which both metal ions have been proposed to be functionally active.3 In addition, it is now widely accepted that a redox inactive CaII ion is necessary for water oxidation to dioxygen in the OEC.4, 5 Examples of these effects are also found in synthetic systems: Yu showed that Group 1 metal ions promote the C–H bond activation in a series of PdII complexes,6 Lau illustrated that Lewis acidic metal ions enhance the reactivity of metal-oxo complexes toward oxidation of alkanes,7 and Collins demonstrated that secondary ions activate O-atom transfer reactions of MnV=O complexes.8 Moreover, Fukuzumi and Nam recently reported that the rates of electron transfer from FeIV=O complexes are significantly altered in the presence of Lewis acidic metal ions, which led to the isolation of a complex containing an FeIV=O---ScIII unit.9 Fukuzumi further demonstrated that the rates for the one-electron conversion of O2 to superoxide ion are correlated to the Lewis acidities of redox inactive metal ions,10 implying that redox inactive metal ions may play a role in the activation of dioxygen.11 We report herein that the rates of O2 reduction by a monomeric MnII complex are accelerated in the presence of Group 2 metal ions. These findings led to the isolation and structural characterization of a discrete heterobimetallic complex containing a MnIII–(μ-OH)–CaII core,12,13 a construct that is related to the active site of the oxygen-evolving complex (OEC) in photosystem II.
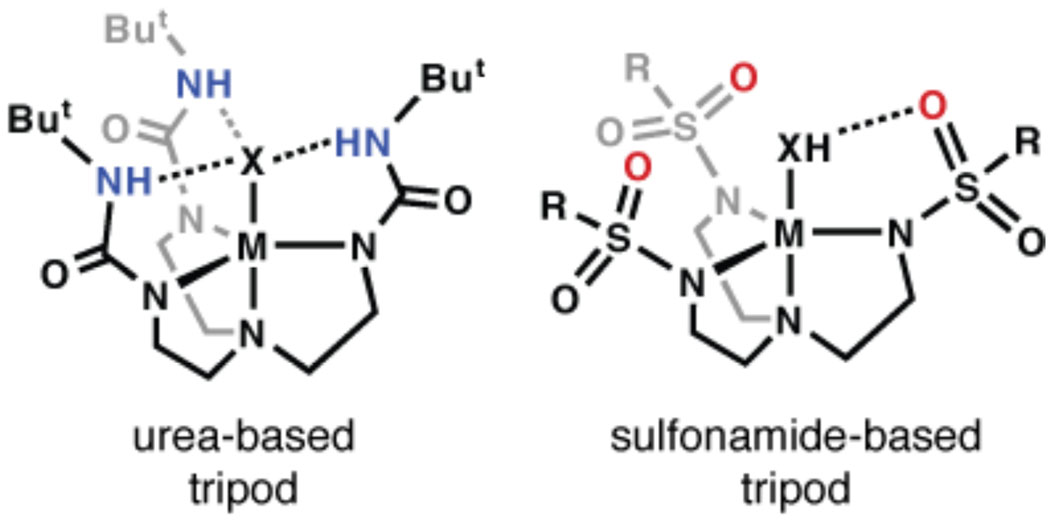
Our group has been investigating the influences of the secondary coordination sphere on metal-mediated processes.14 In particular, we have developed ligands that promote intramolecular hydrogen bonding (H-bonding) networks within the secondary coordination spheres of metal ions. Most of our ligands contain H-bond donors, such as the urea-based ligand [H3buea]3−, whose inward placement of NH groups produce positively polarized cavities (Figure 1). To reverse the polarity of the cavity we designed the sulfonamido-based tripodal ligand N,N′,N″-[2,2',2''-nitrilotris(ethane-2,1-diyl)]tris-(2,4,6-trimethylbenzenesulfonamido) ([MST]3−, Figure 1)15 and show that the SO2R groups can serve as H-bond acceptors. Previously reported ligands with sulfonamido groups are almost exclusively C2 symmetric, such as those of Walsh who showed that both nitrogen and oxygen atoms are capable of coordinating to a metal ion.16 During the course of our studies with the MnII complex [MnIIMST]− we discovered that the oxygen atom of the SO2Ar groups can bind Group 2 metal ions, which appears to be a requirement for the acceleration of the dioxygen activation process.
Figure 1.
Examples of systems containing intramolecular H-bonds.
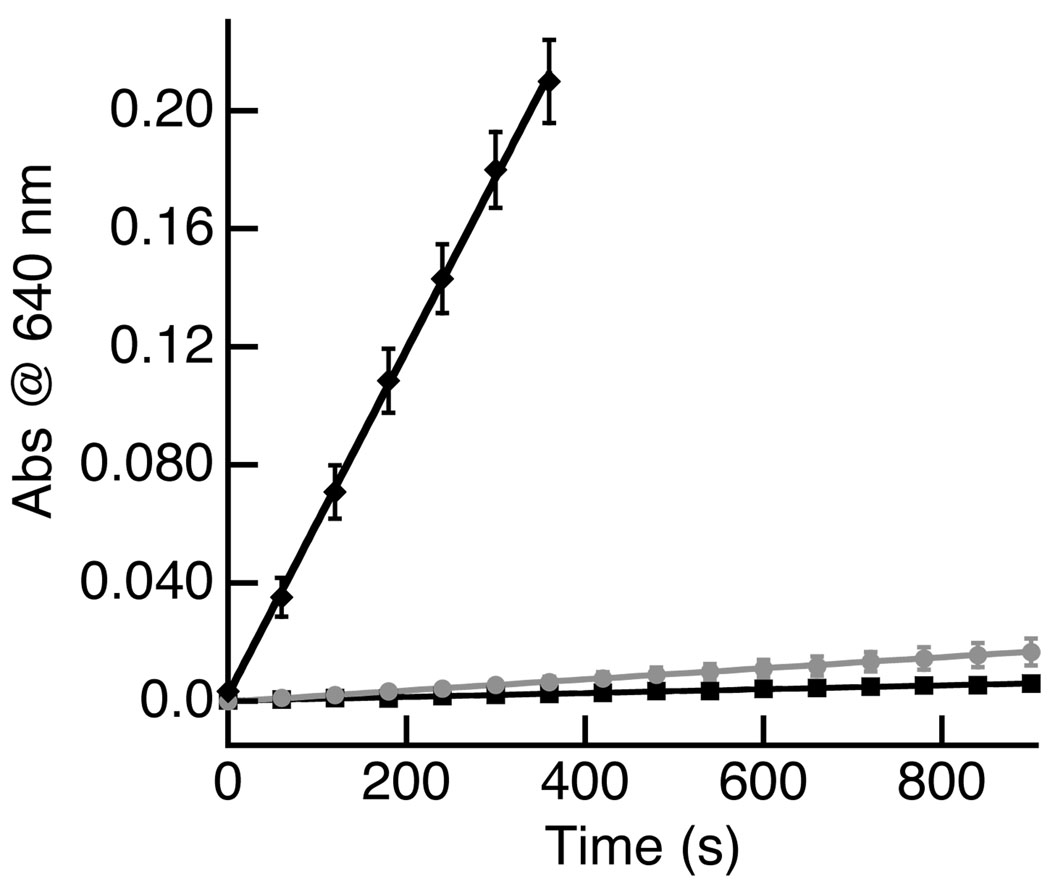
The preparation for [MnIIMST]− was accomplished by treating a DMA solution of H3MST with 3 equiv of NaH, followed by the addition of Mn(OAc)2.17 Metathesis with [NMe4](OAc) afforded [NMe4][MnIIMST] in 90% yield after workup (Scheme 1). This salt showed limited reactivity with dioxygen at 25°C as assayed by optical spectroscopy (Figure 2), having an initial rate of 6.2 × 10−6 s−1: the slow rate of the reaction prevented isolation of any oxidized species. However, we found that the rate of the reaction with dioxygen was accelerated in the presence of Group 2 metal ions. For instance, an initial rate of 5.8 × 10−4 s−1 when the mixture of [NMe4][MnIIMST] and dioxygen was treated with 1 equiv of Ca(OTf)2/15-crown-5 (Figures 2 and 3). Addition of larger amounts of Ca(OTf)2/15-crown-5 did not change this rate substantially, with only a small decrease in the initial rates observed (Figure S1).17 The initial rate for this reaction was also affected when the [NMe4][MnIIMST]/dioxygen mixture was treated with 1 equiv of Ba(OTf)2/18-crown-6, giving an initial rate of 1.8 × 10−5 s−1.18 Although the role of the Group 2 metal ions remains to be clarified, these results clearly indicate that the rate of O2 activation by [MnIIMST]3− is dependent upon the presence of redox inactive metal ions.
Scheme 1.
Reactions involving [MnIIMST]+.
Figure 2.
Initial rate data for the reaction of [MnIIMST]− and O2 in the presence of [NMe4]+ (■, 6.2 × 10−6 s−1), Ca(OTf)2/15-crown-5 (♦, 5.8 × 10−4 s−1), and Ba(OTf)2/18-crown-6 ( , 1.8 × 10−5 s−1). Reactions were done in CH2Cl2 at 25°C.
, 1.8 × 10−5 s−1). Reactions were done in CH2Cl2 at 25°C.
Figure 3.
Electronic absorbance spectra for the reduction of dioxygen in the presence of [MnIIMST]− and Ca(OTf)2/15-crown-5 in CH2Cl2 at 25°C. Spectra were recorded every 30 min. Inset is the spectrum for the isolated product, [15-crown-5⊃CaII-(μ-OH)-MnIIIMST]+.
The major product from the reaction with Ca(OTf)2/15-crown-5 was obtained in yields ranging from 50 to 60% and has a visible absorbance spectrum with features at λmax (εM) = 450 (340), 640 (600) and 800 (sh) nm (Figure 3, inset) that matches the final spectrum obtained from the reaction. Analytical data suggested that a molecular formulation of [15-crown-5⊃CaII-(μ-OH)-MnIIIMST]OTf, which was supported by electrospray ionization mass spectrometry (ESI-MS) experiments. The ESI-MS+ spectrum contained a strong ion having a mass-to-charge ratio (m/z) of 1021.27 when the complex was prepared with 16O2: the mass and calculated isotopic distribution is consistent with [15-crown-5⊃CaII-(μ-OH)-MnIIIMST]+ (Figure S2).17 When the reaction was performed under 18O2, the molecular ion peak shifted by two mass units to a m/z of 1023.27, indicating that the oxygen atom of the hydroxo ligand was derived from the activation of dioxygen. Moreover, our mass spectral studies indicated that the hydroxo ligand undergoes water/hydroxo exchange reactions (Figure S3).17
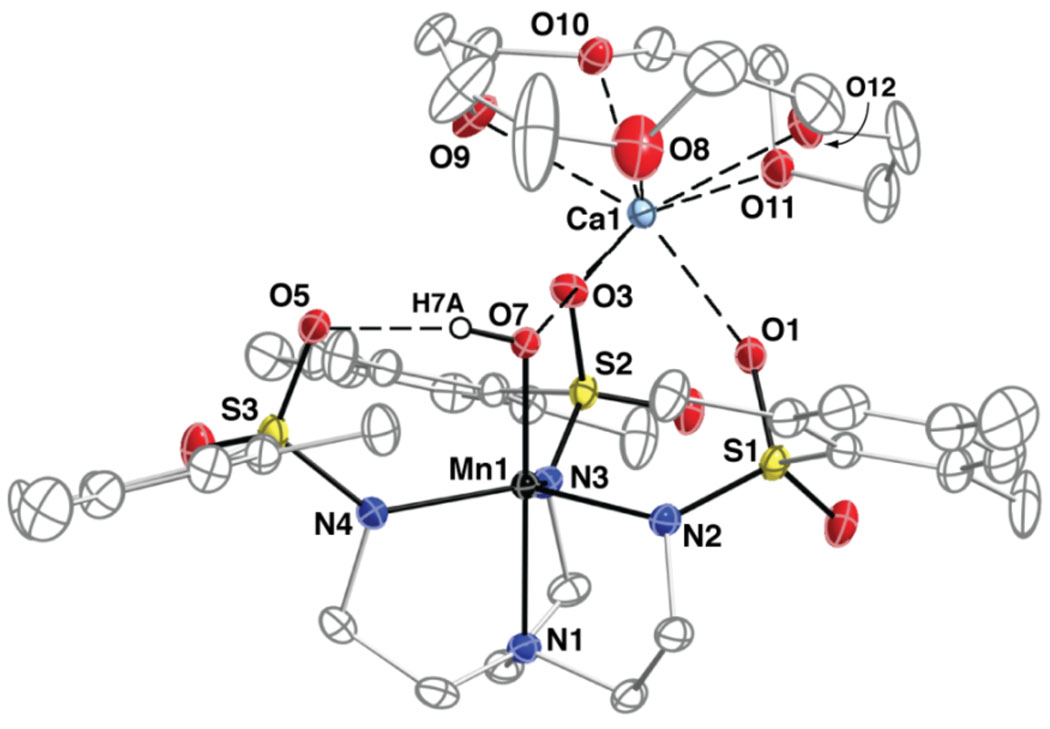
The molecular structure of [15-crown-5⊃CaII-(μ-OH)-MnIIIMST]+ was determined by X-ray diffraction to reveal the predicted heterobimetallic complex (Figure 4). The manganese(III) center has an N4O primary coordination sphere having trigonal bipyramidal geometry. The nitrogen atoms of the [MST]3− ligand coordinate to the manganese(III) ion with an Mn1–N1 bond length of 2.075(2) Å and an average Mn1–Neq bond distance of 2.052(2) Å. The primary sphere of the Mn center is completed by a hydroxo ligand having a Mn1–O7 bond distance of 1.829(2) Å and an N1-Mn1-O7 bond angle of 176.9(1)°. Note that each SO2Ar group adopts a conformation in which one of the S=O bonds is positioned nearly perpendicular to the equatorial plane, with an average Mn-N-S-O torsion angle of 22.8(2)°. This configuration of SO2Ar groups produced a cavity that forms an intramolecular H-bond between the MnIII–OH unit and O5 of the [MST]3− ligand (O7…O5 distance is 2.693(2) Å).19
Figure 4.
Thermal ellipsoid diagram depicting the molecular structure of [15-crown-5⊃CaII-(μ-OH)-MnIIIMST]+. Ellipsoids are drawn at the 50% probability level and only the hydroxo hydrogen atom is shown for clarity. Selected bond length (Å) and angles (°): Mn(1)-O(7), 1.829(2); Mn(1)-N(1), 2.075(2); Mn(1)-N(2), 2.019(2); Mn(1)-N(3), 2.107(2); Mn(1)-N(4), 2.029(2); Mn(1)---Ca(1), 3.7478(6); Ca(1)-O(1), 2.332(2); Ca(1)-O(3), 2.369(2); Ca(1)-O(7), 2.342(2); Ca(1)-O(8), 2.490(3); Ca(1)-O(9), 2.508(2); Ca(1)-O(10), 2.492(2); Ca(1)-O(11), 2.486(2); Ca(1)-O(12), 2.453(2); O(7)-Mn(1)-N(2), 96.95(8); O(7)-Mn(1)-N(4), 96.58(8); N(2)-Mn(1)-N(4), 131.81(10); O(7)-Mn(1)-N(1), 176.94(9); N(2)-Mn(1)-N(1), 81.83(9); N(4)-Mn(1)-N(1), 82.25(9); O(7)-Mn(1)-N(3), 101.09(8); N(2)-Mn(1)-N(3), 109.38(9); N(4)-Mn(1)-N(3), 112.93(9); N(1)-Mn(1)-N(3), 81.97(9); Mn(1)-O(7)-Ca(1), 127.49(9).
The most striking feature of the structure is the placement of a CaII ion within the secondary coordination sphere of the Mn center. The CaII ion interacts with the complex through O7 of the Mn–OH unit and O1 and O3 from two of the sulfonamide groups of the [MST]3− ligand: it appears that these oxygen atoms are positioned such that they form an auxiliary, face-capping site for the binding of additional metal ions. The O7 atom of the hydroxo ligand bridges between the two metal centers with a Mn1-O7-Ca1 bond angle of 127.49(9)° and a calcium-manganese separation of 3.748(2) Å. The Ca1---O7 bond distance is 2.342(2) Å, which is similar to the Ca1---O1 and Ca1---O3 bond lengths of 2.333(2) and 2.370(2) Å. The 5 oxygen atoms of the crown ether fill the remaining coordination sites on Ca1 and the average Ca1---O15-c-5 distance is 2.48 Å. Notice also that the 15-crown-5 ligand adopts an "inverted umbrella" structure, whereby the CaII ion is displaced out of the crown ether toward the [MST]3− ligand.20
To our knowledge, [15-crown-5⊃CaII-(μ-OH)-MnIIIMST]+ represents the first example of a structurally characterized species containing a MnIII–(μ-OH)–CaII core. In fact, discrete CaII–OH systems are rare with the LCaII-(μ-OH)2-CaIIL homodimer of Roesky the only other structurally characterized complex.21 In that complex, each calcium center is 5-coordinate and the Ca–O(H) distances are significantly shorter (less than 2.25 Å) than those observed in [15-crown-5⊃CaII-(μ-OH)-MnIIIMST]+. We are also aware of only three other structurally characterized molecular systems containing calcium/manganese bridged units: the Mn13Ca2 cluster prepared by Christou, the Mn4Ca system of Powell and the Mn3CaNa by Reedijk.22
Studies into the properties of heterometallic complexes with manganese/calcium cores have been sparked by the OEC of photosystem II. Sufficient information is available to show that the active site of the OEC contains a Mn4Ca cluster that is surrounded by a network of H-bonds. Most current mechanistic proposals stipulate that the calcium center has a direct role in water oxidation,4c,5 a premise that is supported by a recent X-ray structure of the OEC at a resolution of 1.9 Å.4f This structure showed that the CaII ion has two bound water molecules and is connected to the manganese centers through oxygen-containing bridging ligands, some of which could possibly be in a manner similar to that found in [15-crown-5⊃CaII-(μ-OH)-MnIIIMST]+. Moreover, the isolation of [15-crown-5⊃CaII-(μ-OH)-MnIIIMST]+ indicates that inclusion of sulfonamido groups into ligands may provide a new synthetic method for the preparation of these types of heterometallic complexes.
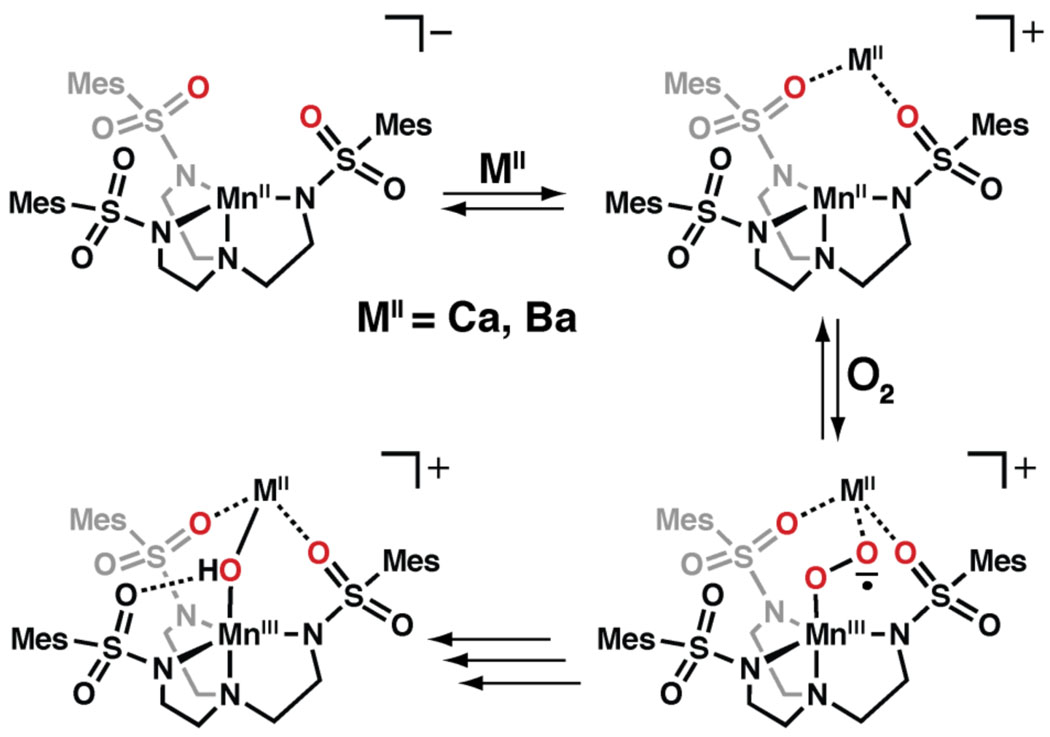
We have presented data that showed Group 2 metal ions have a marked influence on the rate of dioxygen activation by a monomeric MnII complex. Our findings showed an increase in initial rate in the presence of CaII and BaII ions, with CaII ions being faster by a factor of 35. These results complement those reported by Fukuzumi who showed that the rate of electron transfer from [CoII(tetraphenylporphyrin)] to O2 is also accelerated in the presence of CaII and BaII ions: in this one-electron transfer process, CaII ions increased the rate 22 times compared to BaII ions.10a The similarity in rate data to that of our systems offers a possible explanation as to why dioxygen activation with [MnIIMST]− depends on the presence of Group 2 ions (Scheme 2). The reduction of dioxygen to superoxide could be dependent on the prior formation of a heterobimetallic MnIICaII complex, which coordinates dioxygen and then facilitates electron transfer to form a putative superoxido adduct. The rate of intramolecular electron-transfer to generate a putative MnIII–(superoxo)–MII species would depend on the Lewis acidity of the Group 2 metal ion, which helps to alleviate negative charge build-up. This possible function of the redox inactive metal ion is reminiscent of the role that the H-bonding cavities have in metal complexes of our urea-base tripods (Figure 1): in these systems the positively polarized cavities promote the binding and activation of dioxygen through the formation of intramolecular H-bonds.14,23 The results found with [MnIIMST]− illustrate the possibility that redox inactive metal ions can also be used to facilitate the activation of dioxygen, which in turn, may provide another way to control and enhance the reactivity of transition metal complexes.
Scheme 2.
Proposed role of the redox inactive metal ions in the activation of dioxygen.
Supplementary Material
Acknowledgments
ACKNOWLEDGMENT is made to the NIH (GM050781) for financial support. YJP also acknowledges EXAX Inc. for partial support. We thank R. Zarkesh for collecting the X-ray data and Professor L. Doerrer for helpful advice.
Footnotes
Supporting Information Available: Experimental details for all chemical reactions and figures for all spectra. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.(a) Kessar SV, Singh P. Chem. Rev. 1997;97:721–737. doi: 10.1021/cr950082n. [DOI] [PubMed] [Google Scholar]; (b) Mahrwald R. Chem. Rev. 1999;99:1095–1120. doi: 10.1021/cr980415r. [DOI] [PubMed] [Google Scholar]; (c) Corma A, Garcia H. Chem. Rev. 2002;102:3837–3892. doi: 10.1021/cr010333u. [DOI] [PubMed] [Google Scholar]; (d) Corma A, Garcia H. Chem. Rev. 2003;103:4307–4365. doi: 10.1021/cr030680z. 3892. [DOI] [PubMed] [Google Scholar]
- 2.For recent reviews: Fukuzumi S, Kojima T. J. Biol. Inorg. Chem. 2008;13:321–333. doi: 10.1007/s00775-008-0343-1. Fukuzumi S, Ohkubo K. Coord. Chem. Rev. 2010;254:372–385. Fukuzumi S. Prog. Inorg. Chem. 2009;56:49. Fukuzumi S. Pure Appl. Chem. 2003;75:577–587.
- 3.Valentine JS, Doucette PA, Potter SZ. Annu. Rev. Biochem. 2005;74:563–593. doi: 10.1146/annurev.biochem.72.121801.161647. [DOI] [PubMed] [Google Scholar]
- 4.(a) Ferreira KN, Iverson TM, Maghlaoui K, Barber J, Iwata S. Science. 2004;303:1831–1838. doi: 10.1126/science.1093087. [DOI] [PubMed] [Google Scholar]; (b) Yano J, Kern J, Sauer K, Latimer MJ, Pushkar Y, Biesiadka J, Loll B, Saenger W, Messinger J, Zouni A, Yachandra VK. Science. 2006;314:821–825. doi: 10.1126/science.1128186. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Barber J. Inorg. Chem. 2008;47:1700–1710. doi: 10.1021/ic701835r. [DOI] [PubMed] [Google Scholar]; (d) Sauer K, Yano J, Yachandra VK. Co-ord. Chem. Rev. 2008;252:318–335. doi: 10.1016/j.ccr.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Guskov A, Kern J, Gabdulkhakov A, Broser M, Zouni A, Saenger W. Nat. Struct. Mol. Biol. 2009;16:334–342. doi: 10.1038/nsmb.1559. [DOI] [PubMed] [Google Scholar]; (f) Umena Y, Kawakami K, Shen J-R, Kamiya N. Nature. 2011;473:55–60. doi: 10.1038/nature09913. [DOI] [PubMed] [Google Scholar]
- 5.(a) Pecoraro VL, Baldwin MJ, Caudle MT, Hsieh W-Y, Law NA. Pure Appl. Chem. 1998;70:925–929. [Google Scholar]; (b) Cady CW, Crabtree RH, Brudvig GW. Coord. Chem. Rev. 2008;252:444–455. doi: 10.1016/j.ccr.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Brudvig GW. Philos. Trans. R. Soc., B. 2008;8:1211–1219. doi: 10.1098/rstb.2007.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Mullins CS, Pecoraro VL. Co-ord. Chem. Rev. 2008;47:1849–1861. [Google Scholar]; (e) Betley TA, Wu Q, VanVoorhis T, Nocera DG. Inorg. Chem. 2008;47:1894–1861. doi: 10.1021/ic701972n. [DOI] [PubMed] [Google Scholar]
- 6.(a) Giri R, Mangel N, Li J-J, Wang D-H, Breazzano SP, Saunders LB, Yu J-Q. J. Am. Chem. Soc. 2007;129:3510–3511. doi: 10.1021/ja0701614. [DOI] [PubMed] [Google Scholar]; (b) Zhang Y-H, Yu J-Q. J. Am. Chem. Soc. 2009;131:14654–14655. doi: 10.1021/ja907198n. [DOI] [PubMed] [Google Scholar]
- 7.(a) Yiu S-M, Man W-L, Lau T-C. J. Am. Chem. Soc. 2008;130:10821–10827. doi: 10.1021/ja802625e. [DOI] [PubMed] [Google Scholar]; (b) Lam WWY, Yiu S-M, Lee JMN, Yau SKY, Kwong H-K, Lau T-C, Liu D, Lin Z. J. Am. Chem. Soc. 2006;128:2851–2858. doi: 10.1021/ja0552951. [DOI] [PubMed] [Google Scholar]; (c) Yiu S-M, Wu Z-B, Mak C-K, Lau T-C. J. Am. Chem. Soc. 2004;126:14921–14929. doi: 10.1021/ja0487832. [DOI] [PubMed] [Google Scholar]
- 8.Miller CG, Gordon-Wylie SW, Horwitz CP, Strazisar SA, Peraino DK, Clark GR, Weintraub ST, Collins TJ. J. Am. Chem. Soc. 1998;120:11540–11541. [Google Scholar]
- 9.(a) Morimoto Y, Kotani H, Park J, Lee Y-M, Nam W, Fukuzumi S. J. Am. Chem. Soc. 2011;133:403–405. doi: 10.1021/ja109056x. [DOI] [PubMed] [Google Scholar]; (b) Fukuzumi S, Morimoto Y, Kotani H, Naumov P, Lee Y-M, Nam W. Nature Chem. 2010;2:756–759. doi: 10.1038/nchem.731. [DOI] [PubMed] [Google Scholar]; (c) Karlin KD. Nature Chem. 2010;2:711–712. doi: 10.1038/nchem.813. [DOI] [PubMed] [Google Scholar]; (d) Park J, Morimoto Y, Lee Y-M, Nam W, Fukuzumi S. J. Am. Chem. Soc. 2011;133:5236–5239. doi: 10.1021/ja200901n. [DOI] [PubMed] [Google Scholar]
- 10.(a) Fukuzumi S, Ohkubo K. Chem. Eur. J. 2000;6:4532–4535. doi: 10.1002/1521-3765(20001215)6:24<4532::aid-chem4532>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]; (b) Ohkubi K, Menon SC, Orita A, Otera J, Fukuzumi S. J. Org. Chem. 2003;68:4720–4726. doi: 10.1021/jo034258u. [DOI] [PubMed] [Google Scholar]
- 11.Other examples that show the importance of redox inactive metal ions in the binding and activation of small molecules: Darensbourg MY, Darensbourg DJ, Burns D, Drew DA. J. Am. Chem. Soc. 1976;98:3127–3136. Gambarotta Francesco A, Floriani C, Zanazzi PF. J. Am. Chem. Soc. 1982;104:5082–5092. Lee Y, Peters JC. J. Am. Chem. Soc. 2011;133:4438–4446. doi: 10.1021/ja109678y. Lee Y, Mankad NP, Peters JC. Nature Chem. 2010;2:558–565. doi: 10.1038/nchem.660. Betley TA, Peters JC. J. Am. Chem. Soc. 2003;125:10782–10783. doi: 10.1021/ja036687f. Ding K, Brennessel WW, Holland PL. J. Am. Chem. Soc. 2009;131:10804–10805. doi: 10.1021/ja902812y. Ding K, Pierpont AW, Brennessel WW, Lukat-Rodgers G, Rodgers KR, Cundari TR, Bill E, Holland PL. J. Am. Chem. Soc. 2009;131:9471–9472. doi: 10.1021/ja808783u. Smith JM, Sadique AR, Cundari TR, Rodgers KR, Lukat-Rodgers G, Lachicotte RJ, Flaschenriem CJ, Vela J, Holland PL. J. Am. Chem. Soc. 2006;128:756–769. doi: 10.1021/ja052707x.
- 12.Examples of salen-type ligands linked by polyether groups: Horwitz CP, Warden JT, Weintraub ST. Inorg. Chim. Acta. 1996;246:311–320. Turconi S, Horwitz CP, Ciringh Y, Weintraub ST, Warden JT, Nugent JHA, Evans MCW. J. Chem. Soc., Dalton Trans. 1997:4075–4082. Horwitz CP, Ciringh Y, Weintraub ST. Inorg. Chim. Acta. 1999;294:133–139.
- 13.Selected examples of other heterobimetallic complexes: Stephen DW. Coord. Chem. Rev. 1989;95:41–107. Wheatley N, Kalck P. Chem. Rev. 1999;99:3379–3420. doi: 10.1021/cr980325m. Bullock RM, Casey CP. Acc. Chem. Res. 1990;20:167–173. Baranger AM, Bergman RG. J. Am. Chem. Soc. 1994;116:3822–3835. Greenwood BP, Rowe GT, Chen C-H, Foxman BM, Thomas CM. J. Am. Chem. Soc. 2010;132:44–45. doi: 10.1021/ja909310t.
- 14.For reviews: Borovik AS. Acc. Chem. Res. 2005;38:54–61. doi: 10.1021/ar030160q. Shook RL, Borovik AS. Inorg. Chem. 2010;49:3646–3660. doi: 10.1021/ic901550k. Shook RL, Borovik AS. Chem. Commun. 2008:6095–6107. doi: 10.1039/b810957e.
- 15.Sulfonamido tripods have been reported but to our knowledge their metal chemistry has not yet been explored:5e Boon JM, Lambert TN, Smith BD, Beatty AM, Ugrinova V, Brown SN. J. Org. Chem. 2002;67:2168–2174. doi: 10.1021/jo016416s.
- 16.(a) Walsh PJ. Acc. Chem. Res. 2003;36:739–749. doi: 10.1021/ar0300219. [DOI] [PubMed] [Google Scholar]; (b) Walsh PJ, Lurain AE, Balsells J. Chem. Rev. 2003;103:3297–3344. doi: 10.1021/cr0000630. [DOI] [PubMed] [Google Scholar]; (c) Pritchett S, Woodmansee DH, Gantzel P, Walsh PJ. J. Am. Chem. Soc. 1998;120:6423–6424. [Google Scholar]; (d) Nanthakumar A, Miura J, Diltz S, Lee C-K, Aguirre G, Ortega F, Ziller JW, Walsh PJ. Inorg. Chem. 1999;38:3010–3013. doi: 10.1021/ic981093c. [DOI] [PubMed] [Google Scholar]
- 17.Experimental details can be found in supporting information
- 18.The molecular structure of the reaction product was determined by X-ray diffraction methods to be [18-crown-6⊃BaII-(μ-OH)-MnIIIMST]+ which is similar to [15-crown-5⊃CaII-(μ-OH)-MnIIIMST]+.17
- 19.The position of H7A was located from a difference-Fourier map and refined (x,y,z and fixed Uiso)
- 20.Junk PC, Steed JW. J. Chem. Soc., Dalton Trans. 1999:407–414. [Google Scholar]
- 21.Ruspic C, Nembenna S, Hofmeister A, Magull J, Harder S, Roesky HW. J. Am. Chem. Soc. 2006;128:15000–15004. doi: 10.1021/ja065631t. [DOI] [PubMed] [Google Scholar]
- 22.(a) Mishra A, Wernsdorfer W, Abboud KA, Christou G. Chem. Commun. 2005:54–56. doi: 10.1039/b413680b. [DOI] [PubMed] [Google Scholar]; (b) Hewitt IJ, Tang J-K, Madhu NT, Clérac R, Buth G, Anson CE, Powell AK. Chem. Commun. 2006:2650–2652. doi: 10.1039/b518026k. [DOI] [PubMed] [Google Scholar]; (c) Nayak S, Nayek HP, Dehnen S, Powell AK, Reedijk J. Dalton Trans. 2011;40:2699–2702. doi: 10.1039/c0dt01463j. [DOI] [PubMed] [Google Scholar]
- 23.(a) MacBeth CE, Gupta R, Mitchell-Koch KR, Young VG, Jr, Lushington GH, Thompson WH, Hendrich MP, Borovik AS. J. Am. Chem. Soc. 2004;126:2556–2567. doi: 10.1021/ja0305151. [DOI] [PubMed] [Google Scholar]; (b) Lucas RL, Zart MK, Murkerjee J, Sorrell TN, Powell DR, Borovik AS. J. Am. Chem. Soc. 2006;128:15476–15489. doi: 10.1021/ja063935+. [DOI] [PubMed] [Google Scholar]; (c) Shook RL, Peterson SM, Greaves J, Moore C, Rheingold AL, Borovik AS. J. Am. Chem. Soc. 2011;133:5810–5817. doi: 10.1021/ja106564a. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.