Abstract
Introduction
Depending in part on the glutathione to glutathione disulfide ratio, reversible protein glutathionylation to a mixed disulfide may occur. Reversible glutathionylation is important in protecting proteins against oxidative stress, guiding correct protein folding, regulating protein activity, and modulating proteins critical to redox signaling. The potential also exists for irreversible protein glutathionylation via Michael addition of an -SH group to a dehydroalanyl residue, resulting in formation of a stable, non-reducible thioether linkage.
Areas covered
This article reviews factors contributing to reversible and irreversible protein glutathionylation and their biomedical implications. It also examines the possibility that certain drugs such as busulfan may be toxic by promoting irreversible glutathionylation. The reader will gain an appreciation of the protective nature and control of function resulting from reversible protein glutathionylation. The reader is also introduced to the recently identified phenomenon of irreversible protein glutathionylation and its possible deleterious effects.
Expert opinion
The process of reversible protein glutathionylation is now well established but these findings need to be substantiated at the tissue and organ levels, and also with disease state. That being said, irreversible protein glutathionylation can also occur and this has implications in disease and aging. Toxicologists should consider this when evaluating the possible side effects of certain drugs such as busulfan that may generate a glutathionylating species in vivo.
Keywords: Busulfan, glutathione, glutathione disulfide, irreversible protein glutathionylation, lanthionine, redox potential, reversible protein glutathionylation
1. Introduction
It is becoming increasingly apparent that reversible protein glutathionylation (often referred to as S-glutathionylation) is extremely important in a number of biological settings, and several informative reviews on the subject have recently appeared. Along with other authors, we emphasize the importance of reversible glutathionylation. However, a major emphasis of the present review is on the irreversible protein glutathionylation and its possible role in disease, aging and drug interactions – an area that, to our knowledge, has not previously been reviewed.
Glutathione (GSH, γ-glutamylcysteinylglycine) is a tripeptide with many important biological functions, including a) detoxification of reactive electrophiles, b) coenzyme properties (e.g. the glyoxylase reaction), c) protection against oxidative stress, nitrosative stress and radiation damage, and d) protection against cancer [1–15]. GSH is readily oxidized to glutathione disulfide (GSSG), which in turn is easily reduced back to GSH (see below). An extremely important role of the GSH/GSSG redox couple is to provide a means of reversibly glutathionylating proteins. This review summarizes the current state of knowledge of protein glutathionylation (formation of PSSG), with emphasis on its relationship (through disulfide bond formation with cysteinyl moieties on proteins) to redox signaling and disease. The excellent discussions by Schafer and Buettner [4] and Dalle-Donne et al. [9] are especially useful regarding this topic. In addition, the newly identified irreversible glutathionylation of proteins through a stable thioether bond (formation of PSG) and its relevance to age and disease is discussed.
2. The GSH/GSSG redox couple
In order to appreciate reversible protein glutathionylation, it is necessary to briefly describe the glutathione/glutathione disulfide (GSH/GSSG) redox couple. Consider the two-electron reduction of GSSG to GSH (Eq. 1). Under standard conditions (25°C, pH 7.0) the E0' of this couple is -250 mV. Thus, GSH can be readily oxidized to GSSG. The GSH/GSSG ratio adjusts to the overall cellular redox potential, and the intracellular ratio is generally ≥100:1 [4,5,16,17]. Consider also the two-electron reduction of NAD+ (Eq. 2). The E0' of this couple is -316 mV. The E0' for the two-electron reduction of NADP+ (Eq. 3) is similar (−315 mV) [18].
| (1) |
| (2) |
| (3) |
Even though the NAD+ and NADP+ redox systems have such similar E0' values the components are far from equilibrium in the cell. For example, the [NADPH]/[NADP+] ratio is generally ~100:1, whereas the [NADH]/[NAD+] ratio is in the opposite direction, normally in the range of 1:10 to 1:1000 [4]. This imbalance has important consequences for the maintenance of cellular function. The cofactor NADPH serves as a source of electrons for reductive biosynthetic (anabolic) procedures such as fatty acid biosynthesis and drug metabolism, whereas the cofactor NAD+ generally serves as a sink for electrons in catabolic oxidative reactions with concomitant passage of these electrons through the electron transport chain for ATP biosynthesis [4].
The GSH/GSSG system is the major cellular thiol-disulfide redox buffer [4]. GSH is present in mammalian cells in the concentration range of 1 – 12 mM [1,2]. In mammalian tissues ascorbate is also present in relatively high (mM) concentrations, especially in the brain [16,19]. Thus, quantitatively GSH and ascorbate are the most abundant antioxidants and free radical scavengers in the body. As an antioxidant GSH is oxidized to GSSG (the reverse of Eq. 1). Examples of the anti-oxidant properties of GSH include its participation in a) the reduction of hydrogen peroxide (H2O2) to water by catalase (Eq. 4), and b) the reduction of peroxides (ROOH) to the corresponding alcohol (ROH) (Eq. 5) by reactions catalyzed by 1-Cys- and 2-Cys peroxiredoxins (which contain a critical cysteine residue in the active site) [5,20]. These reactions are also catalyzed by a variety of glutathione peroxidases (which contain a selenocysteine residue in the active site) [5,20]. A comparable reaction involving reduction of phospholipid peroxides (Eq. 5 where R = phospholipid) is conducted by the selenoprotein phospholipid hydroperoxide glutathione peroxidase [21].
| (4) |
| (5) |
Because NADPH is a stronger reductant than GSH, GSSG formed as a result of an “oxidative event” is readily reduced back to GSH (Eq. 6) by the enzyme glutathione reductase, a process favored by the relatively high intracellular ratio of NADPH/NADP+ compared to that of NADH/NAD+.
| (6) |
3. Reactions leading to reversible protein glutathionylation
Formation of a protein mixed disulfide containing glutathione (PSSG), a process known as glutathionylation, can occur via several mechanisms as listed below.
3.1. Thiol-disulfide exchange reactions
Glutathionylation of cysteine residues in proteins can occur via direct reaction of the cysteinyl residue in a protein [depicted as PSH (or P'SH when it is necessary to distinguish between –SH groups on two separate proteins)] with GSSG via a thioldisulfide exchange reaction (Eqs. 7–9). However, as noted above, the intracellular ratio of GSH/GSSG is usually ≥100:1. Thus, it has been suggested that thiol-disulfide interchange is an unlikely mechanism for protein glutathionylation, except perhaps under extreme conditions [9]. In this regard, it is estimated that only about 1% of the total glutathione pool in normal liver is present in disulfide linkage to proteins [22]. One such extreme condition may be the respiratory burst of human monocytes, which is accompanied by a marked reduction of intracellular GSH and greatly increased protein glutathionylation [23]. The protein glutathionylation is rapidly reversible and may be a mechanism by which monocytes protect sensitive cysteinyl residues in proteins from more drastic oxidative events [23]. Factors and enzymes that control protein glutathionylation and protein deglutathionylation are covered in section 4.
| (7) |
| (8) |
| (9) |
3.2. Reactions involving thiyl species
Protein glutathionylation may also occur via reactions involving thiyl radical species (GS• or PS•). This is accomplished by one-electron oxidation of PSH or GSH, followed by formation of a mixed disulfide and superoxide anion radical (Eqs. 10, 11). The reaction of proteins with GS• to generate PSSG is catalyzed by glutaredoxins 1 and 2 (Grx1 and Grx2) (also known as thioltransferases), enzymes normally acting as a reductant (i.e. in deglutathionylation) [9]. These reactions are important in maintaining redox homeostasis and signal transduction [24]. Glyceraldehyde 3-phosphate dehydrogenase (GADPH) is an important enzyme in glycolysis. The enzyme has a particularly reactive cysteine residue whose modification is involved in regulation of enzyme activity.
Both GSSG and GSNO can also act as glutathionylation agents. However, glutathionylation of the active cysteine in, for example, GAPDH as catalyzed by Grxs is more effective in the presence of GS• (Eq. 11 where PS• = GAPDH radical) than in the presence of either GSNO or GSSG [25].
| (10) |
| (11) |
3.3. Reaction of a protein sulfenic acid (PSOH) with GSH
In addition to oxidation to the mixed disulfide (PSSP or PSSP'), the thiol group in a cysteinyl residue of a protein (PSH) may be sequentially oxidized to the sulfenic acid (PSOH), then to the sulfinic acid (PSO2H) and finally to the sulfonic acid (PSO3H). The formation of PSOH and PSO2H [26,27], but not PSO3H, is potentially reversible. In some cases, the formation of PSOH in unfolded proteins may help in the correct formation of intramolecular disulfide bonds [28]. In other cases, accumulation of unfolded proteins through excessive oxidation of cysteinyl moieties may cause increased expression of genes involved in growth arrest and apoptosis [29]. This phenomenon may have implications in cancer biology related to decreases in net proliferation of cells, as is discussed in section 6.5 of this review. In some cases (e.g. methionine sulfoxide reductases), a PSOH intermediate is part of a catalytic cycle [30].
The reaction of PSOH with GSH will generate PSSG (Eq. 12), a process that will protect the protein against potential loss of function resulting from further oxidation to PSO2H or PSO3H [7,9], At the same time, a species (PSSG) is generated that can be readily reduced back to PSH, thus conserving the integrity of redox signaling proteins and pre-empting expenditure of cellular energy necessary for de novo protein biosynthesis.
| (12) |
In some cases the protein sulfenic acid is protected against further oxidation by formation of a sulfenyl amide [PSNHC(O)P'] with the carboxamide of a nearby glutamine/asparagine residue. The sulfenyl amide readily reacts with GSH or a cysteine residue to generate PSSG or PSSP(P') [31].
Although, the formation of cysteine sulfenic acid may be deleterious if the sulfenic acid is the start of an irreversible oxidation cascade to the sulfonic acid, recent work suggests that sulfenic acids may participate in redox signaling [32]. For example, global analysis of the “sulfenome” in a tumor cell line identified most known sulfenic acid-modified proteins: 14 in total, plus more than 175 additional potential candidates [32]. Thus, availability of methods for identifying sulfenated proteins will facilitate elucidation of particular proteins within signaling pathways that are not only targets for regulation by oxidation of cysteine moieties, but potential beneficiaries of reversible glutathionylation [33].
3.4. Reactions involving S-nitroso species
There is enormous interest in nitric oxide (NO) as a signaling molecule [e.g. 34]. As discussed by Seth and Stamler [34] NO can react directly with a thiol (RSH) to form a radical anion intermediate that is then oxidized to an S-nitrosothiol (RSNO) species in the presence of an electron acceptor. NO may also dismutate to form NO+ (nitrosyl cation; nitrosonium ion) that will nitrosate (i.e. incorporate NO into) thiols following dimerization (formation of (NO)2) through reaction with aromatic residues. Alternatively, NO may react directly with O2 to generate nitrosating equivalents. Whatever the mechanism, GSH and susceptible protein thiols (PSH) are converted to GSNO and PSNO, respectively (formally depicted in Eqs. 13 and 14). The GSNO thus formed can react with PSH in a “transnitrosation” reaction to generate a nitrosated protein (Eq. 15). The PSNO formed directly with NO-derived species or by transnitrosation can then react with GSSG to generate a glutathionylated protein (Eq. 16; PSSG) [9,35,36].
| (13) |
| (14) |
| (15) |
| (16) |
.
When a cell is exposed to reactive oxygen species (ROS) its route to either survival or apoptosis depends in part on the stage of the cell cycle [35,37]. It has become apparent, in view of interactions among NO, PSH and GSH, that the cell's fate upon exposure to NO also depends on where the cell resides in the cell cycle. In particular, endogenous and exogenous production of NO can induce S-nitrosation of critical cysteinyl moieties on redox active proteins that have the potential of being “rescued” from further oxidation depending on the intracellular GSH/GSSG ratio, the amount of de novo GSH synthesis, and extent of protein-directed glutathionylation. Factors influencing cell cycle control, cytosolic GSH/GSSG redox balance, and glutathionylation of essential cysteinyl moieties on key proteins, such as caspases and actins, can control responses of cells to nitrosative stress. With regard to the situation with actins, several studies have demonstrated that progression of cells into G2/M phase is associated with depolymerization of actin manifested by S-glutathionylation, a reversible post-translational modification that prevents actin polymerization [35–40]. Conversely, increased glucose utilization by cells through the hexose monophosphate pathway that generates NADPH can re-establish cellular reducing capacity by recycling GSSG to GSH and cause deglutathionylation and actin repolymerization. This latter response maintains GSH redox capacity of the cell and enables cells to survive early cytotoxic effects of protein nitrosation.
In a similar fashion, S-nitrosation of cysteinyl moieties on caspases provides protection against caspase-dependent apoptosis. However, caspase-independent programmed cell death may occur at high NO exposure. Under these conditions, caspase activity continues to be inhibited while cell death is induced via a caspase-independent pathway [38]. Thus, protein nitrosation can exert anti-tumor effects through antiproliferative, cytotoxic, and apoptotic processes. Moreover, depending on the position of cells in the cell cycle, programmed cell death may be induced by modulating exposure to nitrosating agents, coupled with a balancing of cytosolic GSH/GSSG levels and glutathionylation of specific proteins.
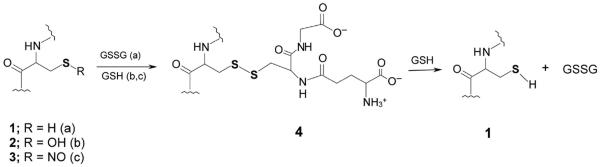
Some processes leading to reversible protein glutathionylation are shown in Figure 1.
Figure 1. Some reactions leading to reversible glutathionylation of proteins and peptides involving a reducible mixed disulfide.
When the GSSG/GSH ratio is favorable, an oxidizable cysteine residue in protein (1) (R = H) may react with GSSG to generate the mixed disulfide (4, PSG, pathway a) and GSH. On the other hand, if the cysteine residue is oxidized to a sulfenic acid (2, R = OH), the protein may react with a GSH equivalent to generate the mixed disulfide (4, pathway b). In another case, the nitrosated cysteine residue (3) (R = NO) may react with GSH to form the mixed disulfide (4, pathway c) and HNO. The mixed disulfide can be converted back to the reduced form (1) by GSH with the formation of GSSG. Note that the mixed disulfide (4) can also be formed from reaction of 1 with GSNO, GSO or GS(O)SG (9). Note that the sulfur atoms are shown bolded for emphasis.
4. Factors that control protein glutathionylation
Schaffer and Buettner have pointed out that the reduction potentials of various redox couples may serve as “nano-switches” [4]. Of particular interest here is the GSH/GSSG couple. Reactions 7 – 9 are freely reversible. Thus, thermodynamically, the extent to which a PSH may be converted to a mixed disulfide will depend in part on the reduction potential of the GSH/GSSG redox pair. Schaffer and Buettner describe two such mechanisms (switches) for protein mixed disulfide (PSSG) formation [4]. A type I nano-witch is represented by Eq. 7, where the equilibrium constant (Eq 17) is:
| (17) |
A type II nano-switch is illustrated by Eq. 18:
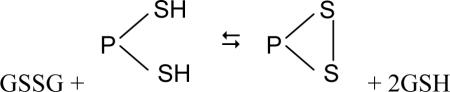 |
(18) |
The equilibrium constant shown in (Eq. 19) is:
| (19) |
Thus, the GSH/GSSG redox state of the cell can result not only in glutathionylation [formation of a mixed disulfide with glutathione; PSSG (Eq. 7)], but also in the formation of an internal disulfide (eqs. 18, 19, where 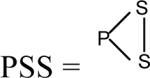 ) or a mixed disulfide between two dissimilar proteins (PSSP'). Moreover, because the GSH term is squared in Eq. 19, switch II is more sensitive to the GSH/GSSG ratio than is switch I. Schaffer and Buettner liken the changes in the switches to rheostats [4]. The switches do not have two positions (i.e. on and off) that may be involved in cell cycle control, as for example as in phosphorylation and dephosphorylation of proteins. Rather the switches act more like dimmers, enabling a gradual activation or inactivation of proteins that depends on the redox status of the cell and degree of oxidative/nitrosative stress. However, it is probable that glutathionylation and phosphorylation/dephosphorylation contribute to the control of mitochondrial bioenergetics, signaling cascades and survival pathways in an interrelated fashion [41,42].
) or a mixed disulfide between two dissimilar proteins (PSSP'). Moreover, because the GSH term is squared in Eq. 19, switch II is more sensitive to the GSH/GSSG ratio than is switch I. Schaffer and Buettner liken the changes in the switches to rheostats [4]. The switches do not have two positions (i.e. on and off) that may be involved in cell cycle control, as for example as in phosphorylation and dephosphorylation of proteins. Rather the switches act more like dimmers, enabling a gradual activation or inactivation of proteins that depends on the redox status of the cell and degree of oxidative/nitrosative stress. However, it is probable that glutathionylation and phosphorylation/dephosphorylation contribute to the control of mitochondrial bioenergetics, signaling cascades and survival pathways in an interrelated fashion [41,42].
Although the glutathionylation reaction is thermodynamically fully reversible, many factors are involved in the kinetic control of glutathionylation. Specific mechanisms and enzymes capable of accelerating formation of PSSG and reductive removal of the GS- moiety from PSSG (deglutathionylation) are only now beginning to be elucidated [6]. Deglutathionylation may be catalyzed by Grx enzymes [6,9], sulfiredoxin [43] and possibly by protein disulfide isomerase (PDI) [44]. Glutathionylation is less well characterized, but is catalyzed by Grx enzymes under certain circumstances (such as in the glutathionylation of GAPDH) and by glutathione S-transferase pi (GSTpi) (an enzyme itself subject to glutathionylation) [45].
The pKa of the sulfhydryl (-SH) group of free cysteine is 8.3. This value, however, can vary enormously in protein cysteine residues, depending in part on a) the location of the -SH moieties within the protein micro-environment, b) the presence of any vicinal amino acids with electron donating or electron withdrawing properties, and c) cooperativity among different cysteine residues in proteins [9]. For example, basic amino acid residues in the vicinity of cysteine residues will lower the pKa value, whereas, acidic amino acid residues will raise the pKa value. Hydrogen bonding to serine or histidine residue or the presence of an adjacent proline residue may also lower the pKa value of the -SH group [9,46]. If the pKa value of the -SH group of the protein-bound cysteine (PSH) is ≥8.3, then the cysteine residue will remain largely uncharged at near neutral physiological pH values, and glutathionylation of this cysteine residue will not be favorable.
Another factor preventing glutathionylation may be low accessibility. If cysteine residues are deeply buried within the protein, then steric factors may prevent ready access of GSSG (or GSH, as in the case relating to PSOH formation) to this residue, even if the pKa value of the -SH group is relatively low [9]. On the other hand, if the pKa value of the -SH group is low and the accessibility to GSSG is high, reactivity of the thiolate anion of the cysteine residue with GSSG [and with ROS and reactive nitrogen species (RNS)] will be favored [9]. Other factors contributing to the ease of protein glutathionylation are also possible. For example, cysteine residues coordinated to a divalent cation (Mg2+, Ca2+, Zn2+) are often reactive toward oxidizing agents, including GSSG [47–50]. Finally, as discussed below, although most cells generally have a high GSH/GSSG ratio, the ratio in the endoplasmic reticulum may be much lower, thereby favoring glutathionylation of proteins in that compartment.
Human cells contain at least 25 selenoproteins [51]. For many of these proteins their biological/enzymatic roles are unknown, whereas others clearly function in biological redox processes [51]. Interestingly, the pKa value of the selenol group (-SeH) of free selenocysteine is 5.2, which is considerably lower than that of the -SH group of free cysteine [52–54]. Thus, the -SeH group of selenocysteine residues will be almost completely ionized to selenolate and therefore, if there are no steric constraints, will be highly susceptible to glutathionylation (formation of PSeSG). This particular mechanism is pertinent to the enzymology associated with glutathione peroxidases, thioredoxin reductase, and other selenoproteins, which contribute to sulfhydryl metabolism [54–56].
5. Biological importance of protein glutathionylation
5.1. Identification of glutathionylated proteins
A number of techniques have been employed to study glutathionylation of proteins and redox proteomics. These include enzymatic procedures, utilizing 35S-cysteine labeled glutathione, use of biotinylated GSSG ethyl ester, incubation with anti-GSH antibodies and subsequent binding to Protein A/G agarose beads, as well as HPLC, spectrophotometric and mass spectrometric methods [9,57–61]. Among these methods, mass spectrometric methods probably provide the most sensitive and useful information regarding actual residues glutathionylated. Biotinylated GSSG ethyl ester appears to be an especially useful reagent. GSH ethyl and GSSG ethyl esters are cell permeable and once inside the cell are “trapped” by hydrolysis of the ester bond [59 and references cited therein]. An advantage of the biotinylated tag is that that glutathionylated proteins formed by sulfide-disulfide interchange reactions with biotinylated GSSG within the cell can be selectively enriched by means of streptavidin affinity chromatography. The biotinylated proteins can be visualized by Western blotting with GSH antibodies [59]. However, it is important to note that the separation of proteins by gel electrophoresis must be carried out under non-denaturing conditions in the absence of added thiols, and N-ethylmaleimide must be added to alkylate free protein thiols [59].
Another method for the quantitation of the total pool of covalently bound glutathionylated proteins involves precipitation from tissue or cell homogenates with metaphosphoric acid (MPA) at a final concentration of 5%. Briefly, precipitated proteins are washed 3 times by resuspension in 5% MPA, followed each time by centrifugation. Pellets are resuspended in 8 M urea/1 mM EDTA and incubated for 10 min at 40°C followed by addition of potassium borohydride to a final concentration of 35 mg/ml. The protein resuspension is incubated for 45 min at 40°C. A few drops of octanol are added to reduce foaming, and proteins in solution are precipitated by addition of MPA to a final concentration of 5%. The mixture is then centrifuged at 14,000 × g and an aliquot of the supernatant fraction is analyzed for GSH by either an enzymatic-recycling method or chromatographic separation on HPLC equipped with electrochemical or coulometric detectors [62].
Despite the fact that many proteins have the potential to undergo glutathionylation, to date only a relatively small number of proteins have been shown to undergoing glutathionylation [9], but the number will no doubt increase as more proteins are studied. As reviewed by Townsend [7] and Dalle-Donne et al. [9], proteins in mammalian cells shown to undergo reversible glutathionylation, often in response to oxidative or nitrosative stress, have been shown to cluster in several categories including 1) mitochondrial enzymes/proteins of energy metabolism [cytochrome c oxidase, mitochondrial isocitrate dehydrogenase, α-ketoglutarate dehydrogenase complex (KGDHC), pyruvate dehydrogenase, complex I, FAD-binding subunit of complex II], 2) cytosolic glycolytic enzymes and other enzymes related to energy metabolism [GAPDH, aldolase, enolase, pyruvate kinase, phosphoglycerate kinase, triosephosphate isomerase, creatine kinase], 3) signaling proteins [p50 and p65 subunits of nuclear factor-kappa B (NF-κB), JNK, protein tyrosine phosphatase 1B (PT1B), PTEN, pyrophosphatase 2A, protein kinase C, protein kinase G, cAMP-dependent protein kinase A, c-abl (ABL1), p53, caspase 3, GTPase, p21 ras, MEKK1], 4) cytoskeletal proteins [actin, myosin, profilin, vimentin, spectrin, tubulin], 5) protein folding and editing enzymes [HSP60, HSP70, 20S chaperonins, PDI, proteasome, ubiquitin conjugating enzyme], 6) ion channels/calcium homeostasis proteins [S100A1, S100B, SERCA, ryanodine receptors I and II, CTFR], and 7) redox balance enzymes [peroxiredoxins, thioredoxin I, GSTs]. It is possible that in some cases, as discussed above, reversible glutathionylation is a safety mechanism that prevents susceptible, but crucial, thiol groups from undergoing a more drastic and irreversible oxidation in the face of an oxidative/nitrosative insult, that would otherwise result in loss of protein function, [7,9]. Some of the cases listed in this paragraph are discussed below. However, it should be kept in mind that although many in vitro studies have demonstrated the feasibility of protein glutathionylation, relatively few studies have documented glutathionylation-dependent changes in protein function under truly physiological settings or that mimic disease states in vivo [8]. Nevertheless, although the field of protein glutathionylation is relatively new, it is burgeoning, so we can expect dramatic changes as this area evolves, including relevant in vivo studies.
5.2. Energy metabolism
Glutathionylation has been suggested to contribute to the coordination of cellular energy metabolism in response to an oxidative/nitrosative stress [9]. Two key enzymes of energy metabolism that may be regulated in part by glutathionylation are discussed here. Most cells contain a large amount of the key glycolytic enzyme GAPDH in the cytosol. However, in the face of an oxidative threat the protein is translocated to the nucleus where it protects the DNA repair enzyme APE1 [63]. GAPDH also binds to the AU-rich elements of the 3'-untranslated region of the gene for endothelin-1 (ET-1) destabilizing the mRNA [64]. ET-1 peptide is a potent vasoconstrictor that is associated with endothelial vasomotor dysfunction and increased cardiovascular risk. Central to the regulatory effects of GAPDH, is modification of the active site Cys152 by glutathionylation, which prevents the enzyme from binding to, for example, ET-1 mRNA [64]. This cysteine residue is also targeted by nitrosation, forming SNO-GAPDH, which transnitrosates nuclear proteins such as the deacetylating enzyme sirtuin-1, histone deacetylase-2 and DNA-activated protein kinase [65]. Thus, posttranslational modification of the -SH moiety of GAPDH greatly expands its role in signal transduction mechanisms.
Another interesting case involves the inhibition of KGDHC activity in isolated rat liver mitochondria exposed to H2O2 in the presence of GSH. Evidence was presented that the inhibition is due to glutathionylation [66]. For example, activity could be restored by Grx [66]. In later work, it was shown that the E2 subunit of KGDHC in isolated mitochondria exposed to H2O2 and GSH is glutathionylated on the covalently bound cofactor lipoamide [67]. Reversible glutathionylation may protect the cofactor from more drastic, and irreversible, oxidation in mitochondria exposed to an oxidative stress [67].
5.3. Signaling, redox regulation, and apoptosis
Several signaling molecules and transcription factors important for cell growth, differentiation and apoptosis may be regulated by reversible glutathionylation [9]. A few examples are discussed here.
Protein kinases appear to be especially prone to targeting by glutathionylation. For example, the kinase activity of p38 MAP kinase [68], MEKK1 [69] and PTP1B [70] are modulated by glutathionylation in response to an oxidative stress in cell model systems. Several authors have suggested that regulation of protein function by glutathionylation/deglutathionylation is akin to regulation of protein function by phosphorylation/dephosphorylation [e.g. 6, 7, 22]. However, as discussed in section 4 of this review, there are notable regulatory differences between the two processes. The human “kinome” contains over 900 tyrosine kinases and their splice variants [71], whereas enzyme-mediated glutathionylation reactions are much more limited. Moreover, protein phosphorylation is more like a true switch, whereas protein glutathionylation is comparable to a rheostat. It is interesting to note that many kinases as well as phosphatases, which are regulatory enzymes, are themselves subjected to possible regulation by glutathionylation [42,72].
NF-κB is a transcription factor involved in the cellular response to a wide variety of factors such as oxidative stress, exposure to cytokines, UV irradiation, microbial infection and oxidized low density lipoprotein (LDL). Glutathionylation and Grx-catalyzed deglutathionylation have been suggested to play important roles in regulating the activation of the NF-κB pathway [73]. In cultured tracheal epithelial cells, Cys179 of the inhibitory κB kinase (IKK) β-subunit of the IKK signalosome is a central target for oxidative inactivation by glutathionylation [73]. The tumor suppressor protein p53 is inhibited by S-glutathionylation in cancer cells by oxidative stress and by treatment with DNA-damaging agents [74]. Glutathionylated p53 is present in human prostate, prostate cancer tissues, melanoma cells and colon cancer cells [74]. The transcription factor PAX-8 is a member of the PAX superfamily of proteins important in regulating gene expression in the thyroid. Glutathionylation occurs on Cys45 and Cys57 in a highly conserved DNA binding motif in the N terminus [75]. A crucial role for glutathionylation and its reversal (by Grx-1) in controlling the activation of interferon regulatory factor (IRF3) and interferon β (IFNβ) gene expression has been suggested [76]. Deglutathionylation of IRF3 is required for interaction with CBP (CREB binding protein), an event essential for transcription of interferon genes [76].
As briefly discussed in section 3.4, some studies suggest that glutathionylation is involved in regulation of apoptosis. It is generally considered that GSH depletion and protein glutathionylation are byproducts of oxidative stress in cells undergoing programmed death. However, recent evidence suggests that protein glutathionylation may be a critical regulatory step in apoptosis and inflammatory responses, often at the level of caspases [10,35,38,62,77–80]. Conversely, Grxs may be protective against apoptosis [10].
5.4. Cell cycle
GSH can be sequestered into the nucleus where it may participate in protein glutathionylation and cell proliferation [13,81,82]. Diaz Vivancos et al. [13] have proposed that this sequestration is intimately linked to the cell cycle. According to these authors “(i) GSH is recruited and sequestered in the nucleus early (G1) in cell proliferation, (ii) as a result of GSH sequestration in the nucleus, the cytoplasm is starved of GSH, (iii) the sharp fall in cytosolic GSH availability and the accompanying change in cytosolic redox state triggers GSH synthesis in the cytoplasm, (iv) the total GSH pool of the cells increases rapidly, (v) the nuclear envelope dissolves at the end of prophase/beginning of metaphase allowing equilibration between the cytosol and nuclear GSH pools during the G2- and M-phases, and (vi) the nuclear envelope re-forms during telophase (before cytokinesis), the cells divide and the cellular GSH pool is re-distributed between the daughter cells”. According to this series of events, redox modification (glutathionylation) of proteins may play a role in the control of protein function during the cell cycle [13]. Corroboration of the cell cycle changes, intracellular compartmentalization, and the dynamic translocation of GSH and glutathionylated proteins within intact cells was elegantly reported by Söderdahl et al. [83].
5.5. Ion channel activity/calcium homeostasis
Protein glutathionylation has been linked to oxidant-mediated vascular potassium-ATP channel regulation [84] and the skeletal muscle calcium-release channel [ryanodine receptor type 1 (RyR1)] [85].
Protein glutathionylation may act as a molecular link between calcium and redox signaling. For example, from cell model studies in which GSH was depleted (by treatment with a mixture of 5-chloro-2-methyl-4-isothiazolin-3-one and 2-methyl-4-isothiazolin-3-one) and in other studies in which GSH was increased (by treatment with glutathione ethyl ester), it was concluded that the degree of GSH depletion is paralleled by protein glutathionylation, which may have a role in increasing cellular calcium levels. This increased mitochondrial and cytosolic calcium load may be factors in apoptosis and necrosis, respectively [80].
Many studies have shown that oxidative stress leads to an elevated level of cellular calcium, increased mitochondrial calcium and cell death. In recent studies of DT40B-lymphocytes in culture, it was shown that stromal interaction molecule 1 (STIM1) is glutathionylated at Cys56 in response to oxidative stress (treatment with lipopolysaccharide or treatment with buthionine sulfoximine to lower GSH), evoking a constitutive calcium entry into mitochondria independent of intracellular stores [86].
5.6. Regulation of cytoskeletal structures
Actin is readily glutathionylated, a process that inhibits actin polymerization. Thus, reversible glutathionylation of actin is a redox-dependent mechanism for regulation of the cytoskeleton structure [87–90]. Interestingly, glutathionylation of actin, a major protein in platelets, does not appear to be related to the GSSG level in these cells [88], but rather to oxidation of a susceptible cysteine residue to a sulfenic acid followed by reaction with GSH [89]. Some evidence suggests that actin glutathionylation is essential for cell spreading and cytoskeleton organization and that this glutathionylation plays a key role in disassembly of actinomyosin complexes during cell adhesion [90]. The possibility has been considered that abnormal glutathionylation of cytoskeletal elements contributes to the pathology of cardiac and skeletal muscles due to ischemia/oxidative stress [91–93] and to muscle pathology in Friedreich ataxia [92].
5.7. Protein folding and integrity
In contrast to the cytosol where the GSH/GSSG ratio is usually >100:1, the ratio in the endoplasmic reticulum (ER) is only about 1:1 to 3:1 [4,7,94]. Correct folding of nascent proteins (protein quality control) is dependent in part upon this controlled redox condition within the ER [7]. Redox conditions within that ER may in turn affect rates of protein glutathionylation [7]. PDI is an enzyme crucial for maintaining the correct disulfide balance and accurate folding in nascent proteins within the ER. Therefore, it is of interest that in human leukemia and ovarian cancer cells, treatment with the anti-cancer agent O(2)-[2,4-dinitro-5-(N-methyl-N-4-carboxyphenylamino)phenyl]1-(N,N-dimethylamino)diazen-1-ium-1,2-diolate (PABA/NO) resulted in an increase in intracellular NO and increased glutathionylation of PDI [95]. This glutathionylation results in loss of activity. Townsend and colleagues suggested that exploitation of PDI inhibition though drug-induced glutathionylation may be a unique strategy to target cancer cells [7,95]. The unfolded protein response (UPR) in the ER is a complex signal cascade induced by stress, including oxidative and nitrosative stress, and the accumulation of misfolded proteins that promotes apoptosis [7]. At least three pathways contribute to apoptosis, and protein components of at least one of these pathways (activation of JNK) can be glutathionylated [7].
The proteasome machinery plays an important role in protein quality control by removing misfolded or otherwise damaged proteins. It is therefore of interest that thimet oligopeptidase (EP24.15), a ubiquitously expressed thiol-rich metallopeptidase that is important in the degradation of peptides generated by the proteasome machinery, is constitutively glutathionylated intracellularly [96]. The findings suggested an unconventional property of protein glutathionylation, namely inducing oligomerization by interprotein thiol-disulfide exchange to a less active enzyme form [96]. These results may be relevant to the dual role of EP24.15 both in degrading antigenic peptides, which results in decreased antigen presentation, and in increasing production of antigenic peptides, for example, under conditions of oxidative stress. It is of further interest that the Rpn1 and Rpn2 subunits of purified human 26S proteasomes undergo glutathionylation, resulting in a less active complex when exposed to H2O2 and GSH [96].
6. Biomedical implications of reversible glutathionylation
Several diseases may be associated with aberrant glutathionylation of proteins [7]. The glutathionylation of actin and cytoskeletal elements in ischemia, heart diseases and Friedreich ataxia has already been mentioned. Other diseases as listed by Townsend [7] are discussed below.
6.1. Alzheimer disease (AD)
Energy metabolism is compromised in AD in part due to low activity of KGDHC [97]. In addition to glutathionylation of lipoamide cofactor the E2 subunit in the presence of H2O2 and GSH, mentioned above, all three subunits of purified KGDHC are readily glutathionylated in the presence of GSSG [97]. It has been suggested that glutathionylation, as a result of oxidative stress, may contribute to the low levels of KGDHC activity in AD brain [97]. Other workers have shown that glutathionylation of tau protein is possible although this process does not seem to contribute to the formation of tau-containing paired helical filaments characteristic of AD brain [98]. In addition, selective glutathionylation of p53 monomers and dimers has been demonstrated in AD brain, possibly contributing to the oxidative stress in AD brain [99].
6.2. Type 2 diabetes
The incidence of glutathionylated hemoglobin HbSSG has been reported to be much higher in diabetic subjects with microangiopathy (69%) as compared to diabetics without angiopathy (22%) and controls (14%) [100].
With the understanding that oxidative-nitrosative stress contributes importantly to the pathophysiology in diabetes, Shelton et al. hypothesized that changes in Grx activity would contribute significantly to the development of diabetic retinopathy [101]. Using retinal homogenates from streptozotocin-diabetic rats as well as retinal cells grown in high glucose medium, these investigators demonstrated that Grx, in contrast to thioredoxin, was upregulated. Increased activity of Gx, which catalyzes deglutathionylation of proteins, led to increased translocation of NF-κB to the nucleus and enhanced expression of ICAM-1, two pro-inflammatory processes implicated in the pathogenesis of retinopathy and targets for glutathionylation. The data suggest that elevated glucose concentrations in retinal glial cells disrupt the glutathionylation-induced regulatory effect on these pro-inflammatory proteins by increasing Grx activity [101].
6.3. Cystic fibrosis
The CFTR (cystic fibrosis transmembrane conductance regulator) channel activity in excised lung tissue is markedly inhibited by several forms of oxidized GSH (GSSG, GSNO and GSH oxidized with diamide) [102]. Several lines of evidence were presented indicating that the likely mechanism for this inhibitory effect is glutathionylation of a CFTR cysteine residue (i.e. formation of a mixed disulfide with GSH). This finding is of interest because reactive GSH species are produced in inflamed epithelia as occurs in cystic fibrosis where they may modulate CFTR activity [102]. However, it is possible that GSNO has beneficial effects at a low concentration. Thus, it was shown that GSNO and some other S-nitrosating agents (but not NO itself), at low micromolar concentrations, increase ΔF508 mutant CFTR and wild-type CFTR expression and maturation [103]. It was suggested that the findings are of relevance to the development of NO donor-based therapies for cystic fibrosis [103].
6.4. Cataracts
GSH is present at high concentrations in the human lens (~6 mM) where, as an antioxidant, it is essential for maintaining transparency [104]. The size of the GSH pool diminishes with age, and loss of GSH is associated with cataract formation. Cataractous lenses exhibit a decreased GSH/GSSG ratio compared with clear lenses [104]. As discussed by Harding and colleagues [104 and references cited therein], the unique development and structure of the lens may explain the need for high levels of GSH. During cataractogenesis, especially in the nucleus part of the lens, the lens proteins unfold and thiols that were buried become exposed and reactive. Some of these thiols then react to form a) mixed disulfides with GSH and cysteine, and b) disulfide-cross-linked aggregates. With increasing severity of the cataract, total protein thiol decreases with a concurrent increase in protein disulfide content. A major glutathionylated protein in the lens was found to have a molecular mass of about 47 kDa, which was shown to be composed of βB1-, βB2- and γS-crystallins [104]. The possibility has been considered that maintaining the GSH status may be helpful in preventing or ameliorating cataract formation [104,105]. In a streptozotocin model of diabetes in rats administration of eye drops containing N-acetyl-L-cysteine and glutathione ethyl ester (compounds readily converted to GSH in vivo) showed significant inhibition of the progression of diabetic cataracts at early (but not late) stages of the diabetes-associated cataractogenesis [105].
6.5. Cancer
Insights into the cellular mechanisms that posttranslationally modify regulatory proteins during recurrent episodes of oxidative and nitrosative stresses, and which initiate and promote carcinogenesis are beginning to emerge. As stated earlier, the majority of redox-sensitive regulatory and cell cycle proteins that are targets of ROS and RNS can be stabilized and recycled via their sulfhydryl moieties. Accordingly, these proteins can be reversibly glutathionylated (depending on the GSH/GSSG ratio) and restored to their reduced state by PDI, Grx, thioredoxin or sulfiredoxin. Thus, glutathionylation of strategic cysteine moieties in metabolic enzymes, kinases, phosphatases, signal proteins and transcription factors has emerged as a central mechanism by which changes in the intracellular redox state may be transformed into functional cellular responses that can include cancer treatment and control. Studies by Velu et al. [106] provide novel insights into the cell death-associated tumor suppressor protein, p53, which is often inactivated during the genesis of cancer as a result of mutational modifications. Tumor suppressor functions of p53 are observed after genomic injury, which, following site specific phosphorylation(s), increases its transcriptional competency by modulating several cell cycle regulators and checkpoint kinases. By contrast, when p53 is glutathionylated, and even though its serine phosphorylated-form is present at nuclear binding sites, glutathionylated-p53 exhibits significantly diminished ability to bind to its consensus DNA sequence. Thus glutathionylation inhibits, albeit temporarily, the tumor suppressor function of p53. The results of this study demonstrate that conversion of reactive cysteines 124 and 141 to mixed disulfides with GSH conveys a negative regulatory function to human p53, and implies that inactivation of this transcription factor may represent an acute defensive response with significant consequences for oncogenesis. Velu et al. [106] speculated that inactivation of p53 through glutathionylation prompts a selective cellular adaptation strategy that suppresses the apoptotic response generated during early phase oxidative stress in an apparent attempt to avert immediate cell death.
The literature is replete with evidence supporting the hypothesis that chronic inflammation contributes significantly to the development of cancer. The inflammatory microenvironment is characterized by the presence of activated leukocytes and macrophages [107] which express pro-inflammatory cytokines such as interleukin 1β (IL-1β) that enhance tumor proliferation, promote angiogenesis, and advance invasive activity within tumors [108]. Following release of IL-1β, this cytokine mediates activation of the transcription factor, NFkB, through a pathway involving phosphorylation, which subsequently results in release and degradation of the NFkB inhibitor, IkB. NFkB regulates the expression of inflammatory and oncogenic genes by translocating to the nucleus and binding its p50 or p65 carboxyl-terminus to transactivation domains on DNA [109]. Thus, greater appreciation is required that integrates our understanding of glutathionylation of NFkB and other transcription factors, adhesion proteins, cytokines and redox-responsive enzymes with diseases such as cancer and chronic inflammation [110].
Studies by Qanungo et al. [111] have shown that Cys59 of the p50-NFkB and Cys38 of the p65-NFkB fractions, which associate near or with DNA-binding loops are targeted by glutathionylation, thereby preventing transcriptional activation. It should be mentioned here that the IkB kinase (IKK), which phosphorylates and causes uncoupling of the IkB inhibitor from NFkB is also controlled via glutathionylation of the Cys179 residue of IKK [112]. The findings regarding glutathionylation of NFkB subunits have therapeutic implications pertinent to hypoxic apoptosis, such as in solid tumors that possess hypoxic cores [111].
Tew and Townsend [15] have suggested that drugs targeting the glutathionome (i.e. the sum total of proteins glutathionylated) may be useful in the treatment of cancer. Studies by Diotte et al. [113] using the anticancer drug doxorubicin (DOX) determined whether glutathionylation of mitochondrial proteins plays a role in mitigating DOX-induced myocardial injury. Using transgenic mice that expressed the human mitochondrial Grx 2, a thioltransferase catalyzing the reduction as well as formation of protein-glutathione mixed disulfides, prevented DOX-induced decreases in NAD- and FAD-linked state 3 respiration and respiratory control ratio in heart mitochondria. Development of tolerance to DOX toxicity was associated with an increase in protein glutathionylation in heart mitochondria. These studies suggest that the efficacy of the chemotherapeutic drug, DOX, whose use is limited due to its often unpredictable cardiotoxicity, may be improved by inducing glutathionylation of heart mitochondrial proteins, thus preventing DOX-induced cardiac injury during treatment of cancer [113].
7. Irreversible protein glutathionylation involving dehydroalanine moieties
7.1. Occurrence of dehydroalanine in nature
Dehydroalanine (2-aminoacrylate; 2,3-didehydroalanine; DHA) residues in proteins/peptides occur naturally. This amino acid is relatively stable in peptide linkage[114], but as an isolated amino acid is rapidly tautomerized and hydrolyzed to pyruvate and ammonia. The vinyl moiety of the DHA residue is conjugated with a carbamoyl carbonyl imparting reactivity as a Michael acceptor. Michael attack by a side group of a cysteine residue in a protein/peptide will give rise to a protein/peptide covalently cross linked by a thioether bond representative of a lanthionine residue. In contrast to the easily reduced disulfide in GSSG, the sulfide in lanthionine is stable to reduction under physiological conditions. Lanthionine linkages are of considerable interest as they are found extensively in a class of ribosome-directed polypeptide-based antibiotics known as lantibiotics [115]. Of relevance to the current review, however, is the presence of such linkages in lens proteins.
7.2. Irreversible glutathionylation of lens proteins
The lens nucleus contains proteins that are present from birth. Moreover, the outer fibrous cells no longer make proteins after the lens has been fully shaped [104, and references therein]. Thus, it is not surprising perhaps that after many decades some nonenzymatic posttranslational protein modifications occur in the lens. One such modification is the loss of H2S or H2O from cysteine or serine residues, respectively, resulting in the formation of DHA residues (Figure 2). Michael addition by cysteine, histidine, or lysine residues will result in proteins cross linked internally (or to other proteins) by lanthionine bridges, histidinolalanine linkages or lysylalanine cross links, respectively [116,117]. Linetsky and colleagues showed that lanthionine and histidinoalanine residues in hydrolyzed proteins are present at higher concentrations in cataractous lenses than in normal lenses; lysylalanine could only be detected in cataractous lenses [116]. Loss of H2O from a threonine residue yields a dehydrobutyrine (2,3-didehydributyrine) residue that can undergo Michael chemistry with a cysteine residue to generate a 3-methyl lanthionine link. This derivative occurs in the antimicrobial peptide nisin [118], which is used extensively in the food industry as a preservative, but, to our knowledge, dehydrobutyrine has not been identified in lens proteins.
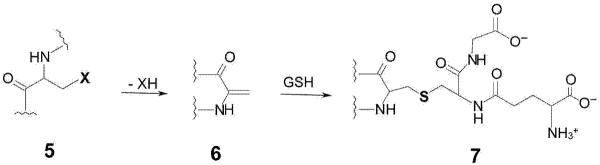
Figure 2. Formation of a dehydroalanyl residue in proteins and peptides followed by irreversible glutathionylation.
A protein/peptide (5) containing a leaving group (X) in the β position of an amino acid residue may undergo spontaneous β elimination to generate a protein/peptide containing a dehydroalanyl residue (6). Michael addition of the sulfhydryl group of GSH will generate of an adduct containing a non-reducible thioether bond (7), resulting in irreversible glutathionylation. Residues such as cysteine (X = -SH) and serine (X = -OH) are expected to be relatively stable. However, for proteins with very long turnover times, such as those found in the lens, β-elimination reactions may occur as a consequence of aging. Moreover, other residues such as selenocysteine, serine O-phosphate and serine O-sulfate are expected to be more labile and amenable to β elimination.
Lanthionine residues may be formed in lens tissues not only via Michael addition of a protein cysteine residue to a DHA residue, but also by attack of either free cysteine or the cysteine moiety of GSH. In a later study, Linetsky and LeGrand showed that increased lanthionine occurs in the aged and cataractous human lens and that a considerable portion results from addition of GSH to a DHA residue, resulting in non-reducible glutathionylation in which the cross link is a lanthionine [117].
7.3. Does irreversible glutathionylation occur in other tissues?
This question is important, but to our knowledge has not been adequately addressed. At this point, a discussion of DHA is appropriate. The synthesis of peptides containing DHA residues and other dehydro amino acids was accomplished more than 60 years ago [119 and references cited therein]. This early work by Jesse Greenstein and colleagues seems largely to have been overlooked. Perhaps, it is time to rethink the biological importance of dehydro amino acid residues. It is relatively easy to generate DHA residues in proteins/peptides. For example, DHA residues are formed in model peptides containing a reactive S-nitrosocysteine by treatment with phosphine under relatively mild conditions [120]. Base-induced β-elimination of phosphate from phosphoserine and phosphothreonine residues followed by addition of an affinity tag has been used as a strategy for enriching phosphorylated proteins from complex mixtures [121]. In other studies, DHA residues have been observed as artifacts in proteome analysis [122], as common post-translational modifications in human serum albumin [123], and after loss of selenium following oxidation of selenocysteine moieties from selenoproteins [124]. Heat and alkali treatment of foods result in the formation of DHA residues and resulting cross-linked amino acids in proteins, as well as the epimerization of L-amino acid stereoisomers [125]. Electrophilic addition at the α,β-unsaturated moiety of DHA residues by a variety of nucleophiles is an entry to functionalized proteins [121,126–128]. Lanthionine bridges can also form in proteins during derivatization steps before mass spectral analysis, presumably through intramolecular condensation of a cysteine residue with a DHA [129]. In a recent article, Jeong et al. [130] described the use of sensitive mass spectrometry techniques to show several modifications of a redox-active cysteine residue in GAPDH. These modifications included conversion to serine, DHA and a cysteine thiosulfinate residue.
Proteins in other tissues are generally not as long lived on average as those in the lens. The average protein turnover in the whole adult human body has been reported to be about 1.5 – 6 g/kg/d, which represents ≤0.1% of the total protein pool [131]. Accordingly, on average, proteins, and in particular skeletal muscle proteins, must have a half life of ≥500 days and presumably some may have much longer half lives. Thus, the relative ease with which DHA residues can be generated in model systems and the relatively long half life of a portion of the human body pool of proteins, make it almost certain that DHA will occur in vivo in other tissues. In this context, a recent study on the inactivation of GPx1, the principal enzyme responsible for peroxide elimination in human red blood cells (RBCs), revealed that H2O2 exposure converts the selenocysteine residue at the active site to a DHA residue [132]. Given that DHA-GPx1 formation is irreversible and its content increases with increasing RBC age, the content of DHA-GPx1 within the RBC has been suggested to reflect total oxidative stress experienced by the cell during its lifetime [132]. In light of the relatively high levels of endogenous GSH in blood (~1 mM) [133], it is conceivable that a Michael addition reaction will occur. This will result in the formation of lanthionine cross-link in GPx1 protein and the possibility of non reducible glutathionylation of this protein occurring within aging RBCs. We hypothesize that these modifications will increase with age as occurs in the lens.
7.4. Metabolism of busulfan
Busulfan is a bifunctional alkylating agent used for the treatment of hematological and other malignancies prior to stem cell transplantation [134]. The structures of busulfan and related anti-cancer agents are shown in Figure 3. The compound is converted in vivo to a glutathione S-conjugate [L-γ-glutamyl-β-(S-tetrahydrothiophenium)-L-alanylglycine; GS+THT] by direct interaction with GSH [135] and enzymatic catalysis by GSTs, especially GST A1-1 [135–141]. GS+THT undergoes a base-catalyzed β-elimination reaction to yield γ-glutamyldehydroalanylglycine (EdAG) and tetrahydrothiophene (THT) [139, 140]. This reaction, however, occurs readily in vitro at physiological pH values and temperature [141]. EdAG was identified as a metabolite of busulfan in a human liver cytosol fraction [141]. THT formed directly from EdAG or from a β-elimination reaction of the corresponding cysteine S-conjugate [140] and THT oxidation products are the major metabolites of busulfan in vivo [139].
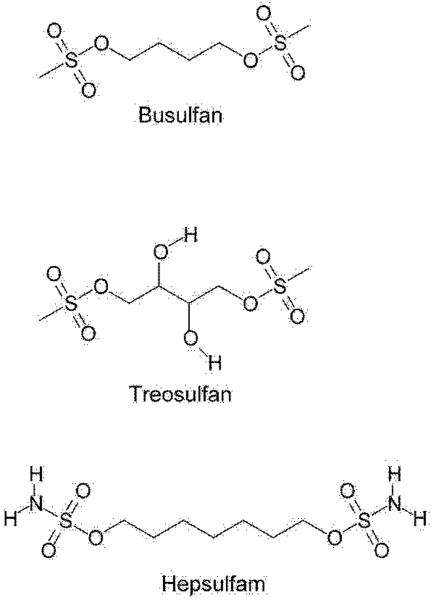
Figure 3. Structures of busulfan, treosulfan and hepsulfam.
Note the reactive groups at both ends that are involved in DNA cross linking.
We recently showed that EdAG condenses with GSH in a Michael addition reaction to produce a lanthionine-containing thioether (GSG), which is a non-reducible analog of glutathione disulfide (GSSG) [141]. EdAG was less cytotoxic than busulfan to C6 rat glioma cells. GSH and EdAG were equally effective in displacing a GST isozyme (human GSTA1-1) from a GSH-agarose column [141].
7.5. Is the toxicity of busulfan related to irreversible glutathionylation?
High doses of busulfan can cause hepatic veno-occlusive disease (HVOD) [142] and cataracts [143]. HVOD is caused by the destruction of sinusoidal endothelial cells and the surrounding centrilobular hepatocytes [144]. Injury to sinusoidal endothelial cells and hepatocytes of the liver acinus is considered to be the initial event in the development of HVOD. Studies have shown that this region is rich in cytochrome P450 and GST, but contains levels of GSH that are lower than at other sites [145]. Sinusoidal endothelial cells are more sensitive to damage than hepatocytes because the concentration of GSH in sinusoidal endothelial cells, which is needed for detoxification, is less than half that in hepatocytes. In cell culture studies when precursor amino acids are added to culture media, hepatocytes adequately synthesize GSH while sinusoidal endothelial cells exhibit limited capacity [146,147]. It was proposed that HVOD associated with conditioning regimens of busulfan in bone transplant patients is related to metabolites of busulfan rather than to the parent compound [148]. DeLeve and Wang [149] suggested that this complication may be caused either directly through oxidative stress or indirectly through GSH depletion. We hypothesize that busulfan-induced HVOD may be related in part to the nonenzymatic decomposition of GS+THT to EdAG. Interestingly, it has recently been shown that busulfan treatment of mice resulted in up-regulation of GSH synthesis and increased toxicity of busulfan [150]. We suggest that higher titers of GSH will result in higher levels of GS+THT [141]. An increase in GS+THT would lead to increased nonenzymatic formation of EdAG, which, in turn, would lead to nonreducible glutathionylation of proteins (formation of PSG) associated with the circulatory system. Thus, increased nonreducible glutathionylation may contribute to busulfan-induced HVOD. A possible mechanisms leading to busulfan-induced irreversible glutathionylation of proteins is shown in Figure 4. It is also possible that increased synthesis of GSH, oxidative stress and relatively high GST levels in sinusoidal endothelial cells will promote reversible glutathionylation of proteins (formation of PSSG) when these cells are exposed to busulfan.
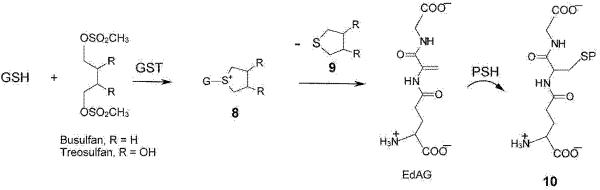
Figure 4. Formation of glutathione S-conjugates of busulfan and facile decomposition to a dehydroalanyl analogue of GSH.
Busulfan (R = H) reacts with GSH in a reaction that occurs spontaneously under physiological conditions, but which is accelerated by GSTs, to form the glutathione S-conjugate 8. This glutathione S-conjugate contains an unusual sulfonium moiety (most glutathione S-conjugates are thioethers). The cyclic sulfonium contains an exceptionally good leaving group. Thus, tetrahydrothiophene (9) is readily eliminated generating a dehydroalanyl analogue of GSH (γ-glutamyldehydroalanylglycine, EdAG). Michael addition of GSH to this compound results in the formation of GSG, a GSSG analogue in which the reducible disulfide bond is replaced by a non-reducible thioether. We suggest that the reaction of EdAG with an exposed cysteine residue of a susceptible residue (PSH) may result in irreversible glutathionylation, generating a protein linked to a glutathione molecule through a non-reducible thioether bond (10). This reaction is identical to that depicted in figure 2 (6 → 7), except that the Michael acceptor and donor are reversed. Although the possible formation of a sulfonium conjugate of treosulfan has not yet been investigated, given the similarity in structure of treosulfan to busulfan, we predict that it will form under in vivo conditions.
Non-reducible glutathionylation may also contribute to the busulfan-induced cataractogenesis. Note that in the studies of Linetsky and LeGrand [117] it was assumed that the irreversible glutathionylation of lens proteins is due to Michael addition of the thiol group in GSH to protein dehydroalanyl residues. However, irreversible protein glutathionylation is also theoretically possible by Michael addition of a protein thiol to the dehydroalanyl residue of EdAG (Figure 4). Evidence for this possibility is the formation of GSG from direct interaction of GSH with EdAG [141].
In summary, busulfan may be toxic in part by assisting in the conversion of GSH from a nucleophile to an electrophilic metabolite (EdAG) (umpulong chemistry) thereby depleting cellular GSH concentrations. Moreover, irreversible non-reducible glutathionylation of GSH will result in formation of thioether-linked GSG. The latter metabolite may interfere with GST function and act as a competitive inhibitor of glutathione reductase, the enzyme necessary to convert GSSG disulfide back to GSH. Although not yet demonstrated directly, EdAG is chemically capable of irreversibly glutathionylating proteins (formation of PSG).
7.6. Possibility of other drugs/xenobiotics promoting irreversible glutathionylation
Treosulfan is an analogue of busulfan in which the C4 butane backbone contains two hydroxyl groups (Figure 3). Like busulfan, treosulfan acts as a DNA cross-linking agent. A major carcinogenic metabolite is thought to be the hydrolysis product 1,2:3,4-diepoxybutane [151]. Treosulfan shows preclinical promise against Ewing sarcoma [152], and is cytotoxic to human prostate cancer cells [153]. Very little is known about its metabolism. We strongly suspect that treosulfan will react with GSH and cysteine to generate S-conjugates. By analogy with the conjugates formed in the major biotransformation pathway of busulfan in humans, the S-conjugates formed from treosulfan should be sulfonium compounds in which the sulfur is part of a five-membered ring. However, the conjugates will have two ring-positioned hydroxyl groups that are lacking in the busulfan conjugates. The resulting 3,4-dihydroxy tetrahydrothiophenium intermediate formed in the reaction of treosulfan with GSH could produce EdAG in an elimination reaction. Since 3,4-dihydroxy tetrahydrothiophene may be an even better leaving group than is tetrahydrothiophene, we predict that the treosulfan glutathione S-conjugate will undergo a facile non-enzymatic β-elimination reaction to yield thiophene and EdAG (Figure 4).
Another busulfan analogue, namely hepsulfam, has also been evaluated as a DNA cross-linking agent and as an anti-cancer agent. Armstrong et al. [154] showed that several human breast cancer cells in culture are able to convert hepsulfam to the corresponding monoglutathione S-conjugate with varying efficiency. The only major GST detected, and only in some cell lines, was GST-Pi. Thus, formation of the glutathione S-conjugate was presumably catalyzed by this GST isozyme. The amount of GST-Pi isozyme correlated with sensitivity to hepsulfam in the three most resistant cell lines, but was undetectable in the three most sensitive cell lines [154]. When the cells were treated with buthionine sulfoximine to lower the intracellular concentration of GSH, the cells became more sensitive to hepsulfam. Presumably, even if the diglutathionyl conjugate were formed it is unlikely that a cyclic sulfonium would be formed because this would entail formation of an unlikely eight-membered ring.
Westerhof et al. [155] synthesized a number of analogs of busulfan and hepsulfam in which the terminal H3CO2SO- and H2NO2SO- groups, respectively, were retained but the central portion was changed. Among 25 different compounds tested, a remarkably broad range of potencies was found, with PL63 [cis-1,2-(2-hydroxyethyl) cyclohexane dimethanesulfonate] proving to be the most effective in providing for hematopoietic engraftment [155]. The authors pointed out that pharmacokinetics rather than structural properties, such as distance and orientation of the two alkylating groups, are likely the more important determinants in the effectiveness of the drugs [155]. In this respect, it is worth pointing out that although PL63 is theoretically capable of forming a glutathione S-conjugate, it is most unlikely that a cyclic sulfonium S-conjugate of this compound can be formed. We suggest that when considering the biological action of the busulfan and hepsulfam analogues, the ability to form cyclic sulfonium S-conjugates should be considered in the evaluation.
Several 1,2-dihaloethanes are known to form glutathione S-conjugates in a manner exactly analogous to the formation of GS+THT from busulfan (and from 1,4-dihalobutanes [156]) except that a three-membered sulfonium ring structure (thiirane) is formed rather than a sulfonium containing a five-membered ring (reviewed by Anders [157]). The thiirane glutathione S-conjugate is known to be directly toxic by forming adducts with DNA [158]. With regard to the glutathione S-conjugate, since the thiirane grouping is likely to be an excellent nucleofuge [see ref. 159 for a discussion of nucleofugality], we suggest that the glutathione S-conjugate will undergo a spontaneous β-elimination reaction to generate EdAG, which may also contribute to the toxicity of 1,2-dihaloethanes. It is also possible that the corresponding cysteine S-conjugate would undergo spontaneous cysteine S-conjugate β-lyase-assisted β elimination. If these β-elimination reactions were to occur in vivo, the finding would be of toxicological interest because 1,2-dihaloethanes are produced on an industrial scale [157] and are genotoxic [158].
8. Irreversible protein glutathionylation involving 4-oxo-2-nonenal
The polyunsaturated lipid peroxidation product 4-oxo-2-nonenal (ONE) is a highly reactive protein cross-linking agent [160]. It has recently been shown that addition of GSH to ONE results in the formation of a reactive 4-ketoaldehyde that also has the potential to cross link proteins [160]. Zhu et al. [160] showed that almost every lysine residue was cross linked when 0.25 mM bovine β-lactoglobulin was incubated in 100 mM potassium phosphate buffer containing 0.25–2 mM ONE, and 1 mM GSH (an antielectrophile) at 37°C for 24 h. The authors suggested “stable antielectrophile-ONE-protein cross-links may serve as biomarkers of oxidative stress and may represent a novel mechanism of irreversible protein glutathionylation” [160].
9. Conclusion
As judged by the number of publications just within the last two years on the topic, it is obvious that the field of reversible protein glutathionylation is undergoing a veritable explosion. Reversible glutathionylation impinges on many aspects of biology, from regulation of protein function to roles in the cell cycle and apoptosis. The importance of aberrant protein glutathionylation in cancer and other diseases is an area of increasing interest. It is hoped that understanding the processes may lead to new therapeutic regimens.
This review provides a novel dimension that has not been included in previous reviews on protein glutathionylation by other authors. We have emphasized here that, in addition to reversible protein glutathionylation, irreversible protein glutathionylation is also a possibility, albeit a possibility that has been largely overlooked by the scientific community. Although irreversible protein glutathionylation has only been conclusively demonstrated to occur in human lens, we emphasize the possibility that this process is likely to occur in other tissues perhaps in RBCs, particularly with aging and following treatment with certain drugs and environmental toxins such as certain halogenated alkanes.
10. Expert opinion
The process of reversible protein glutathionylation is now well established, and research in the field is burgeoning. However, as recognized by several authors cited herein, much of the work in the area has been carried out with isolated enzymes or with cells in culture, and the findings need to be corroborated at the tissue and organ levels, and also with disease state. In the near future, we can look forward to improved methodology that will permit the realistic assessment of the glutathione redox state in tissues in vivo under normal and pathological conditions. In this regard it of interest that nuclear magnetic resonance (NMR) pulsing techniques are being developed for the detection and estimation of GSH in normal and cancerous tissues [e.g. 161–165]. Moreover, glutathionylation of a protein will increase the mass of the protein by 305 kDa and introduce a net negative charge. Such changes are easily identifiable by modern mass spectrometric techniques. NMR techniques are currently not sensitive enough to detect normal in vivo levels of GSSG, which are usually much lower than those of GSH. Nevertheless, in vivo analysis of GSH levels coupled with in vitro sensitive mass spectral proteomics (and other methods listed in reference 59) will be crucial in defining the glutathionome. As Tew and Townsend suggest in a recent review [166], defining the glutathionome has the potential of providing opportunities for target identification for therapeutic intervention (particularly cancer treatment), perhaps with a relevance that parallels ongoing efforts with the kinome. Due to the high GSH/GSSG ratio, the number of glutathionylated proteins in vivo is probably not large (possibly of the order of hundreds) under normal conditions compared to the number of phosphorylatable proteins [166]. Nevertheless, as with phosphorylation, glutathionylation is crucial to cell signaling and cell survival, and may be subtly altered in a variety of disease settings and treatments [166].
A major goal of the present review is to also emphasize the fact that irreversible protein glutathionylation can also occur. Although irreversible (non reducible) protein glutathionylation has thus far only been observed in aged and cataracterous human lenses, we emphasize the possibility that this process may occur in other human tissues as well, especially in tissues that contain slowly turning over proteins and a peroxidative environment. We also caution that some drugs and xenobiotics (e.g. busulfan, and possibly treosulfan and dihaloethanes) may be toxic in part by forming reactive sulfonium glutathione S-conjugates that can generate EdAG. Toxicologists should be aware that generation EdAG may possibly result in a) irreversible protein glutathionylation, b) decreases in tissue GSH pools, c) interference with GSTs, and d) formation of a novel thioether-analogue of glutathione, namely GSG, that may exhibit antagonistic effects with the disulfide analogue GSSG.
Acknowledgments
AJL Cooper's laboratory is supported National Institutes of Health grant (RO1 ES08421) while PS Callery is supported by a grant from the National Institute of Justice grant IJ-CX-K014.
Abbreviations
- DHA
dehydroalanine
- EdAG
γ-glutamyldehydroalanylglycine
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- Gpx
glutathione peroxidase
- Grx
glutaredoxin
- GSH
glutathione
- GSNO
nitrosated glutathione
- GSSG
glutathione disulfide
- GST
glutathione S-transferase
- GS+THT
L-γ-glutamyl-β-(S--tetrahydrothiophenium)-L-alanylglycine
- HVOD
hepatic veno-occlusive disease
- KGDHC
α-ketoglutarate dehydrogenase complex
- MPA
metaphosphoric acid
- NFkB
nuclear factor-kappa B
- NMR
nuclear magnetic resonance
- PDI
protein disulfide isomerase
- PSG
irreversibly (thioether-linked) glutathionylated protein
- PSNO
nitrosated protein
- PSSG
reversibly (disulfide-linked) glutathionylated protein
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
- THT
tetrahydrothiophene.
Footnotes
Declaration of Interest JT Pinto has received no funding and declares no conflict of interest in the preparation of this manuscript.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Meister A. Biochemistry of Glutathione. In: Greenberg DM, editor. Metabolism of Sulfur Compounds, Metabolic Pathways. 3rd Edition Vol. 7. 1975. pp. 101–188. [Google Scholar]; •An earlier account of the biological importance of GSH by a pioneer in the field.
- 2.Meister A, Anderson MA. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]; •A good review of earlier work on the biological importance of GSH.
- 3.Sies H. Glutathione and its role in cellular functions. Free Radic Biol Med. 1999;27:916–921. doi: 10.1016/s0891-5849(99)00177-x. [DOI] [PubMed] [Google Scholar]
- 4.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]; ••Excellent account of the importance of the GSH/GSSG redox couple in controlling the cellular redox environment including thiol/disulfide switches in proteins and the relevance to biological processes.
- 5.Jacob C, Giles GI, Giles NM, et al. Sulfur and selenium: the role of oxidation state in protein structure and function. Angew Chem Int Ed Engl. 2003;42:4742–4758. doi: 10.1002/anie.200300573. [DOI] [PubMed] [Google Scholar]
- 6.Shelton MD, Chock PB, Mieyal JJ. Glutaredoxin: role in reversible protein S-glutathionylation and regulation of redox signal transduction and protein translocation. Antioxid Redox Signal. 2005;7:348–366. doi: 10.1089/ars.2005.7.348. [DOI] [PubMed] [Google Scholar]
- 7.Townsend DM. S-Glutathionylation: indicator of cell stress and regulator of the unfolded protein response. Mol Interv. 2007;7:313–324. doi: 10.1124/mi.7.6.7. [DOI] [PMC free article] [PubMed] [Google Scholar]; •A good introduction to the biological importance of protein glutathionylation and possible biomedical implications.
- 8.Mieyal JJ, Gallogly MM, Qanungo S, et al. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid Redox Signal. 2008;10:1941–1988. doi: 10.1089/ars.2008.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalle-Donne I, Rossi R, Colombo G, et al. Protein S-glutathionylation: a regulatory device from bacteria to humans. Trends Biochem Sci. 2009;34:85–96. doi: 10.1016/j.tibs.2008.11.002. [DOI] [PubMed] [Google Scholar]; ••An excellent account of the important biological roles of protein glutathionylation in nature.
- 10.Dalle-Donne I, Colombo G, Gagliano N, et al. S-Glutathiolation in life and death decisions of the cell. Free Radic Res. 2010 Sep 6; doi: 10.3109/10715762.2010.515217. Epub ahead of print. [DOI] [PubMed] [Google Scholar]; •A timely review on the recent findings of an important role for GSH in apoptosis.
- 11.Cooper AJL, Hanigan MH. Enzymes involved in processing of glutathione conjugates. In: McQueen CA, Guengerich FP, editors. Comprehensive Toxicology 2nd Edition: Volume 4. Biotransformations. Elsevier Press; Oxford: 2010. pp. 323–365. [Google Scholar]; •This chapter describes the role of glutathione and the mercapturate pathway in the detoxification of potentially harmful endogenous and exogenous electrophiles.
- 12.Markovic J, García-Gimenez JL, Gimeno A, et al. Role of glutathione in cell nucleus. Free Radic Res. 2010;44:721–733. doi: 10.3109/10715762.2010.485989. [DOI] [PubMed] [Google Scholar]
- 13.Diaz Vivancos P, Wolff T, Markovic J, et al. A nuclear glutathione cycle within the cell cycle. Biochem J. 2010;431:169–178. doi: 10.1042/BJ20100409. [DOI] [PubMed] [Google Scholar]; •A good rsummary of the potential role of GSH in the cell cycle.
- 14.Garcia J, Han D, Sancheti H, et al. Regulation of mitochondrial glutathione redox status and protein glutathionylation by respiratory substrates. J Biol Chem. 2010 Oct 11; doi: 10.1074/jbc.M110.164160. [DOI] [PMC free article] [PubMed] [Google Scholar]; •This paper describes the important role of glutathionylation of mitochondrial proteins as regulators of mitochondrial redox status and maintenance of mitochondrial NADPH levels.
- 15.Tew KD, Townsend DM. Redox platforms in cancer drug discovery and development. Curr Opin Chem Biol. 2010 Nov 11; doi: 10.1016/j.cbpa.2010.10.016. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]; •An excellent suggestion that intervention in protein glutathionylation may be of relevance in cancer treatment.
- 16.Cooper AJL, Pulsinelli WA, Duffy TE. Glutathione and ascorbate during ischemia and postischemic reperfusion in rat brain. J Neurochem. 1980;35:1242–1245. doi: 10.1111/j.1471-4159.1980.tb07882.x. [DOI] [PubMed] [Google Scholar]
- 17.Akerboom TP, Bilzer M, Sies H. The relationship of biliary glutathione disulfide efflux and intracellular glutathione disulfide content in perfused rat liver. J Biol Chem. 1982;257:4248–4252. [PubMed] [Google Scholar]
- 18.Rodkey FL. Oxidation-reduction potentials of the triphosphopyridine nucleotide system. J Biol Chem. 1955;213:777–786. [PubMed] [Google Scholar]
- 19.Harrison FE, May JM. Vitamin C function in the brain: vital role of the ascorbate transporter SVCT2. Free Radic Biol Med. 2009;46:719–30. doi: 10.1016/j.freeradbiomed.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hofmann B, Hecht HJ, Flohé L. Peroxiredoxins. Biol Chem. 2002;383:347–364. doi: 10.1515/BC.2002.040. [DOI] [PubMed] [Google Scholar]
- 21.Mauri P, Benazzi L, Flohé L, et al. Versatility of selenium catalysis in PHGPx unraveled by LC/ESI-MS/MS. Biol Chem. 2003;384:575–488. doi: 10.1515/BC.2003.065. [DOI] [PubMed] [Google Scholar]
- 22.Fratelli M, Goodwin LO, Ørom UA, et al. Gene expression profiling reveals a signaling role of glutathione in redox regulation. Proc Natl Acad Sci U S A. 2005;102:13998–14003. doi: 10.1073/pnas.0504398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seres T, Ravichandran V, Moriguchi T, et al. Protein S-thiolation and dethiolation during the respiratory burst in human monocytes. A reversible post-translational modification with potential for buffering the effects of oxidant stress. J Immunol. 1996;156:1973–1980. [PubMed] [Google Scholar]
- 24.Gallogly MM, Starke DW, Leonberg AK, et al. Kinetic and mechanistic characterization and versatile catalytic properties of mammalian glutaredoxin 2: implications for intracellular roles. Biochemistry. 2008;47:11144–11157. doi: 10.1021/bi800966v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Starke DW, Chock PB, Mieyal JJ. Glutathione-thiyl radical scavenging and transferase properties of human glutaredoxin (thioltransferase). Potential role in redox signal transduction. J Biol Chem. 2003;278:14607–14613. doi: 10.1074/jbc.M210434200. [DOI] [PubMed] [Google Scholar]
- 26.Lei K, Townsend DM, Tew KD. Protein cysteine sulfinic acid reductase (sulfiredoxin) as a regulator of cell proliferation and drug response. Oncogene. 2008;27:4877–4887. doi: 10.1038/onc.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jönsson TJ, Murray MS, Johnson LC, et al. Reduction of cysteine sulfinic acid in peroxiredoxin by sulfiredoxin proceeds directly through a sulfinic phosphoryl ester intermediate. J Biol Chem. 2008;283:23846–23851. doi: 10.1074/jbc.M803244200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rehder DS, Borges CR. Cysteine sulfenic acid as an intermediate in disulfide bond formation and nonenzymatic protein folding. Biochemistry. 2010;49:7748–7755. doi: 10.1021/bi1008694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boot-Handford RP, Briggs MD. The unfolded protein response and its relevance to connective tissue diseases. Cell Tissue Res. 2010;339:197–211. doi: 10.1007/s00441-009-0877-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ranaivoson FM, Neiers F, Kauffmann B, et al. Methionine sulfoxide reductase B displays a high level of flexibility. J Mol Biol. 2009;394:83–93. doi: 10.1016/j.jmb.2009.08.073. [DOI] [PubMed] [Google Scholar]
- 31.Gates KS, Tanner JJ, Parsons ZD, et al. Redox regulation of protein tyrosine phosphatases: Structural and chemical aspects. Antioxid Redox Signal. 2010 Oct 4; doi: 10.1089/ars.2010.3611. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 32.Leonard SE, Reddie KG, Carroll KS. Mining the thiol proteome for sulfenic acid modifications reveals new targets for oxidation in cells. ACS Chem Biol. 2009;4:783–799. doi: 10.1021/cb900105q. [DOI] [PubMed] [Google Scholar]
- 33.Reddie KG, Seo YH, Muse WB, III, et al. A chemical approach for detecting sulfenic acid-modified proteins in living cells. Mol Biosyst. 2008;4:521–531. doi: 10.1039/b719986d. [DOI] [PMC free article] [PubMed] [Google Scholar]; •Provides a method for identifying sulfenated proteins that are targets for S-glutathionylation
- 34.Seth D, Stamler JS. The SNO-proteome: causation and classifications. Curr Opin Chem Biol. 2010 Nov 17; doi: 10.1016/j.cbpa.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]; • A good review of protein S-nitrosation.
- 35.Jarry A, Charrier L, Bou-Hanna C, et al. Position in cell cycle controls the sensitivity of colon cancer cells to nitric oxide-dependent programmed cell death. Cancer Res. 2004;64:4227–4234. doi: 10.1158/0008-5472.CAN-04-0254. [DOI] [PubMed] [Google Scholar]
- 36.Rössig L, Fichtlscherer B, Breitschopf K, et al. Nitric oxide inhibits caspase-3 by S-nitrosation in vivo. J Biol Chem. 1999;274:6823–6826. doi: 10.1074/jbc.274.11.6823. [DOI] [PubMed] [Google Scholar]
- 37.Klein JA, Ackerman SL. Oxidative stress, cell cycle, and neurodegeneration. J Clin Invest. 2003;111:785–793. doi: 10.1172/JCI18182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang Z, Pinto JT, Deng H, Richie JP., Jr. Inhibition of caspase-3 activity and activation by protein glutathionylation. Biochem Pharmacol. 2008;75:2234–2244. doi: 10.1016/j.bcp.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giustarini D, Milzani A, Aldini G, et al. S-nitrosation versus S-glutathionylation of protein sulfhydryl groups by S-nitrosoglutathione. Antioxid Redox Signal. 2005;7:930–939. doi: 10.1089/ars.2005.7.930. [DOI] [PubMed] [Google Scholar]
- 40.Martínez-Ruiz A, Lamas S. Signalling by NO-induced protein S-nitrosylation and S-glutathionylation: convergences and divergences. Cardiovasc Res. 2007;75:220–228. doi: 10.1016/j.cardiores.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 41.Foster DB, Van Eyk JE, Marbán E, et al. Redox signaling and protein phosphorylation in mitochondria: progress and prospects. J Bioenerg Biomembr. 2009;41:159–168. doi: 10.1007/s10863-009-9217-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Townsend DM, Tew KD. Pharmacology of a mimetic of glutathione disulfide, NOV-002. Biomed Pharmacother. 2009;63:75–78. doi: 10.1016/j.biopha.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Findlay VJ, Townsend DM, Morris TE, et al. A novel role for human sulfiredoxin in the reversal of glutathionylation. Cancer Res. 2006;66:6800–6806. doi: 10.1158/0008-5472.CAN-06-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peltoniemi MJ, Karala AR, Jurvansuu JK, et al. Insights into deglutathionylation reactions. Different intermediates in the glutaredoxin and protein disulfide isomerase catalyzed reactions are defined by the γ-linkage present in glutathione. J Biol Chem. 2006;281:33107–33114. doi: 10.1074/jbc.M605602200. [DOI] [PubMed] [Google Scholar]
- 45.Townsend DM, Manevich Y, He L, et al. Novel role for glutathione S-transferase pi. Regulator of protein S-glutathionylation following oxidative and nitrosative stress. J Biol Chem. 2009;284:436–445. doi: 10.1074/jbc.M805586200. [DOI] [PMC free article] [PubMed] [Google Scholar]; •An interesting suggestion that GSTs play a role in protein glutathionylation.
- 46.Schneider A, Brandt W, Wessjohann LA. Influence of pH and flanking serine on the redox potential of S-S and S-Se bridges of Cys-Cys and Cys-Sec peptides. Biol Chem. 2007;388:1099–1101. doi: 10.1515/BC.2007.114. [DOI] [PubMed] [Google Scholar]
- 47.Wang PF, Flynn AJ, Naor MM, et al. Exploring the role of the active site cysteine in human muscle creatine kinase. Biochemistry. 2006;45:11464–11472. doi: 10.1021/bi0607002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gopalakrishna R, Jaken S. Protein kinase C signaling and oxidative stress. Free Radic Biol Med. 2000;28:1349–1361. doi: 10.1016/s0891-5849(00)00221-5. [DOI] [PubMed] [Google Scholar]
- 49.Kambe T, Song T, Takata T, Hatano N, Miyamoto Y, Nozaki N, Naito Y, Tokumitsu H, Watanabe Y. Inactivation of Ca2+/calmodulin-dependent protein kinase I by S-glutathionylation of the active-site cysteine residue. FEBS Lett. 2010;584:2478–2484. doi: 10.1016/j.febslet.2010.04.059. [DOI] [PubMed] [Google Scholar]
- 50.Singleton WC, McInnes KT, Cater MA, et al. Role of glutaredoxin1 and glutathione in regulating the activity of the copper-transporting P-type ATPases, ATP7A and ATP7B. J Biol Chem. 2010;285:27111–27121. doi: 10.1074/jbc.M110.154468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gromer S, Eubel JK, Lee BL, et al. Human selenoproteins at a glance. Cell Mol Life Sci. 2005;62:2414–2437. doi: 10.1007/s00018-005-5143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; • A good review of selenoproteins.
- 52.Huber RE, Criddle RS. Comparison of the chemical properties of selenocysteine and selenocystine with their sulfur analogs. Arch Biochem Biophys. 1967;122:164–173. doi: 10.1016/0003-9861(67)90136-1. [DOI] [PubMed] [Google Scholar]
- 53.Wessjohann LA, Schneider A, Abbas M, et al. Selenium in chemistry and biochemistry in comparison to sulfur. Biol Chem. 2007;388:997–1006. doi: 10.1515/BC.2007.138. [DOI] [PubMed] [Google Scholar]; •A very good comparison of the properties of sulfur versus selenium.
- 54.Reeves MA, Hoffmann PR. The human selenoproteome: recent insights into functions and regulation. Cell Mol Life Sci. 2009;66:2457–2478. doi: 10.1007/s00018-009-0032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; •Summarizes properties of selenoproteins and their distribution, localization, and regulation of expression
- 55.Lu J, Holmgren A. Selenoproteins. J Biol Chem. 2009;284:723–727. doi: 10.1074/jbc.R800045200. [DOI] [PubMed] [Google Scholar]
- 56.Sarma BK, Mugesh G. Thiol cofactors for selenoenzymes and their synthetic mimics. Org Biomol Chem. 2008;6:965–974. doi: 10.1039/b716239a. [DOI] [PubMed] [Google Scholar]
- 57.Brennan JP, Miller JI, Fuller W, et al. The utility of N,N-biotinyl glutathione disulfide in the study of protein S-glutathiolation. Mol Cell Proteomics. 2006;5:215–225. doi: 10.1074/mcp.M500212-MCP200. [DOI] [PubMed] [Google Scholar]
- 58.Eaton P. Protein thiol oxidation in health and disease: techniques for measuring disulfides and related modifications in complex protein mixtures. Free Radic Biol Med. 2006;40:1889–1899. doi: 10.1016/j.freeradbiomed.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 59.Priora R, Coppo L, Salzano S, et al. Measurement of mixed disulfides including glutathionylated proteins. Methods Enzymol. 2010;473:149–159. doi: 10.1016/S0076-6879(10)73007-X. [DOI] [PubMed] [Google Scholar]
- 60.Aesif SW, Janssen-Heininger YM, Reynaert NL. Protocols for the detection of S-glutathionylated and S-nitrosylated proteins in situ. Methods Enzymol. 2010;474:289–296. doi: 10.1016/S0076-6879(10)74017-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hu Y, Wang T, Liao X, et al. Anti-oxidative stress and beyond: multiple functions of the protein glutathionylation. Protein Pept Lett. 2010;17:1234–1244. doi: 10.2174/092986610792231573. [DOI] [PubMed] [Google Scholar]
- 62.Huang Z, Pinto JT, Deng H, et al. Inhibition of caspase-3 activity and activation by protein glutathionylation. Biochem Pharmacol. 2008;75:2234–2244. doi: 10.1016/j.bcp.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Azam S, Jouvet N, Jilani A, et al. Human glyceraldehyde-3-phosphate dehydrogenase plays a direct role in reactivating oxidized forms of the DNA repair enzyme APE1. J Biol Chem. 2008;283:30632–30641. doi: 10.1074/jbc.M801401200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodríguez-Pascual F, Redondo-Horcajo M, Magán-Marchal N, et al. Glyceraldehyde-3-phosphate dehydrogenase regulates endothelin-1 expression by a novel, redox-sensitive mechanism involving mRNA stability. Mol Cell Biol. 2008;28:7139–7155. doi: 10.1128/MCB.01145-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kornberg MD, Sen N, Hara MR, et al. GAPDH mediates nitrosylation of nuclear proteins. Nat Cell Biol. 2010;12:1094–1100. doi: 10.1038/ncb2114. [DOI] [PMC free article] [PubMed] [Google Scholar]; •Paper presents a novel mechanism of nitrosation and expands understanding of signal transduction mechanisms
- 66.Nulton-Persson AC, Starke DW, Mieyal JJ, et al. Reversible inactivation of α-ketoglutarate dehydrogenase in response to alterations in the mitochondrial glutathione status. Biochemistry. 2003;42:4235–4242. doi: 10.1021/bi027370f. [DOI] [PubMed] [Google Scholar]
- 67.Applegate MA, Humphries KM, Szweda LI. Reversible inhibition of α-ketoglutarate dehydrogenase by hydrogen peroxide: glutathionylation and protection of lipoic acid. Biochemistry. 2008;47:473–478. doi: 10.1021/bi7017464. [DOI] [PubMed] [Google Scholar]
- 68.Templeton DJ, Aye MS, Rady J, et al. Purification of Reversibly Oxidized Proteins (PROP) Reveals a Redox Switch Controlling p38 MAP Kinase Activity. PLoS One. 2010;5:e15012. doi: 10.1371/journal.pone.0015012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cross JV, Templeton DJ. Oxidative stress inhibits MEKK1 by site-specific glutathionylation in the ATP-binding domain. Biochem J. 2004;381:675–683. doi: 10.1042/BJ20040591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rinna A, Torres M, Forman HJ. Stimulation of the alveolar macrophage respiratory burst by ADP causes selective glutathionylation of protein tyrosine phosphatase 1B. Free Radic Biol Med. 2006;41:86–91. doi: 10.1016/j.freeradbiomed.2006.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anamika K, Garnier N, Srinivasan N. Functional diversity of human protein kinase splice variants marks significant expansion of human kinome. BMC Genomics. 2009;10:622. doi: 10.1186/1471-2164-10-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maher P. Redox control of neural function: background, mechanisms, and significance. Antioxid Redox Signal. 2006;8:1941–1970. doi: 10.1089/ars.2006.8.1941. [DOI] [PubMed] [Google Scholar]
- 73.Reynaert NL, van der Vliet A, Guala AS, et al. Dynamic redox control of NF-κB through glutaredoxin-regulated S-glutathionylation of inhibitory κB kinase β. Proc Natl Acad Sci USA. 2006;103:13086–13091. doi: 10.1073/pnas.0603290103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yusuf MA, Chuang T, Bhat GJ, et al. Cys-141 glutathionylation of human p53: Studies using specific polyclonal antibodies in cancer samples and cell lines. Free Radic Biol Med. 2010;49:908–917. doi: 10.1016/j.freeradbiomed.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Codutti L, van Ingen H, Vascotto C, et al. The solution structure of DNA-free Pax-8 paired box domain accounts for redox regulation of transcriptional activity in the pax protein family. J Biol Chem. 2008;283:33321–33328. doi: 10.1074/jbc.M805717200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Prinarakis E, Chantzoura E, Thanos D, et al. S-glutathionylation of IRF3 regulates IRF3-CBP interaction and activation of the IFNβ pathway. EMBO J. 2008;27:865–875. doi: 10.1038/emboj.2008.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Franco R, Cidlowski JA. Apoptosis and glutathione: beyond an antioxidant. Cell Death Differ. 2009;16:1303–1314. doi: 10.1038/cdd.2009.107. [DOI] [PubMed] [Google Scholar]
- 78.Pan S, Berk BC. Glutathiolation regulates tumor necrosis factor-α-induced caspase-3 cleavage and apoptosis: key role for glutaredoxin in the death pathway. Circ Res. 2007;100:213–219. doi: 10.1161/01.RES.0000256089.30318.20. [DOI] [PubMed] [Google Scholar]
- 79.Meissner F, Molawi K, Zychlinsky A. Superoxide dismutase 1 regulates caspase-1 and endotoxic shock. Nat Immunol. 2008;9:866–872. doi: 10.1038/ni.1633. [DOI] [PubMed] [Google Scholar]
- 80.Frosali S, Leonini A, Ettorre A, et al. Role of intracellular calcium and S-glutathionylation in cell death induced by a mixture of isothiazolinones in HL60 cells. Biochim Biophys Acta. 2009;1793:572–583. doi: 10.1016/j.bbamcr.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 81.Markovic J, Borrás C, Ortega A, et al. Glutathione is recruited into the nucleus in early phases of cell proliferation. J Biol Chem. 2007;282:20416–20424. doi: 10.1074/jbc.M609582200. [DOI] [PubMed] [Google Scholar]
- 82.Markovic J, Mora NJ, Broseta AM, et al. Nuclear GSH depletion impairs cell proliferation in 3T3 fibroblasts. PLoS ONE. 2009;29:e6413. doi: 10.1371/journal.pone.0006413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Söderdahl T, Enoksson M, Lundberg M, et al. Visualization of the compartmentalization of glutathione and protein-glutathione mixed disulfides in cultured cells. FASEB J. 2003;17:124–126. doi: 10.1096/fj.02-0259fje. [DOI] [PubMed] [Google Scholar]; •This paper presents, using staining and confocal microscopy, the compartmentalization of S-glutathionylation within intact cells.
- 84.Yang Y, Shi W, Cui N, et al. Oxidative stress inhibits vascular KATP channels by S-glutathionylation. J Biol Chem. 2010;285:38641–38648. doi: 10.1074/jbc.M110.162578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aracena-Parks P, Goonasekera SA, Gilman CP, et al. Identification of cysteines involved in S-nitrosylation, S-glutathionylation, and oxidation to disulfides in ryanodine receptor type 1. J Biol Chem. 2006;281:40354–40368. doi: 10.1074/jbc.M600876200. [DOI] [PubMed] [Google Scholar]
- 86.Hawkins BJ, Irrinki KM, Mallilankaraman K, et al. S-Glutathionylation activates STIM1 and alters mitochondrial homeostasis. J Cell Biol. 2010;190:391–405. doi: 10.1083/jcb.201004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang J, Boja ES, Tan W, et al. Reversible glutathionylation regulates actin polymerization in A431 cells. J Biol Chem. 2001;276:47763–47766. doi: 10.1074/jbc.C100415200. [DOI] [PubMed] [Google Scholar]
- 88.Dalle-Donne I, Giustarini D, Colombo R, et al. S-glutathionylation in human platelets by a thiol-disulfide exchange-independent mechanism. Free Radic Biol Med. 2005;38:1501–1510. doi: 10.1016/j.freeradbiomed.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 89.Johansson M, Lundberg M. Glutathionylation of β-actin via a cysteinyl sulfenic acid intermediary. BMC Biochem. 2007;8:26. doi: 10.1186/1471-2091-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fiaschi T, Cozzi G, Raugei G, et al. Redox regulation of β-actin during integrin-mediated cell adhesion. J Biol Chem. 2006;281:22983–22991. doi: 10.1074/jbc.M603040200. [DOI] [PubMed] [Google Scholar]
- 91.Passarelli C, Di Venere A, Piroddi N, et al. Susceptibility of isolated myofibrils to in vitro glutathionylation: Potential relevance to muscle functions. Cytoskeleton. 2010;67:81–89. doi: 10.1002/cm.20425. [DOI] [PubMed] [Google Scholar]
- 92.Sparaco M, Gaeta LM, Santorelli FM, Passarelli C, et al. Friedreich's ataxia: oxidative stress and cytoskeletal abnormalities. J Neurol Sci. 2009;287:111–118. doi: 10.1016/j.jns.2009.08.052. [DOI] [PubMed] [Google Scholar]
- 93.Chen FC, Ogut O. Decline of contractility during ischemia-reperfusion injury: actin glutathionylation and its effect on allosteric interaction with tropomyosin. Am J Physiol Cell Physiol. 2006;290:C719–C727. doi: 10.1152/ajpcell.00419.2005. [DOI] [PubMed] [Google Scholar]
- 94.Hwang C, Sinskey AJ, Lodish HF. Oxidized redox state of glutathione in the endoplasmic reticulum. Science. 1992;257:1496–1502. doi: 10.1126/science.1523409. [DOI] [PubMed] [Google Scholar]
- 95.Townsend DM, Manevich Y, He L, et al. Nitrosative stress-induced S-glutathionylation of protein disulfide isomerase leads to activation of the unfolded protein response. Cancer Res. 2009;69:7626–7634. doi: 10.1158/0008-5472.CAN-09-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Demasi M, Piassa Filho GM, Castro LM, et al. Oligomerization of the cysteinyl-rich oligopeptidase EP24.15 is triggered by S-glutathionylation. Free Radic Biol Med. 2008;44:1180–1190. doi: 10.1016/j.freeradbiomed.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 97.Shi Q, Xu H, Kleinman WA, et al. Novel functions of the α-ketoglutarate dehydrogenase complex may mediate diverse oxidant-induced changes in mitochondrial enzymes associated with Alzheimer's disease. Biochim Biophys Acta. 2008;1782:229–238. doi: 10.1016/j.bbadis.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dinoto L, Deture MA, Purich DL. Structural insights into Alzheimer filament assembly pathways based on site-directed mutagenesis and S-glutathionylation of three-repeat neuronal Tau protein. Microsc Res Tech. 2005;67:156–163. doi: 10.1002/jemt.20195. [DOI] [PubMed] [Google Scholar]
- 99.Di Domenico F, Cenini G, Sultana R, et al. Glutathionylation of the pro-apoptotic protein p53 in Alzheimer's disease brain: implications for AD pathogenesis. Neurochem Res. 2009;34:727–733. doi: 10.1007/s11064-009-9924-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sampathkumar R, Balasubramanyam M, Sudarslal S, et al. Increased glutathionylated hemoglobin (HbSSG) in type 2 diabetes subjects with microangiopathy. Clin Biochem. 2005;38:892–899. doi: 10.1016/j.clinbiochem.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 101.Shelton MD, Kern TS, Mieyal JJ. Glutaredoxin regulates nuclear factor κ-B and intercellular adhesion molecule in Müller cells: model of diabetic retinopathy. J Biol Chem. 2007;282:12467–12474. doi: 10.1074/jbc.M610863200. [DOI] [PubMed] [Google Scholar]
- 102.Wang W, Oliva C, Li G, et al. Reversible silencing of CFTR chloride channels by glutathionylation. J Gen Physiol. 2005;125:127–141. doi: 10.1085/jgp.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zaman K, Carraro S, Doherty J, et al. S-Nitrosylating agents: a novel class of compounds that increase cystic fibrosis transmembrane conductance regulator expression and maturation in epithelial cells. Mol Pharmacol. 2006;70:1435–1442. doi: 10.1124/mol.106.023242. [DOI] [PubMed] [Google Scholar]
- 104.Craghill J, Cronshaw AD, Harding JJ. The identification of a reaction site of glutathione mixed-disulphide formation on γS-crystallin in human lens. Biochem J. 2004;379:595–600. doi: 10.1042/BJ20031367. [DOI] [PMC free article] [PubMed] [Google Scholar]; •The discussion provides an excellent reference to human lens biology.
- 105.Zhang S, Chai FY, Yan H, et al. Effects of N-acetylcysteine and glutathione ethyl ester drops on streptozotocin-induced diabetic cataract in rats. Mol Vis. 2008;14:862–870. [PMC free article] [PubMed] [Google Scholar]
- 106.Velu CS, Niture SK, Doneanu CE, et al. Human p53 is inhibited by glutathionylation of cysteines present in the proximal DNA-binding domain during oxidative stress. Biochemistry. 2007;46:7765–7780. doi: 10.1021/bi700425y. [DOI] [PMC free article] [PubMed] [Google Scholar]; •Mechanistic approach to role of glutathionylation in cell signaling
- 107.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 108.Voronov E, Shouval DS, Krelin Y, et al. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci USA. 2003;100:2645–2650. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jung YJ, Isaacs JS, Lee S, et al. IL-1β-mediated up-regulation of HIF-1α via an NFκB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J. 2003;17:2115–2117. doi: 10.1096/fj.03-0329fje. [DOI] [PubMed] [Google Scholar]
- 110.Shelton MD, Mieyal JJ. Regulation by reversible S-glutathionylation: molecular targets implicated in inflammatory diseases. Mol Cells. 2008;25:332–346. [PMC free article] [PubMed] [Google Scholar]; •Good review of molecular targets of glutathionylation during inflammation.
- 111.Qanungo S, Starke DW, Pai HV, et al. Glutathione supplementation potentiates hypoxic apoptosis by S-glutathionylation of p65-NFκB. J Biol Chem. 2007;282:18427–18436. doi: 10.1074/jbc.M610934200. [DOI] [PubMed] [Google Scholar]; •Illustrates mechanism of S-glutathionylation control during conditions of hypoxia.
- 112.Reynaert NL, van der Vliet A, Guala AS, et al. Dynamic redox control of NF-κB through glutaredoxin-regulated S-glutathionylation of inhibitory κB kinase beta. Proc Natl Acad Sci USA. 2006;103:13086–13091. doi: 10.1073/pnas.0603290103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Diotte NM, Xiong Y, Gao J, et al. Attenuation of doxorubicin-induced cardiac injury by mitochondrial glutaredoxin 2. Biochim Biophys Acta. 2009;1793:427–438. doi: 10.1016/j.bbamcr.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 114.Walk TB, Süssmuth R, Kempter C, et al. Identification of unusual amino acids in peptides using automated sequential Edman degradation coupled to direct detection by electrospray-ionization mass spectrometry. Biopolymers. 1999;49:329–340. doi: 10.1002/(SICI)1097-0282(19990405)49:4<329::AID-BIP7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 115.Asaduzzaman SM, Sonomoto K. Lantibiotics: diverse activities and unique modes of action. J Biosci Bioeng. 2009;107:475–487. doi: 10.1016/j.jbiosc.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 116.Linetsky M, Hill JM, LeGrand RD, et al. Dehydroalanine crosslinks in human lens. Exp Eye Res. 2004:499–512. doi: 10.1016/j.exer.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 117.Linetsky M, LeGrand RD. Glutathionylation of lens proteins through the formation of thioether bond. Mol Cell Biochem. 2005;272:133–144. doi: 10.1007/s11010-005-6908-1. [DOI] [PubMed] [Google Scholar]; ••The first demonstration of irreversible protein glutathionylation in vivo
- 118.Bo Li B, van der Donk WA. Identification of essential catalytic residues of the cyclase NisC involved in the biosynthesis of nisin. J Biol Chem. 2007;282:21169–21175. doi: 10.1074/jbc.M701802200. [DOI] [PubMed] [Google Scholar]
- 119.Meister A, Greenstein JP. Glycyl dehydropeptides of leucine, valine, and isoleucine. J Biol Chem. 1952;195:849–854. [PubMed] [Google Scholar]
- 120.Wang H, Zhang J, Xian M. Facile formation of dehydroalanine from S-nitrosocysteines. J Am Chem Soc. 2009;131:13238–13239. doi: 10.1021/ja905558w. [DOI] [PubMed] [Google Scholar]
- 121.McLachlin DT, Chait BT. Improved β-elimination-based affinity purification strategy for enrichment of phosphopeptides. Anal Chem. 2003;75:6826–6836. doi: 10.1021/ac034989u. [DOI] [PubMed] [Google Scholar]
- 122.Herbert B, Hopwood F, Oxley D, et al. β-Elimination: an unexpected artefact in proteome analysis. Proteomics. 2003;3:826–831. doi: 10.1002/pmic.200300414. [DOI] [PubMed] [Google Scholar]
- 123.Bar-Or R, Rael LT, Bar-Or D. Dehydroalanine derived from cysteine is a common post-translational modification in human serum albumin. Rapid Commun Mass Spectrom. 2008;22:711–716. doi: 10.1002/rcm.3421. [DOI] [PubMed] [Google Scholar]
- 124.Ma S, Caprioli RM, Hill KE, et al. Loss of selenium from selenoproteins: Conversion of selenocysteine to dehydroalanine in vitro. J Am Soc Mass Spectrom. 2003;14:593–600. doi: 10.1016/S1044-0305(03)00141-7. [DOI] [PubMed] [Google Scholar]
- 125.Friedman M. Chemistry, biochemistry, nutrition, and microbiology of lysinoalanine, lanthionine, and histidinoalanine in food and other proteins. J Agric Food Chem. 1999;47:1295–1319. doi: 10.1021/jf981000+. [DOI] [PubMed] [Google Scholar]
- 126.Snow JT, Finley JW, Friedman M. Relative reactivities of sulfhydryl groups with N-acetyl dehydroalanine and N-acetyl dehydroalanine methyl ester. Int J Pept Protein Res. 1976;8:57–64. doi: 10.1111/j.1399-3011.1976.tb02481.x. [DOI] [PubMed] [Google Scholar]
- 127.Friedman M, Finley JW, Yeh LS. Reactions of proteins with dehydroalanines. Adv Exp Med Biol. 1977;86B:213–224. doi: 10.1007/978-1-4757-9113-6_15. [DOI] [PubMed] [Google Scholar]
- 128.Bernardes GJ, Chalker JM, Errey JC, et al. Facile conversion of cysteine and alkyl cysteines to dehydroalanine on protein surfaces: versatile and switchable access to functionalized proteins. J Am Chem Soc. 2008;130:5052–5053. doi: 10.1021/ja800800p. [DOI] [PubMed] [Google Scholar]
- 129.Amini A, Nilsson E. Quantitative analysis of polypeptide pharmaceuticals by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J Pharm Biomed Anal. 2008;46:411–417. doi: 10.1016/j.jpba.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 130.Jeong J, Jung Y, Na S, et al. Novel oxidative modifications in redox-active cysteine residues. Mol Cell Proteomics. 2010 Dec 10; doi: 10.1074/mcp.M110.000513. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stein TP, Leskiw MJ, Buzby GP, et al. Measurement of protein synthesis rates with [15N]glycine. Am J Physiol. 1980;239:E294–E300. doi: 10.1152/ajpendo.1980.239.4.E294. [DOI] [PubMed] [Google Scholar]
- 132.Cho CS, Lee S, Lee GT, et al. Irreversible inactivation of glutathione peroxidase 1 and reversible inactivation of peroxiredoxin II by H2O2 in red blood cells. Antioxid Redox Signal. 2010;12:1235–1246. doi: 10.1089/ars.2009.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 134.Iwamoto T, Hiraku Y, Oikawa S, et al. DNA intrastrand cross-link at the 5'-GA-3' sequence formed by busulfan and its role in the cytotoxic effect. Cancer Sci. 2004;95:454–458. doi: 10.1111/j.1349-7006.2004.tb03231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ritter CA, Bohnenstengel F, Hofmann U, et al. Determination of tetrahydrothiophene formation as a probe of in vitro busulfan metabolism by human glutathione S-transferase A1-1: use of highly sensitive gas chromatographic-mass spectrometric method. J Chromatog B Biomed Sci Appl. 1999;730:25–31. doi: 10.1016/s0378-4347(99)00170-x. [DOI] [PubMed] [Google Scholar]
- 136.Czerwinski M, Gibbs JP, Slattery JT. Busulfan conjugation by glutathione S-transferases μ, μ, and π. Drug Metab Dispos. 1996;24:1015–1019. [PubMed] [Google Scholar]
- 137.Gibbs JP, Czerwinski M, Slattery JT. Busulfan-glutathione conjugation catalyzed by human liver cytosolic glutathione S-transferases. Cancer Res. 1996;56:3678–3681. [PubMed] [Google Scholar]
- 138.Ritter CA, Sperker B, Grube M, et al. Overexpression of glutathione S-transferase A1-1 in ECV 304 cells protects against busulfan mediated G2-arrest and induces tissue factor expression. Br J Pharmacol. 2002;137:1100–1106. doi: 10.1038/sj.bjp.0704972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Roberts JJ, Warwick GP. The mode of action of alkylating agents. III. The formati o n o f 3-hydroxytetrahydrothiophene-1:1-dioxide from 1:4-dimethanesulphonyloxybutane (myleran), S-β-L-alanyl-tetrahydrothiophenium mesylate, tetrahydrothiophene and tetrahydrothiophene-1:1-dioxide in the rat, rabbit and mouse. Biochem Pharmacol. 1961;6:217–227. [Google Scholar]
- 140.Cooper AJL, Younis IR, Niatsetskaya ZV, et al. Metabolism of the cysteine S-conjugate of busulfan involves a β-lyase reaction. Drug Metab Dispos. 2008;36:1546–1552. doi: 10.1124/dmd.108.020768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Younis IR, Elliott M, Peer CJ, et al. Dehydroalanine analog of glutathione: an electrophilic busulfan metabolite that binds to human glutathione S-transferase A1-1. J Pharmacol Exp Ther. 2008;327:770–776. doi: 10.1124/jpet.108.142208. [DOI] [PMC free article] [PubMed] [Google Scholar]; ••Demonstration of the formation of a dehydroalanyl analogue of GSH, the formation of GSG, and inhibition of a GST by GSG
- 142.Kalayoglu-Besisik S, Yenerei MN, Caliskan Y, et al. Time-related changes in the incidence, severity, and clinical outcome of hepatic veno-occlusive disease in hematopoietic cell transplantation patients during the past 10 years. Transplant Proc. 2005;37:2285–2289. doi: 10.1016/j.transproceed.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 143.Holmström G, Borgström B, Calissendorff B. Cataract formation after bone marrow transplantation: relation to conditioning regimen. Acta Ophthalmol Scand. 2002;80:211–215. doi: 10.1034/j.1600-0420.2002.800217.x. [DOI] [PubMed] [Google Scholar]
- 144.Wadleigh M, Ho V, Momtaz P, et al. Hepatic veno-occlusive disease: pathogenesis, diagnosis and treatment. Curr Opin Hematol. 2003;10:451–462. doi: 10.1097/00062752-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 145.Kera Y, Penttila KE, Lindros KO. Glutathione replenishment capacity is lower in isolated perivenous than in periportal hepatocytes. Biochem J. 1988;254:411–417. doi: 10.1042/bj2540411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Deleve LD. Dacarbazine toxicity in murine liver cells: a model of hepatic endothelial injury and glutathione defense. J Pharmacol Exp Ther. 1994;268:1261–1270. [PubMed] [Google Scholar]
- 147.Deleve LD. Glutathione defense in non-parenchymal cells. Semin Liver Dis. 1998;18:403–413. doi: 10.1055/s-2007-1007173. [DOI] [PubMed] [Google Scholar]
- 148.Srivastava A, Poonkuzhali B, Shaji RV, et al. Glutathione S-transferase M1 polymorphism: a risk factor for hepatic venoocclusive disease in bone marrow transplantation. Blood. 2004;104:1574–1577. doi: 10.1182/blood-2003-11-3778. [DOI] [PubMed] [Google Scholar]
- 149.DeLeve LD, Wang X. Role of oxidative stress and glutathione in busulfan toxicity in cultured murine hepatocytes. Pharmacology. 2000;60:143–154. doi: 10.1159/000028359. [DOI] [PubMed] [Google Scholar]
- 150.Bouligand J, Deroussent A, Simonnard N, et al. Induction of glutathione synthesis explains pharmacodynamics of high-dose busulfan in mice and highlights putative mechanisms of drug interaction. Drug Metab Dispos. 2007;35:306–314. doi: 10.1124/dmd.106.012880. [DOI] [PubMed] [Google Scholar]
- 151.Zeiger E, Pagano DA. Mutagenicity of the human carcinogen treosulphan in Salmonella. Environ Mol Mutagen. 1989;13:343–346. doi: 10.1002/em.2850130411. [DOI] [PubMed] [Google Scholar]
- 152.Werner S, Mendoza A, Hilger RA, et al. Preclinical studies of treosulfan demonstrate potent activity in Ewing's sarcoma. Cancer Chemother Pharmacol. 2008;62:19–31. doi: 10.1007/s00280-007-0566-9. [DOI] [PubMed] [Google Scholar]
- 153.Feyerabend S, Feil G, Krug J, et al. Cytotoxic effects of treosulfan on prostate cancer cell lines. Anticancer Res. 2007;27:2403–2408. [PubMed] [Google Scholar]
- 154.Armstrong DK, Gordon GB, Hilton J, et al. Hepsulfam sensitivity in human breast cancer cell lines: the role of glutathione and glutathione S-transferase in resistance. Cancer Res. 1992;52:1416–1421. [PubMed] [Google Scholar]
- 155.Westerhof GR, Ploemacher RE, Boudewijn A, et al. Comparison of different busulfan analogues for depletion of hematopoietic stem cells and promotion of donor-type chimerism in murine bone marrow transplant recipients. Cancer Res. 2000;60:5470–5478. [PubMed] [Google Scholar]
- 156.Onkenhout W, van Loon WM, Buijs W, et al. Biotransformation and quantitative determination of sulfur-containing metabolites of 1,4-dibromobutane in the rat. Drug Metab Dispos. 1986;14:608–612. [PubMed] [Google Scholar]
- 157.Anders MW. Chemical toxicology of reactive intermediates formed by the glutathione-dependent bioactivation of halogen-containing compounds. Chem Res Toxicol. 2008;21:145–159. doi: 10.1021/tx700202w. [DOI] [PubMed] [Google Scholar]; •A good review of how the formation of glutathioneS-conjugates with certain halogenated xenobiotics can lead to bioactivation (toxification) rather than detoxification.
- 158.Guengerich FP. Metabolism and genotoxicity of dihaloalkanes. Adv Pharmacol. 1994;27:211–236. doi: 10.1016/s1054-3589(08)61034-0. [DOI] [PubMed] [Google Scholar]
- 159.Bentley TW. Additivity rules using similarity models for chemical reactivity: calculation and interpretation of electrofugality and nucleofugality. Chemistry. 2006;25:6514–6520. doi: 10.1002/chem.200600517. [DOI] [PubMed] [Google Scholar]; •A good review for those interested in the chemistry of elimination reactions.
- 160.Zhu X, Gallogly MM, Mieyal JJ, et al. Covalent cross-linking of glutathione and carnosine to proteins by 4-oxo-2-nonenal. Chem Res Toxicol. 2009;22:1050–1059. doi: 10.1021/tx9000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Choi C, Dimitrov IE, Douglas D, et al. Improvement of resolution for brain coupled metabolites by optimized 1H MRS at 7T. NMR Biomed. 2010;23:1044–1052. doi: 10.1002/nbm.1529. [DOI] [PubMed] [Google Scholar]; •This article highlights the increasing use of NMR techniques to noninvasively measure GSH in human brain.
- 162.Choi IY, Lee SP, Denney D, Lynch S. Lower levels of glutathione in the brains of secondary progressive multiple sclerosis patients measured by 1H magnetic resonance chemical shift imaging at 3 T. Mult Scler. 2010 Oct 4; doi: 10.1177/1352458510384010. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Matsuzawa D, Hashimoto K. Magnetic Resonance Spectroscopy Study of the Antioxidant Defense System in Schizophrenia. Antioxid Redox Signal. 2010 Dec 4; doi: 10.1089/ars.2010.3453. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 164.Wright AJ, Fellows GA, Griffiths JR, et al. Ex-vivo HRMAS of adult brain tumours: metabolite quantification and assignment of tumour biomarkers. Mol Cancer. 2010;9:66. doi: 10.1186/1476-4598-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Thelwall PE, Yemin AY, Gillian TL, et al. Noninvasive in vivo detection of glutathione metabolism in tumors. Cancer Res. 2005;65:10149–10153. doi: 10.1158/0008-5472.CAN-05-1781. [DOI] [PubMed] [Google Scholar]
- 166.Tew KD, Townsend DM. Redox platforms in cancer drug discovery and development. Curr Opin Chem Biol. 2011;15:156–161. doi: 10.1016/j.cbpa.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]