Abstract
Background
Granulocyte colony-stimulating factor (G-CSF) is effective in accelerating neutrophil recovery after intensive chemotherapy for acute myeloid leukemia (AML). However, the optimal G-CSF dosage for patients with AML has not been determined. To our knowledge, G-CSF dosages have not been compared in a randomized AML study.
Methods
Patients enrolled on the St. Jude AML97 protocol who remained on study after window therapy were eligible to participate. The effect of the dosage of G-CSF given after induction chemotherapy courses 1 and 2 was analyzed in 46 patients randomly assigned in a double-blinded manner to receive 5 or 10 μg/kg/day of G-CSF. The number of days of G-CSF treatment, neutropenia (absolute neutrophil count < 0.5 × 109/L), and hospitalization; the number of episodes of febrile neutropenia, grade 2-4 infection, and antimicrobial therapy; transfusion requirements; the cost of supportive care; and survival were compared between the two study arms.
Results
We found no statistically significant difference between the two arms in any of the endpoints measured.
Conclusions
The higher G-CSF dosage (10 μg/kg/day) offers no greater benefit than the lower dosage (5 μg/kg/day) in patients undergoing intensive chemotherapy for AML.
Keywords: acute myeloid leukemia, granulocyte colony-stimulating factor, dosage, children, randomized trial
INTRODUCTION
Intensive chemotherapy1-4 and advances in supportive care 5, 6 have improved the rates of remission and long-term survival in children with acute myeloid leukemia (AML). Granulocyte-colony stimulating factor (G-CSF) is effective in accelerating neutrophil recovery in patients undergoing intensive chemotherapy for AML.7, 8 In a number of adult AML studies, prophylactic use of 5 μg/kg/day of G-CSF after induction and consolidation therapy reduced the duration of neutropenia, infection, and antibiotic use.9-11 However, the duration of febrile episodes, the frequency of documented infection, the duration of hospitalization, and overall survival were not affected. It is unknown whether higher doses can extend the benefits of G-CSF beyond reduction of the duration of neutropenia.
G-CSF has generally been used at dosages of 10 μg/kg/day or more in the setting of autologous bone marrow stem cell collection,12, 13 but no studies have compared different dosages of G-CSF in patients receiving chemotherapy for AML. We report here the results of a randomized, double-blind trial comparing the effects of two different doses (5 μg/kg/day or 10 μg/kg/day) of G-CSF after induction treatment in children with newly diagnosed AML.
PATIENTS AND METHODS
Patients
Between March 1997 and June 2002, 102 children with previously untreated AML or myelodysplastic syndrome (MDS) were enrolled on the single-institution AML97 protocol at St. Jude Children’s Research Hospital.3 Patients with acute promyelocytic leukemia were not eligible. The protocol was amended in May 1999 to compare the effects of two dosages of G-CSF given after remission induction chemotherapy. Patients enrolled on AML97 who remained on study after window therapy were eligible.
The study design specified double-blind random assignment of at least 36 patients to receive G-CSF (filgrastim) at 5 μg/kg/day or 10 μg/kg/day IV after induction courses 1 and 2. This number would provide 90% power to detect a 5-day difference in the number of neutropenic days (primary outcome measure) at an alpha level of 0.05 (1-sided test). Secondary outcomes compared between the treatment arms were number of days of G-CSF treatment and hospitalization; the cumulative number of episodes of febrile neutropenia, grade 2-4 infection, antibiotic therapy, IV antibiotic therapy, and antifungal therapy; the number of red blood cell and platelet transfusions; the cost of supportive care; and the estimates of event-free survival and survival. The study was approved by the St. Jude Institutional Review Board, and signed informed consent was obtained from patients, parents, or guardians, as appropriate.
Therapy
The AML97 treatment protocol has been previously described.3 Briefly, patients who agreed to participate in “upfront window” therapy were randomly assigned to receive either 5 daily short (2-hour) infusions of cytarabine (500 mg/m2/day; arm A) or a 5-day continuous infusion of cytarabine (500 mg/m2/day; arm B); both arms received five daily 30-minute infusions of cladribine (9 mg/m2). Induction chemotherapy courses 1 and 2 comprised daunorubicin (30 mg/m2/day, continuous infusion, days 1–3), cytarabine (250 mg/m2/day, continuous infusion, days 1–5), and etoposide (200 mg/m2/day, continuous infusion, days 4 and 5) (DAV 1 and DAV 2). Patients with high-risk AML (megakaryoblastic leukemia; refractory anemia with excess blasts in transformation; secondary AML; AML with −7, 5q-, or t(9;22); or persistent leukemia after DAV 1) were eligible for allogeneic hematopoietic stem cell transplantation (HSCT) after DAV 2. Patients with the t(8;21) or inv(16) were considered to have low-risk AML and were not eligible for allogeneic HSCT. All other patients were considered to have standard-risk AML and were eligible for HSCT if a matched sibling donor was available. Patients who did not undergo allogeneic HSCT received two courses of consolidation chemotherapy consisting of cytarabine (3 g/m2/day every 12 hours on days 1, 2, 8, and 9) and L-asparaginase (6000 U/m2 after the fourth and eighth doses of cytarabine), followed by mitoxantrone (10 mg/m2/day on days 1–5) and cytarabine (1 g/m2/day every 12 hours on days 1–3).
All patients received one intrathecal treatment with cytarabine at diagnosis. Patients without central nervous system (CNS) disease received four triple intrathecal chemotherapy treatments with methotrexate, hydrocortisone, and cytarabine (with doses adjusted based on age), beginning during DAV 1. Patients with CNS leukemia received triple intrathecal therapy weekly until the cerebrospinal fluid was clear of leukemia cells (minimum, four doses), and then four additional doses.
Random assignment to G-CSF dosage
After May 1999, patients were randomly assigned before the start of DAV1 to receive 5 μg/kg/day or 10 μg/kg/day of IV G-CSF after DAV 1 and DAV 2. Daily 30-minute G-CSF IV infusions began 24 hours after the last day of each chemotherapy cycle and continued until the absolute neutrophil count remained ≥ 0.5 × 109/L for 2 days. The next chemotherapy cycle was started at least 24 hours after discontinuation of G-CSF. G-CSF was not administered to patients scheduled to undergo HSCT after DAV 2 or to patients who had a poor response to DAV1 and therefore were taken off AML97 protocol.
Statistical analysis
Patient features were compared between G-CSF treatment arms by the exact chi-squared test. Outcome variables were measured during the period beginning with the end of each DAV course and ending with the start of the subsequent chemotherapy course. The median number of days of G-CSF treatment in the two arms was compared separately for each induction cycle by using the Wilcoxon rank-sum test. A repeated-measures mixed-effects model based on normal distribution was used to analyze the effect of G-CSF dosage on number of days of neutropenia (absolute neutrophil count <0.5 × 109/L) and hospitalization, and on the cost of supportive care, adjusting for chemotherapy course effect and modeling the correlation with an autoregressive structure.14 The supportive care charges in DAV1 and DAV2 were divided into six categories: antimicrobial agents, laboratory tests, diagnostic imaging tests, room/procedure charges, transfusion charges, and other general supportive care. The charges during induction therapy were calculated and log-transformed. Proportional means models were used to compare the cumulative number of episodes of febrile neutropenia, grade 2-4 infection (National Cancer Institute Common Toxicity Criteria version 2.0), antibiotic therapy, IV antibiotic therapy, and antifungal therapy, and the number of red blood cell and platelet transfusions, with G-CSF treatment as fixed covariate.15
Event-free survival was defined as the time between G-CSF randomization and relapse, death, secondary malignancy, or last follow-up. Remission induction failure was treated as an event at time 0. The Kaplan-Meier method was used to estimate the probability of event-free survival and survival; standard error was determined by the method of Peto and Pike.16 Survival distributions were compared by using the Mantel-Haenszel log-rank test.17 All analyses were performed using SAS software (SAS Institute, Cary, NC), Windows version 9.1, and StatXact (Cytel Corporation, Cambridge, MA) Windows version 7.1.
RESULTS
Patient characteristics
Of 55 patients approached for enrollment, five declined and three did not remain on the protocol after window therapy. Forty-seven patients were randomly assigned to G-CSF 5 or 10 μg/kg/day. One patient was excluded from analysis because of treatment with granulocyte-macrophage colony-stimulating factor (GM-CSF) at the physician’s discretion; 46 patients were analyzed for DAV 1 and 36 for DAV 2. Median age was 9.03 years (range, 0.05–21 years) and median WBC count was 12.3 × 109/L (range, 1.2–166.8 × 109/L). Patient characteristics did not differ significantly according to G-CSF arm (Table 1).
Table 1.
Patient characteristics
| Clinical features | Total (n=46) |
G-CSF treatment group | p-value* | ||
|---|---|---|---|---|---|
| 5 μg/kg/day (n=23) |
10 μg/kg/day (n=23) |
||||
| WBC (×109/L) |
<50 | 33 | 14 | 19 | 0.19 |
| ≥50 | 13 | 9 | 4 | ||
| Cytogenetics | Normal | 10 | 3 | 7 | 0.38 |
| inv(16) | 4 | 2 | 2 | ||
| t(8;21) | 5 | 1 | 4 | ||
| t(9;11) | 6 | 4 | 2 | ||
| Other | 17 | 10 | 7 | ||
| Sex | Female | 25 | 14 | 11 | 0.55 |
| Male | 21 | 9 | 12 | ||
| FAB group | M1 | 10 | 4 | 6 | 0.86 |
| M2 | 9 | 4 | 5 | ||
| M4 | 11 | 7 | 4 | ||
| M5 | 8 | 4 | 4 | ||
| M7 | 7 | 3 | 4 | ||
| MDS | 1 | 1 | 0 | ||
| Risk group | High | 16 | 7 | 9 | 0.76 |
| Other | 30 | 16 | 14 | ||
| Race | White | 32 | 14 | 18 | 0.34 |
| Non-white | 14 | 9 | 5 | ||
| Window therapy | Arm A | 23 | 12 | 11 | 0.54 |
| Arm B | 20 | 11 | 9 | ||
| Not randomized | 3 | 0 | 3 | ||
| Window response | CR | 27 | 13 | 14 | 0.75 |
| PR | 9 | 6 | 3 | ||
| NR | 7 | 4 | 3 | ||
| DAV1 response | CR | 40 | 20 | 20 | 0.35 |
| PR | 2 | 2 | 0 | ||
| NR | 4 | 1 | 3 | ||
| DAV2 response | CR | 33 | 16 | 17 | 0.49 |
| PR | 2 | 2 | 0 | ||
| NR | 1 | 0 | 1 | ||
Exact chi-squared test
Abbreviations: G-CSF, granulocyte colony-stimulating factor; WBC, white blood cell count; FAB, French-American-British; MDS, myelodysplastic syndrome; CR, complete response; PR, partial response; NR, no response; DAV, daunorubicin, cytarabine and etoposide
Duration of G-CSF treatment and neutropenia
There were no significant differences between the two G-CSF treatment groups in the duration of G-CSF treatment after DAV1 or DAV2 (Table 2). The number of neutropenic days also did not differ significantly in the two treatment arms (Table 2).
Table 2.
Comparison of the two G-CSF arms
| G-CSF treatment group | p-value | |||
|---|---|---|---|---|
| 5μg/kg/day | 10μg/kg/day | |||
| Days of G-CSF treatment a | ||||
| DAV1 | N | 23 | 23 | 0.86 |
| Median (range) | 13 (6–26) | 14 (6–30) | ||
| DAV2 | N | 18 | 18 | 0.57 |
| Median (range) | 15 (10–33) | 14 (8–29) | ||
| Days of neutropenia b | ||||
| DAV1 | Median (range) | 13 (6–27) | 12 (6–29) | 0.54 |
| DAV2 | Median (range) | 11 (4–25) | 10 (5–27) | |
| Days of hospitalization b | ||||
| DAV1 | Median (range) | 0 (0–18) | 0 (0–25) | 0.48 |
| DAV2 | Median (range) | 2 (0–23) | 0 (0–22) | |
|
Adverse events, antimicrobials,
transfusions c |
Cumulative mean function estimate at last event (95% confidence interval) |
|||
| Febrile neutropenia (episodes) | 1.91 (1.35–2.71) | 1.60 (1.22–2.09) | 0.37 | |
| Grade 2-4 infection (episodes) | 1.10 (0.60–2.02) | 1.43 (0.77–2.63) | 0.48 | |
| Antibiotic therapy (episodes) | 4.27 (3.15–5.79) | 4.55 (3.52–5.88) | 0.72 | |
| IV antibiotic therapy (episodes) | 3.30 (2.49–4.39) | 4.02 (2.95–5.46) | 0.27 | |
| Antifungal therapy (episodes) | 2.48 (1.69–3.64) | 1.80 (1.20–2.68) | 0.20 | |
| Red blood cell transfusion (number) | 7.08 (5.55–9.04) | 7.05 (5.48–9.06) | 0.98 | |
| Platelet transfusion (number) | 10.21 (6.74–15.49) | 10.48 (7.39–14.87) | 0.98 | |
| Supportive care costs b | Median (range) $U.S. | |||
| Antimicrobials | 1462 (92–14779) | 1249 (14–2689) | 0.61 | |
| Laboratory tests | 7335 (852–14853) | 7319 (1280–12916) | 0.90 | |
| Diagnostic imaging | 420 (113–3549) | 902.24 (117–4660) | 0.16 | |
| Room/procedure | 5009 (582–15712) | 3974 (399–7864) | 0.21 | |
| Transfusion | 2693 (1071–6678) | 2098 (446–5433) | 0.19 | |
| Other support | 2690 (170–9068) | 3059 (160–10082) | 0.97 | |
| Total | 18648 (1434–60040) | 19108 (6014–33119) | 0.99 | |
Wilcoxon rank-sum test
Repeated-measures mixed-effects model
Proportional means model
Abbreviations: G-CSF, granulocyte colony-stimulating factor; DAV, daunorubicin, cytarabine and etoposide; IV, intravenous
Episodes of febrile neutropenia and infection and days of hospitalization
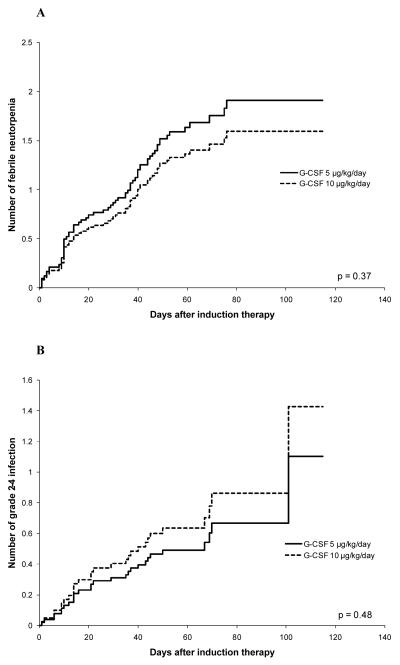
There was no evidence that the number of febrile neutropenic episodes or episodes of grade 2-4 infection differed significantly between the 5 and 10 μg/kg/day G-CSF arms (Table 2, Figure 1A and B). The number of hospitalization days was also not found to differ significantly between the two arms (Table 2).
Figure 1.
Comparison of cumulative mean function estimates of number of episodes of febrile neutropenia (A) and grade 2-4 documented infection (B) in the two G-CSF arms (5 μg/kg/day and 10 μg/kg/day).
Episodes of antibiotic and antifungal therapy
There was no evidence that the 5 μg/kg/day G-CSF arm differed significantly from the 10 μg/kg/day arm in the number of episodes of antibiotic therapy, IV antibiotic therapy, or antifungal therapy (Table 2).
Transfusions
The number of transfusions of red blood cells and of platelets was not found to differ significantly between the two G-CSF arms (Table 2).
Cost of supportive care
We found no evidence that patients treated with 5 μg/kg/day G-CSF differed significantly from those treated with 10 μg/kg/day G-CSF in total supportive care costs or in any of the six categories of supportive care costs (Table 2).
Event-free and overall survival
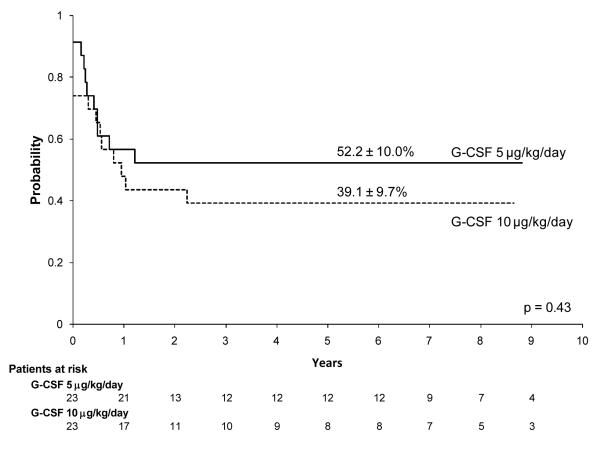
We observed no significant differences between the 5 μg/kg/day and 10 μg/kg/day G-CSF arms in the proportion of complete responses (Table 1) or in estimates of event-free survival or overall survival. The 6-year event-free survival estimate was 52.2% ± 10.0% for patients treated with 5 μg/kg/day G-CSF and 39.1% ± 9.7% for those treated with 10 μg/kg/day G-CSF (p = 0.43; Fig. 2). The 6-year survival estimate was 65.2% ± 9.6% for patients treated with 5 μg/kg/day G-CSF and 52.2% ± 11.4% for those treated with 10 μg/kg/day G-CSF (p = 0.45).
Figure 2.
Event-free survival distributions of patients treated with G-CSF 5 μg/kg/day vs. 10μg/kg/day on the AML97 protocol.
DISCUSSION
Our prospective, randomized trial of two dosages of prophylactic G-CSF (5 μg/kg/day vs. 10 μg/kg/day) in children receiving intensive induction chemotherapy for AML showed no significant difference in any of the outcomes measured. We acknowledge that statistical power for secondary outcomes may have been limited. However, this study is, to our knowledge, the first randomized study comparing the dosage of G-CSF in AML.
Several adult studies have shown that G-CSF can modestly reduce (by 5-6 days) the duration of neutropenia when begun shortly after induction chemotherapy.9, 10, 18, 19 However, it is not clear whether this acceleration of neutrophil recovery is clinically meaningful. Endpoints such as duration of hospitalization and frequency of severe infection have been reduced only variably and modestly. Further, in the majority of these studies, growth factor therapy did not alter the likelihood of complete remission, disease-free survival, or overall survival.
The BFM 98 study randomly assigned children with AML to receive G-CSF (5 μg/kg/day) or no G-CSF, starting on day 15 of the first and second induction therapy courses.20 The duration of neutropenia was significantly less in the G-CSF arm after the first induction course (cytarabine, idarubicin, and etoposide) (median, 18.0 vs. 23.0 days; p = 0.02) and second induction course (cytarabine and mitoxantrone) (11.0 vs. 16.0 days; p = 0.0005), but the duration of thrombocytopenia, the frequency of grade 3-4 infection, and 5-year event-free survival did not differ significantly between the arms. In another pediatric AML study, CCG 2891, patients who received intensive chemotherapy and 5 μg/kg/day of G-CSF were compared to historical controls treated similarly but without G-CSF.21 Although the duration of neutropenia, number of hospital days, and delays in planned therapy were significantly reduced, the frequency of severe infection and estimates of event-free survival and survival were not affected.
Studies in healthy hematopoietic stem cell donors who received G-CSF suggest a relationship between the G-CSF dosage and the level of circulating progenitor CD34+ cells 4 to 7 days after administration.12, 13 All volunteers who received 10 μg/kg/day and underwent a single leukapheresis mobilized more than the minimum target number of stem cells, whereas volunteers receiving lower daily doses of G-CSF did not consistently do so.12 However, higher G-CSF dosage did not have a comparable effect in patients undergoing autologous stem cell harvest after cytotoxic chemotherapy, probably because of their limited number of progenitor cells.22 Similarly, higher daily doses of G-CSF (2 to 16 μg/kg) did not induce greater improvement in neutrophil recovery than lower doses in adults and children with solid malignancies and lymphoma who had undergone chemotherapy or autologous HSCT.23-25 These findings are consistent with our results, which failed to show any benefit provided by the higher G-CSF dosage of 10 μg/kg/day.
Although we found no statistically significant difference between the study arms in event-free survival and survival, both of these estimates were lower in patients who received 10 μg/kg/day. Increased relative expression of class IV G-CSF receptor isoform in AML uncouples the proliferative and maturational G-CSF receptor signaling pathways, and higher doses of G-CSF may enhance this effect.26, 27 In the BFM 98 study, patients with standard-risk AML that overexpressed the maturation-defective G-CSF receptor isoform IV had a significantly higher incidence of relapse with G-CSF treatment.28 Further studies of the function and expression patterns of G-CSF receptor and its isoforms in AML cells are warranted.
Improved supportive care is likely to enhance treatment outcome in patients undergoing AML therapy.1, 5, 6 The lower rate of early death in recent BFM-AML trials than in previous trials probably reflects both experience and improved supportive care.5 However, infectious mortality, particularly that caused by invasive bacterial and fungal infections, remains unacceptably high.29, 30 We recently reported that the routine prophylactic use of 1) vancomycin, oral ciprofloxacin or cephalosporin, and voriconazole or 2) cefepime and voriconazole reduced morbidity and dramatically decreased the incidence of septicemia and the number of hospital days.14 Improved 3-year event-free survival (63.0% ± 4.1%) and survival (71.1% ± 3.8%) in our recent multi-institutional AML02 study could be partly attributable to better supportive care.31 Likewise, the excellent survival rates in the Japanese Childhood AML Cooperative Study Group (5-year event-free survival, 61.6% and survival, 75.6%) were associated with routine inpatient care throughout the treatment course, allowing close patient monitoring and ready access to antibiotics.4
In conclusion, we suggest that first priority be given to the use of prophylactic antibiotics and close patient monitoring (by inpatient care or frequent outpatient visits) and that growth factors (e.g., G-CSF 5 μg/kg/day) be reserved for patients with extremely prolonged neutropenia and/or clinically complicated infection ideally after confirming the expression pattern of G-CSF receptor isoform IV in AML cells. A recent survey of Children’s Oncology Group and BFM group institutions revealed systematic differences in infection-related supportive care practices for pediatric AML patients.6 To improve supportive care in AML, harmonized use and/or randomized studies of antibacterial and antifungal prophylaxis and criteria for growth factor use and for discharge after chemotherapy are needed.
Condensed abstract.
This double-blind study analyzed the effect of two randomly assigned dosages (5 and 10 μg/kg/day) of granulocyte colony-stimulating factor (G-CSF) given after induction chemotherapy in 46 pediatric patients with acute myeloid leukemia. The higher G-CSF dosage offered no greater benefit than the lower dosage in the number of days of G-CSF treatment, neutropenia, or hospitalization; the number of episodes of febrile neutropenia, grade 2-4 infection, or antimicrobial therapy; transfusion requirements; the cost of supportive care; or survival.
ACKNOWLEDGEMENT
We acknowledge the expertise of Ms. Sharon Naron in editorial review of the manuscript.
Supported in part by Cancer Center Support Grant CA 21765 from the National Institutes of Health and by the American Lebanese Syrian Associated Charities (ALSAC). Dr. Ching-Hon Pui is an American Cancer Society Professor.
Footnotes
The authors have no conflicts of interest, including specific financial interests, relationships, or affiliations relevant to the subject of this manuscript.
REFERENCES
- 1.Creutzig U, Zimmermann M, Lehrnbecher T, et al. Less toxicity by optimizing chemotherapy, but not by addition of granulocyte colony-stimulating factor in children and adolescents with acute myeloid leukemia: results of AML-BFM 98. J Clin Oncol. 2006;24:4499–4506. doi: 10.1200/JCO.2006.06.5037. [DOI] [PubMed] [Google Scholar]
- 2.Lange BJ, Smith FO, Feusner J, et al. Outcomes in CCG-2961, a children’s oncology group phase 3 trial for untreated pediatric acute myeloid leukemia: a report from the children’s oncology group. Blood. 2008;111:1044–1053. doi: 10.1182/blood-2007-04-084293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubnitz JE, Crews KR, Pounds S, et al. Combination of cladribine and cytarabine is effective for childhood acute myeloid leukemia: results of the St Jude AML97 trial. Leukemia. 2009;23:1410–1416. doi: 10.1038/leu.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsukimoto I, Tawa A, Horibe K, et al. Risk-stratified therapy and the intensive use of cytarabine improves the outcome in childhood acute myeloid leukemia: the AML99 trial from the Japanese Childhood AML Cooperative Study Group. J Clin Oncol. 2009;27:4007–4013. doi: 10.1200/JCO.2008.18.7948. [DOI] [PubMed] [Google Scholar]
- 5.Creutzig U, Zimmermann M, Reinhardt D, Dworzak M, Stary J, Lehrnbecher T. Early deaths and treatment-related mortality in children undergoing therapy for acute myeloid leukemia: analysis of the multicenter clinical trials AML-BFM 93 and AML-BFM 98. J Clin Oncol. 2004;22:4384–4393. doi: 10.1200/JCO.2004.01.191. [DOI] [PubMed] [Google Scholar]
- 6.Lehrnbecher T, Ethier MC, Zaoutis T, et al. International variations in infection supportive care practices for paediatric patients with acute myeloid leukaemia. Br J Haematol. 2009;147:125–128. doi: 10.1111/j.1365-2141.2009.07844.x. [DOI] [PubMed] [Google Scholar]
- 7.Ravandi F. Role of cytokines in the treatment of acute leukemias: a review. Leukemia. 2006;20:563–571. doi: 10.1038/sj.leu.2404152. [DOI] [PubMed] [Google Scholar]
- 8.Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187–3205. doi: 10.1200/JCO.2006.06.4451. [DOI] [PubMed] [Google Scholar]
- 9.Dombret H, Chastang C, Fenaux P, et al. A controlled study of recombinant human granulocyte colony-stimulating factor in elderly patients after treatment for acute myelogenous leukemia. AML Cooperative Study Group. N Engl J Med. 1995;332:1678–1683. doi: 10.1056/NEJM199506223322504. [DOI] [PubMed] [Google Scholar]
- 10.Heil G, Hoelzer D, Sanz MA, et al. A randomized, double-blind, placebo-controlled, phase III study of filgrastim in remission induction and consolidation therapy for adults with de novo acute myeloid leukemia. The International Acute Myeloid Leukemia Study Group. Blood. 1997;90:4710–4718. [PubMed] [Google Scholar]
- 11.Harousseau JL, Witz B, Lioure B, et al. Granulocyte colony-stimulating factor after intensive consolidation chemotherapy in acute myeloid leukemia: results of a randomized trial of the Groupe Ouest-Est Leucemies Aigues Myeloblastiques. J Clin Oncol. 2000;18:780–787. doi: 10.1200/JCO.2000.18.4.780. [DOI] [PubMed] [Google Scholar]
- 12.Grigg AP, Roberts AW, Raunow H, et al. Optimizing dose and scheduling of filgrastim (granulocyte colony-stimulating factor) for mobilization and collection of peripheral blood progenitor cells in normal volunteers. Blood. 1995;86:4437–4445. [PubMed] [Google Scholar]
- 13.Dreger P, Haferlach T, Eckstein V, et al. G-CSF-mobilized peripheral blood progenitor cells for allogeneic transplantation: safety, kinetics of mobilization, and composition of the graft. Br J Haematol. 1994;87:609–613. doi: 10.1111/j.1365-2141.1994.tb08321.x. [DOI] [PubMed] [Google Scholar]
- 14.Kurt B, Flynn P, Shenep JL, et al. Prophylactic antibiotics reduce morbidity due to septicemia during intensive treatment for pediatric acute myeloid leukemia. Cancer. 2008;113:376–382. doi: 10.1002/cncr.23563. [DOI] [PubMed] [Google Scholar]
- 15.Lin DY. Proportional means regression for censored medical costs. Biometrics. 2000;56:775–778. doi: 10.1111/j.0006-341x.2000.00775.x. [DOI] [PubMed] [Google Scholar]
- 16.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 18.Usuki K, Urabe A, Masaoka T, et al. Efficacy of granulocyte colony-stimulating factor in the treatment of acute myelogenous leukaemia: a multicentre randomized study. Br J Haematol. 2002;116:103–112. doi: 10.1046/j.1365-2141.2002.03251.x. [DOI] [PubMed] [Google Scholar]
- 19.Goldstone AH, Burnett AK, Wheatley K, Smith AG, Hutchinson RM, Clark RE. Attempts to improve treatment outcomes in acute myeloid leukemia (AML) in older patients: the results of the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98:1302–1311. doi: 10.1182/blood.v98.5.1302. [DOI] [PubMed] [Google Scholar]
- 20.Lehrnbecher T, Zimmermann M, Reinhardt D, Dworzak M, Stary J, Creutzig U. Prophylactic human granulocyte colony-stimulating factor after induction therapy in pediatric acute myeloid leukemia. Blood. 2007;109:936–943. doi: 10.1182/blood-2006-07-035915. [DOI] [PubMed] [Google Scholar]
- 21.Alonzo TA, Kobrinsky NL, Aledo A, Lange BJ, Buxton AB, Woods WG. Impact of granulocyte colony-stimulating factor use during induction for acute myelogenous leukemia in children: a report from the Children’s Cancer Group. J Pediatr Hematol Oncol. 2002;24:627–635. doi: 10.1097/00043426-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Martin-Murea S, Voso MT, Hohaus S, et al. The dose of granulocyte colony-stimulating factor administered following cytotoxic chemotherapy is not related to the rebound level of circulating CD34+ haemopoietic progenitor cells during marrow recovery. Br J Haematol. 1998;101:582–585. doi: 10.1046/j.1365-2141.1998.00730.x. [DOI] [PubMed] [Google Scholar]
- 23.Toner GC, Shapiro JD, Laidlaw CR, et al. Low-dose versus standard-dose lenograstim prophylaxis after chemotherapy: a randomized, crossover comparison. J Clin Oncol. 1998;16:3874–3879. doi: 10.1200/JCO.1998.16.12.3874. [DOI] [PubMed] [Google Scholar]
- 24.Bolwell B, Goormastic M, Dannley R, et al. G-CSF post-autologous progenitor cell transplantation: a randomized study of 5, 10, and 16 micrograms/kg/day. Bone Marrow Transplant. 1997;19:215–219. doi: 10.1038/sj.bmt.1700645. [DOI] [PubMed] [Google Scholar]
- 25.Cairo MS, Shen V, Krailo MD, et al. Prospective randomized trial between two doses of granulocyte colony-stimulating factor after ifosfamide, carboplatin, and etoposide in children with recurrent or refractory solid tumors: a children’s cancer group report. J Pediatr Hematol Oncol. 2001;23:30–38. doi: 10.1097/00043426-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 26.White SM, Ball ED, Ehmann WC, Rao AS, Tweardy DJ. Increased expression of the differentiation-defective granulocyte colony-stimulating factor receptor mRNA isoform in acute myelogenous leukemia. Leukemia. 1998;12:899–906. doi: 10.1038/sj.leu.2401062. [DOI] [PubMed] [Google Scholar]
- 27.White SM, Alarcon MH, Tweardy DJ. Inhibition of granulocyte colony-stimulating factor-mediated myeloid maturation by low level expression of the differentiation-defective class IV granulocyte colony-stimulating factor receptor isoform. Blood. 2000;95:3335–3340. [PubMed] [Google Scholar]
- 28.Ehlers S, Herbst C, Zimmermann M, et al. Granulocyte colony-stimulating factor (G-CSF) treatment of childhood acute myeloid leukemias that overexpress the differentiation-defective G-CSF receptor isoform IV is associated with a higher incidence of relapse. J Clin Oncol. 2010;28:2591–2597. doi: 10.1200/JCO.2009.25.9010. [DOI] [PubMed] [Google Scholar]
- 29.Lehrnbecher T, Varwig D, Kaiser J, Reinhardt D, Klingebiel T, Creutzig U. Infectious complications in pediatric acute myeloid leukemia: analysis of the prospective multi-institutional clinical trial AML-BFM 93. Leukemia. 2004;18:72–77. doi: 10.1038/sj.leu.2403188. [DOI] [PubMed] [Google Scholar]
- 30.Sung L, Lange BJ, Gerbing RB, Alonzo TA, Feusner J. Microbiologically documented infections and infection-related mortality in children with acute myeloid leukemia. Blood. 2007;110:3532–3539. doi: 10.1182/blood-2007-05-091942. [DOI] [PubMed] [Google Scholar]
- 31.Rubnitz J, Inaba H, Dahl G, et al. Minimal Residual Disease-Directed Therapy for Childhood Acute Myeloid Leukemia: Results of the AML02 Multicenter Trial. Lancet Oncol. 2010 doi: 10.1016/S1470-2045(10)70090-5. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]