Summary
Focal cortical epilepsy is currently most effectively studied in humans. However, improvement in cortical monitoring and investigational device development is limited by lack of an animal model mimicking human acute focal cortical epileptiform activity under epilepsy surgery conditions. Therefore, we assessed the swine model for translational epilepsy research. Swine were used due to their cost effectiveness, convoluted cortex, and comparative anatomy similar to humans. Focal subcortical injection of benzyl-penicillin produced clinical seizures correlating with epileptiform activity demonstrating temporal and spatial progression. Swine were evaluated under 5 different anesthesia regimens. Of the 5 regimens, conditions similar to human intraoperative anesthesia, including continuous fentanyl with low dose isoflorane, was the most effective for eliciting complex, epileptiform activity after benzyl-penicillin injection. The most complex epileptiform activity (spikes, and high frequency activity) was then repeated reliably in 9 animals, utilizing 14 swine total. There were 20.1 ± 10.8 (95% CI: 11.8–28.4) epileptiform events with greater than 3.5 hertz activity occurring per animal. Average duration of each event was 46.3 ± 15.6 (95% CI: 44.0 to 48.6) seconds, ranging from 20 to 100 seconds. In conclusion, the acute swine model of focal cortical epilepsy surgery provides an animal model mimicking human surgical conditions with a large brain, gyrated cortex, and is relatively cheap among animal models. Therefore, we feel this model provides a valuable, reliable, and novel platform for translational studies of implantable hardware for intracranial monitoring.
Keywords: Epilepsy, Animal Model, Electroencephalography, Swine, Pig, Translational Research
Introduction
Experimental implants for intracranial electroencephalography (iEEG) currently are restricted by like-device exemptions, therefore limiting novel device implantation (Van Gompel, et al. 2008). Important questions related to implant development will continue to be impeded without development of a cost effective experimental animal model mimicking intraoperative conditions for human monitoring. Swine (pig) animal models poses fewer barriers and emulate the complexity of intracranial monitoring because pigs are readily available, require less procurement and maintenance expense than other large animal species, are faraway cheaper than non-human primates, and are gyrencephalic making it a recent popular alternative for large animal neuroscience research (Wakeman, et al. 2006). Comparatively, adult pig brains (~160 g) are larger in size to that of available non-human primates such as rhesus monkeys (~100 g) and baboons (~140 g). Moreover, the availability of a detailed pig brain atlas makes stereotaxic implantation possible, such as directed implantation of depth electrodes (Felix, et al. 1999). Finally, pig brain development is complete by 5 months, enabling the use of younger animals (20–50 kg) with adult-sized brains reducing maintenance cost (Dobbing 1964). Therefore, we undertook this investigation to establish the viability of the pig as an animal model of intraoperative monitoring for investigational device implantation.
Methods
Pigs
All studies were performed with approval of the Institutional Animal Care and Use Committee. 30–35kg castrated male domestic swine (Large-white/Landrace/Duroc cross) were utilized for this study.
Surgery
Each pig, case 1–5, was subjected to a different protocol as described below along with its effect on epileptiform activity. All pigs were induced with intramuscular injection of Telazol (tiletamine and zolazepam) (5–6 mg/Kg) and Xylazine (2mg/Kg). After prone positioning, craniectomy, and dural opening, different implants were assessed as illustrated in figure 1. All animals prior to administration of benzyl-penicillin received a carrier control injection of 1X PBS (Gibco Invitrogen, Carlsbad CA). Epileptiform activity was induced by subcortical injection (depth of injection was 5 mm below the cortical surface) of 5 µl Benzyl-penicillin (PCN) 2–3 hours after induction of anesthesia, stock solution of 1100 Units/µl (Penna-G, Sigma, St. Louis MO) solved in 1X PBS. Pigs were euthanized with pentobarbital at the end of acute experiments.
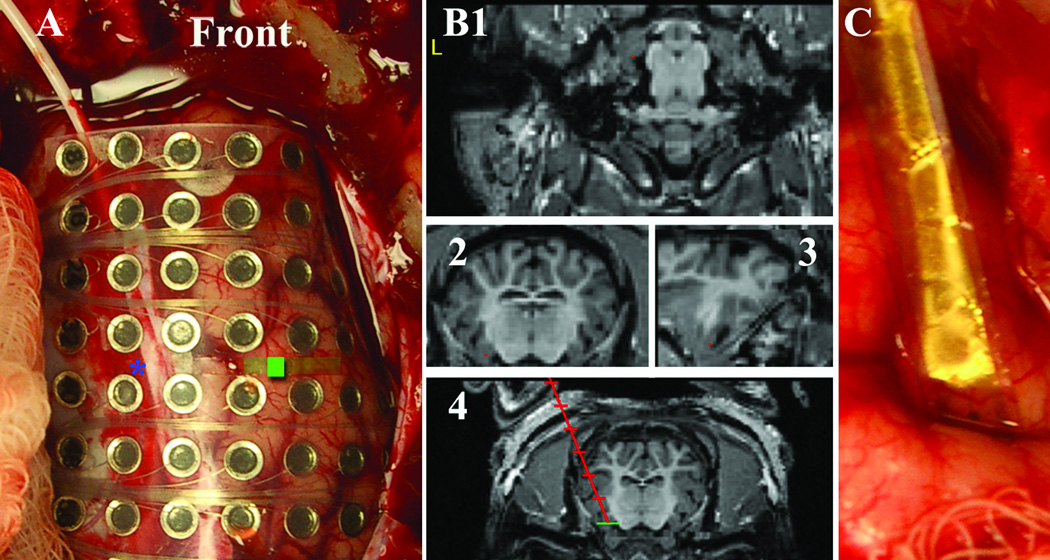
Figure 1.
(A) intraoperative photo demonstrating implantation of an 8×8 grid. Front is toward the snout. Blue asteric is the tip of an implanted depth electrode. The green window is the benzyl-penicillin injection window. (B) 1. Axial 2. Coronal 3. Sagital MRI after stereotactic localization with red point at the head of the hippocampus for depth planning 4. Trajectory view of planned depth insertion to the head of the hippocampus. (C) Experimental polyamide microwire grid placed intragyrally in the frontal cortex.
EEG
Electroencephalography (EEG) was acquired on a 128 channel Neuralynx (Bozeman, MT) recording system (1–32,500 Hz broad band acquisition). Epileptiform activity was defined as follows: grade I = basic background without epileptiform spikes; grade II = mostly normal background, high-voltage spikes less than 1 Hz; grade III = high-voltage spiking greater than 1 Hz with high voltage spiking; and grade IV, high-frequency >3.5 Hz and attenuated voltage synchronized polyspike or paroxysmal sharp waves lasting ≥6 sec.
Results
Anesthesia
Anesthesia setup (AS) 1: Maintenance anesthesia was performed with isoflurane as the sole agent. Single agent isoflurane necessitated between 2–4% inhaled concentration to maintain anesthesia. EEG demonstrated severely suppressed cortical activity. Grade II activity was achieved, however with little variation and low voltage. AS2: After induction, morphine 0.25 mg/kg IM was given prior to incision to augment anesthesia, then continuous a continuous IV was run with the following agents: Morphine 0.24 mg/kg/hr (analgesic dosage 0.1–1mg/kg), Lidocaine 0.3mg/kg/hr (antiarrhythmic dose = 5 mg/kg), and Ketamine 0.6mg/kg/hr (anesthetic dose = 20–30 mg/kg). Nitrous oxide 2 to 1 with oxygen was used to reduce isoflurane requirements. Isoflurane was maintained at 1% for this procedure. Under this protocol, grade II activity was dominant with rare episodes of grade III. AS3: After induction, this animal was given a bolus dose (100 mcg) fentanyl during the initial craniotomy. Thereafter was run on fentanyl at 75–125 mcg/hr continuously. There was no isoflurane used. This simple protocol produced excellent variation in epileptiform activity, cycling between grade II–IV, often with runs of grade 4 followed by termination of activity of up to 30 seconds (figure 2). Clinical seizures were present with grade IV activity, therefore the animal received pancuronium for safety and to reduce artifact. After this clinical confirmation, all animals were given pancuronium at injection of penicillin, and pain was assessed by a greater than 10% change in baseline heart rate or blood pressure. AS4: This animal was performed in a manner similar to AS3, however pancuronium was added to the continuous fentanyl after administration of benzyl-penicillin. Further, the animal was maintained on 0.5% isoflurane. This gave similar results to AS3 with good variation between grade II–IV activity. AS5: Case 5 was performed in the same manner as Case 4 with the exception of the addition of continuous ketamine as in Case 2 (ketamine 1 mg/kg/hr). Variation between grade II and III was infrequent, there were no episodes of grade IV activity, and therefore felt to be inferior to AS4.
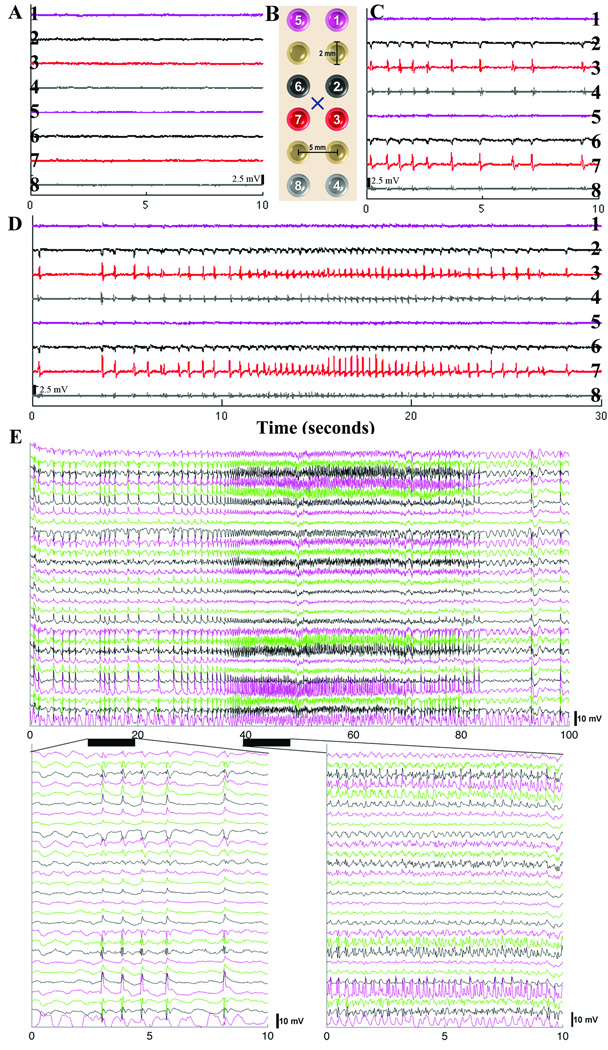
Figure 2.
Representative referential (subgaleal reference) EEG signal. The figure schematic (B) demonstrates the relationship of select EEG electrodes (colored and numbered: purple (1,5) contralateral hemisphere, black (6,2) medial to injection site, red (7,3) lateral to injection site, grey (8,4) distal and lateral to injection site all on frontal cortex) in figure parts A, C, and D to the benzyl-penicillin injection site (X) (black hash represents 2.5 mV). (A) Baseline EEG (grade I) tracing prior to benzyl-penicillin injection. (C) EEG recording after injection of penicillin. Note minimal response on contralateral hemisphere (purple), however between red and black electrodes there is phase reversal as expected. Distal recordings (grey) show diminished voltage. (D) Event of Grade IV activity. (E) Separate epileptiform event showing 100 seconds of EEG data from 28 channels (chosen as those with largest voltage changes) again demonstrating the temporal and spatial dynamics of these events (color coding does not correspond to a specific electrode). Below are two 10 second expanded views of the above event demonstrating the EEG waveform. X-axis is time in seconds in A through E.
Repeatability
Nine pigs were sequentially anesthetized according to AS4. Epileptiform activity at or greater than grade III was observed in all 9 animals and showed variation in epileptiform activity (figure 2) at a dose of 5500 units of benzyl-penicillin (PCN). 550 units of benzyl-penicillin was the lowest effective dose for generating observable epileptiform activity as established by serial dilution testing in 2 animals.
Seizure characteristics
9 Animals were used for EEG analysis. There were 181 total grade 4 events in these 9 animals, between 3 to 39 grade 4 events occurred per animal in an 8 hour time window. 20.1 ± 10.8 (95% CI: 11.8–28.4) grade 4 events occurred per animal. Average duration was 46.3 ± 15.6 (95% CI: 44.0 to 48.6) seconds, range was 20 to 100 seconds.
Discussion
Implantable technology to support iEEG or other investigative techniques is lagging behind acquisition and recording technologies (Van Gompel, et al. 2008). Exploration of novel implantable devices is limited by the absence of an appropriate animal model for development. Although the human brain is 5 to 10 times larger than animals of equivalent weight such as swine, these animals can be used to understand the complex interactions that occur during epileptiform activity in convoluted cortical surfaces.
The use of swine as a model of epilepsy is not new, however the description of its EEG characteristics is. Dr. Terndrup and various co-authors described in the late 80’s and mid 90’s the use of assorted chemoconvulsants, most notably subcortical PCN injection, to demonstrate physiologic changes in vital signs occurring during convulsive activity in pigs (Leaming, et al. 1999, Terndrup, et al. 1999, Terndrup & Fordyce 1995, Terndrup, et al. 1995, Terndrup, et al. 1993, Terndrup, et al. 1994). Further, due to easy glottal access, several glottal parameters were investigated in relation to clinical events (Leaming, et al. 1999, Terndrup, et al. 1995). Interestingly, the cortical seizure activity was not assessed beyond its presence by a single tungsten wire electrode; therefore the spatial and temporal variability has not been understood. Further, they noted vigorous motor seizures with high doses of PCN necessary to evaluate parameters of interest to anesthesiologists (Leaming, et al. 1999, Terndrup, et al. 1999, Terndrup, et al. 1995). In these studies, subcortical injections of PCN were higher, 100 microliters (1,000,000 Units/ml) and several injections were used to induce activity (Terndrup, et al. 1999). We have found that this is far more than needs to be injected to induce activity, and that repeated injections lead to separate foci of activity, which can complicate the analysis of epileptiform activity (Figure 2). Further, with lower doses of PCN there is a focus of epileptiform activity that one can expect to vary with time from grade II to IV activity, if the pig is left without neuromuscular blockade vigorous motor activity is seen with grade IV activity.
Subcortical injection of GABAa antagonists (PCN) has also been described to produce epileptiform activity in a variety of animal models such as cat, dog, rat, mouse etc (Binnie, et al. 1985, Steriade & Contreras 1998). Beside non-human primates, all of these models are limited by small size or lissencephalic cortex. Although we have not tested other chemoconvulsants such as kainic acid, PTZ (pentylenetetrazol), etc., it may be reasonable to assume that these could be also investigated to provide other models of focal cortical epileptiform activity. These approaches offer the advantage of spatial and temporal interrogation of multi-array devices such as those seen in figure 1. Further, investigation of questions that cannot be addressed in human subjects at this time, such as sulcal recordings (figure 1) may be undertaken.
Conclusion
This acute translational model demonstrates clinical (convulsions), temporal, and spatial evolution of an epileptiform focus under conditions similar to intraoperative anesthesia for EEG monitoring. This model may be useful in evaluating devices for implantation. Finally, the pig is an economically advantageous model for investigative iEEG. In conclusion, focal PCN injection in the large animal model, swine, is a viable translational model to assess human cortical recording investigative devices.
Acknowledgments
This work was supported by: Dr. Lee has received support from NIH (K08 NS 52232 award to KHL) and Mayo Foundation (2008–2010 Research Early Career Development Award for Clinician Scientists award) to support this work. Dr. Worrell has received support from NIH (R01 NS063039-01). Dr. Van Gompel has received support from Bernard and Irene Waterman Award in Individualized Medicine and the Epilepsy Foundation.
Financial and Material Support: This work was supported by: NIH (K08 NS 52232 award to KHL), Mayo Foundation (2008–2010 Research Early Career Development Award for Clinician Scientists award to KHL), NIH (R01 NS063039-01 award to GAW) and Bernard and Irene Waterman Award in Individualized Medicine (award to JJVG). This project was further funded by the Epilepsy Foundation (award to JJVG).
Footnotes
Previous Presentations: none
Disclosure of Conflicts of Interest:
We have no conflicts of interest to disclose
“We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.”
References
- Binnie CD, Van Emde Boas W, Wauquier A. Geniculate spikes during epileptic seizures induced in dogs by pentylenetetrazol and bicuculline. Electroencephalogr Clin Neurophysiol. 1985;61:40–49. doi: 10.1016/0013-4694(85)91071-5. [DOI] [PubMed] [Google Scholar]
- Dobbing J. The Influence of Early Nutrition on the Development and Myelination of the Brain. Proc R Soc Lond B Biol Sci. 1964;159:503–509. doi: 10.1098/rspb.1964.0016. [DOI] [PubMed] [Google Scholar]
- Felix B, Leger ME, Albe-Fessard D, Marcilloux JC, Rampin O, Laplace JP. Stereotaxic atlas of the pig brain. Brain Res Bull. 1999;49:1–137. doi: 10.1016/s0361-9230(99)00012-x. [DOI] [PubMed] [Google Scholar]
- Leaming JM, Terndrup TE, Ognibene S. Glottal patency during experimental cortical seizures in piglets. Acad Emerg Med. 1999;6:682–687. doi: 10.1111/j.1553-2712.1999.tb00435.x. [DOI] [PubMed] [Google Scholar]
- Steriade M, Contreras D. Spike-wave complexes and fast components of cortically generated seizures. I. Role of neocortex and thalamus. J Neurophysiol. 1998;80:1439–1455. doi: 10.1152/jn.1998.80.3.1439. [DOI] [PubMed] [Google Scholar]
- Terndrup TE, Darnall R, Knuth SL, Bartlett D., Jr Effects of experimental cortical seizures on respiratory motor nerve activities in piglets. J Appl Physiol. 1999;86:2052–2058. doi: 10.1152/jappl.1999.86.6.2052. [DOI] [PubMed] [Google Scholar]
- Terndrup TE, Fordyce WE. Respiratory drive during status epilepticus and its treatment: comparison of diazepam and lorazepam. Epilepsy Res. 1995;20:21–30. doi: 10.1016/0920-1211(94)00061-z. [DOI] [PubMed] [Google Scholar]
- Terndrup TE, Kadison A, Woo P. Glottal patency during experimental seizures in piglets. Pediatr Res. 1995;38:932–937. doi: 10.1203/00006450-199512000-00017. [DOI] [PubMed] [Google Scholar]
- Terndrup TE, Paskanik AM, Fordyce WE, Kanter RK. Development of a piglet model of status epilepticus: preliminary results. Ann Emerg Med. 1993;22:164–170. doi: 10.1016/s0196-0644(05)80196-9. [DOI] [PubMed] [Google Scholar]
- Terndrup TE, Starr F, Fordyce WE. A piglet model of status epilepticus: comparison of cardiorespiratory and metabolic changes with two methods of pentylenetetrazol administration. Ann Emerg Med. 1994;23:470–479. doi: 10.1016/s0196-0644(94)70065-6. [DOI] [PubMed] [Google Scholar]
- Van Gompel JJ, Stead SM, Giannini C, Meyer FB, Marsh WR, Fountain T, So E, Cohen-Gadol A, Lee KH, Worrell GA. Phase I trial: safety and feasibility of intracranial electroencephalography using hybrid subdural electrodes containing macro- and microelectrode arrays. Neurosurg Focus. 2008;25:E23. doi: 10.3171/FOC/2008/25/9/E23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeman DR, Crain AM, Snyder EY. Large animal models are critical for rationally advancing regenerative therapies. Regen Med. 2006;1:405–413. doi: 10.2217/17460751.1.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]