Abstract
Background and Objectives
Ambulatory arterial stiffness index (AASI) is well known as a predictor of cardiovascular mortality in hypertensive patients. Mathematically, AASI reflect the standard deviation (SD) of blood pressure (BP) variation. AASI is measured higher levels in non-dipper than dipper. Thus, AASI has a possibility of not only reflecting arterial stiffness but also BP variability and/or autonomic nervous dysfunction.
Subjects and Methods
Consecutive data from 418 untreated hypertensive patients were analyzed retrospectively. We examined the association between the 24-hour ambulatory BP monitoring (ABPM) parameters and AASI.
Results
AASI had a simple correlation with age (R=0.189, p<0.001), relative wall thickness (RWT) (R=0.115, p=0.019), left ventricular mass index (LVMI) (R=0.192, p<0.001), average systolic BP (SBP) (R=0.232, p<0.001), average pulse pressure (PP) (R=0.363, p<0.001), SD of diastolic BP (DBP) (R=-0.352, p<0.001), SD of PP (R=0.330, p<0.001), SD of heart rate (HR) (R=-0.268, p<0.001), and nocturnal dipping (R=-0.137, p=0.005). In multiple linear regression analysis model including clinical parameters and 24 hour-ABPM parameters, independent predictors of AASI were SD of PP (β=1.246, p<0.001), SD of DBP (β=-1.067, p<0.001), SD of SBP (β=-0.197, p<0.001), and non-dipper (β=0.054, p=0.033).
Conclusion
AASI is closely correlated with BP variability. The result of this study shows that AASI is not only a parameter for arterial stiffness, but also a parameter for BP variability.
Keywords: Blood pressure; Blood pressure monitoring, ambulatory; Autonomic nervous system diseases
Introduction
Ambulatory arterial stiffness index (AASI) is a marker of arterial stiffness suggested by Li et al.1) AASI is a well known predictor of cardiovascular mortality in hypertensive patients.2) In the dynamic relation between systolic and diastolic blood pressure (BP), for a given increase in diastolic BP (DBP), the increase in systolic BP (SBP) is smaller in a compliant artery than in a stiff artery.3) According to this basic principle, Li et al.1) calculated the regression slope of DBP on SBP from the BP value of 24 hour ambulatory BP monitoring (ABPM), to quantify the increase in DBP for a given SBP change. One minus the slope corresponds to AASI, which is known to reflect arterial stiffness.1)
However, the biological mechanisms underlying AASI as a parameter of arterial stiffness is not yet fully understood. First, by using ABPM, AASI is inevitably influenced by BP variability component. In other words, with a mathematical consideration, the standard deviation (SD) of DBP as a numerator of the regression slope is positively correlated with the regression slope of DBP on SBP. Consequently, SD of DBP is inversely correlated with AASI (1-regression slope).
Second, BP variability has also been documented to be a prognostic marker in some reports.4-7) According to a previous report, AASI has only weak correlations with the parameters of arterial stiffness.8) There appears to be a possibility that AASI, as a prognostic marker, is confounded by BP variability.
Moreover, some studies showed that AASI is strongly influenced by factors unrelated to arterial stiffness, i.e., nocturnal BP fall.8),9) According to Schillaci et al.8) AASI is higher in non-dippers than dippers. AASI has a possibility of reflecting not only arterial stiffness, but also diurnal BP variability and/or autonomic nervous dysfunction. Therefore, we hypothesized that BP variability is a significant biologic mechanism underlying AASI, and elected to study the relationship between AASI and BP variability in hypertensive patients using ABPM data.
Subjects and Methods
Study population
The dataset of 644 consecutive patients was acquired from the ABPM database. The patients had undergone both ABPM and echocardiography at the Hanyang University Hospital, Seoul, the Republic of Korea from February 16th, 2006 to December 31st, 2008. Among these 644 patients, only hypertensive patients who had not taken medication were enrolled. Hypertension was defined as clinic BP of at least 140/90 mmHg, or 24 hour average BP of at least 135/85 mmHg, or antihypertensive drug treatment in patients with history of hypertension. Finally, consecutive data from 418 patients were analyzed retrospectively.
Height, weight, clinical BP and heart rate (HR) were measured before ABPM was performed. Diabetes was defined as a self-reported history of being told by a physician that diabetes was present, or having a fasting glucose of 126 mg/dL or greater. Clinical information was collected by reviewing medical records. The study protocol was approved by the institutional review board at the Hanyang University Hospital.
Measurement of office blood pressure and 24-hour ambulatory blood pressure monitoring
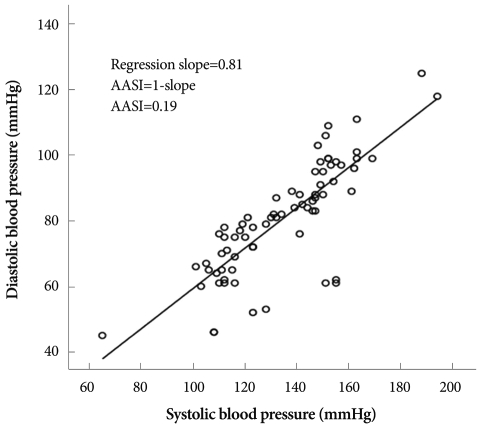
Clinic BP was measured by a mercury sphygmomanometer, and defined as the average of at least 2 measurements recorded 3 minutes apart. Five physicians were involved in the measurement of clinic BP. Standardization quality was not validated. ABPM was recorded during a routine day by a TM-2430 device (A&D, Saitama, Japan). The device was applied to the non-dominant arm for 24 hours. BP was measured every 15 minutes during daytime, and every 30 minutes at nighttime. Patients were instructed to maintain their usual activities during monitoring, and to stay calm when the device started to work. Daytime and nighttime periods were defined individually according to the patients' self-reported data. As displayed in Fig. 1, raw data were examined by a scatter plot and the regression slope of DBP on SBP was computed to obtain an AASI (1-slope).3) The average mean arterial pressure (AMAP), SBP, DBP, pulse pressure (PP) and HR were calculated. The SDs of SBP, DBP, PP and HR were also calculated as parameters of BP and HR variability. Nocturnal dipping (%) is defined by percent decrease in nocturnal systolic BP compared to daytime systolic BP. When patients exhibited nocturnal dipping of less than 10%, they were defined as non-dippers.
Fig. 1.
Derivation of the ambulatory arterial stiffness index (AASI) from a 24 hour ambulatory blood pressure recording.
Measurement of echocardiography
Echocardiograms were carried out using a HP Sonos 2500 (Hewlett-Packard, Santa Clara, CA, USA) machine equipped with a 2.5 MHz probe. Left ventricular mass index (LVMI)10-12) and relative wall thickness (RWT)13),14) are associated with arterial stiffness. We have examined LVMI and RWT to elucidate the relationship between AASI and LV structural change due to arterial stiffness. Left ventricular mass (LVM) was calculated from 2D-guided M-mode echocardiographic measurement of the left ventricle. Measurement of the left ventricle internal dimension, interventricular septal thickness, and posterior wall thickness were made during diastole according to methods established by the American Society of Echocardiography. LVM was calculated using the Devereux equation.15) LVMI was calculated by dividing LVM by height2.7 to minimize effects of age, gender, ethnicity, and overweight status.16),17) RWT was calculated as the ratio of 2×(posterior wall thickness/end diastolic diameter) to assess the concentric LV remodeling.18)
Statistical analysis
All data are presented as means±SDs. Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) (version 13.0; SPSS Inc., Chicago, IL, USA). A p<0.05 was considered statistically significant.
Student's t-test was used for comparison of the measured values from the two groups divided according to sex and according to nocturnal BP dipping status. Pearson correlation coefficients between AASI and clinical parameters or 24 hour-ABPM parameters were calculated.
Multiple linear regression analyses were performed to adjust associated variables. Two multiple linear regression analysis models were used to estimate the independent correlation of AASI with clinical parameters and 24 hour-ABPM parameters. The first model included age, sex, height, weight, LVMI, RWT, diabetes mellitus, lipid profile, and 24 hour-ABPM parameters. Twenty-four hour ABPM parameters, included average MAP, average SBP, SD of SBP, average DBP, SD of DBP, average PP, SD of PP, average HR, SD of HR, and dipping status. In the second model, as another method to adjust the BP level, we introduced coefficient of variation (CV). CV was defined as the ratio of the SD to the mean. The criterion for entry into stepwise multiple linear regression analysis was p<0.05, while removal criterion was p>0.10.
Results
General characteristics of study subjects
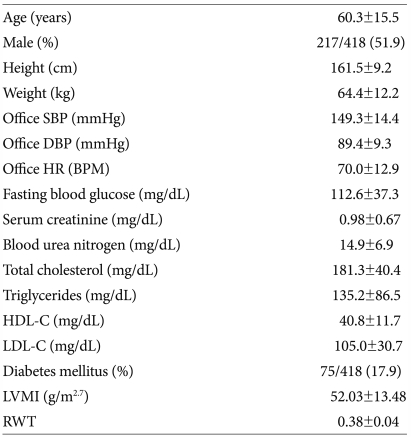
The clinical characteristics of the study population are summarized in Table 1. The mean (±SD) age of the subjects was 60.3±15.5, and proportion of male was 51.9%. The mean (±SD) office SBP was 149.3±14.4 mmHg and the mean (±SD) office DBP was 89.4±9.3 mmHg.
Table 1.
Clinical characteristics of patients
Data are reported as mean (±SD) or percentage. SBP: systolic blood pressure, DBP: diastolic blood pressure, HR: heart rate, BPM: beats per minute, HDL-C: high density lipoprotein-cholesterol, LDL-C: low density lipoprotein-cholesterol, LVMI: left ventricular mass index, RWT: relative wall thickness
The results of 24 hour ambulatory blood pressure monitoring
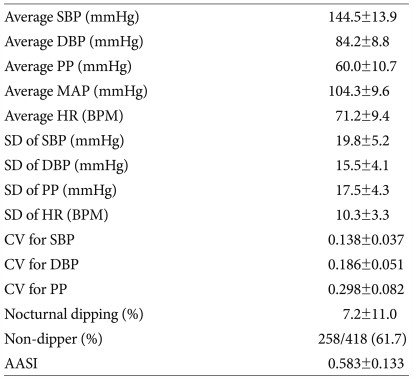
Twenty-four hour ABPM parameters of the study population are listed in Table 2. The mean (±SD) of average SBP was 144.5±13.9 mmHg, the mean (±SD) of average DBP was 84.2±8.8 mmHg, and the mean (±SD) of average PP was 60.0±10.7 mmHg. The proportion of non-dippers was 61.7%, and the mean (±SD) of AASI was 0.583±0.133.
Table 2.
24-hour ABPM parameters
Data are reported as mean (±SD) or percentage. ABPM: ambulatory BP monitoring, SBP: systolic blood pressure, DBP: diastolic blood pressure, PP: pulse pressure, MAP: mean arterial pressure, HR: heart rate, BPM: beats per minute, SD: standard deviation, CV: coefficient of variation, AASI: ambulatory arterial stiffness index
Crude association between ambulatory arterial stiffness index and clinical parameters
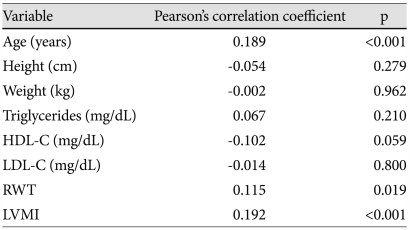
AASI was not different between female and male (0.576±0.126 vs. 0.589±0.139; p=0.311). Bivariate correlations of AASI with clinical parameters are listed in Table 3. AASI had significant correlation with age (R=0.189, p<0.001), RWT (R=0.115, p=0.019), and LVMI (R=0.192, p<0.001).
Table 3.
Bivariate correlation of AASI with clinical parameters
AASI: ambulatory arterial stiffness index, HDL-C: high density lipoprotein-cholesterol, LDL-C: low density lipoprotein-cholesterol, RWT: relative wall thickness, LVMI: left ventricular mass index
Crude association between ambulatory arterial stiffness index and 24 hour ambulatory blood pressure monitoring parameters
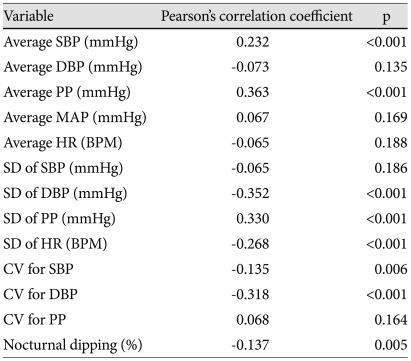
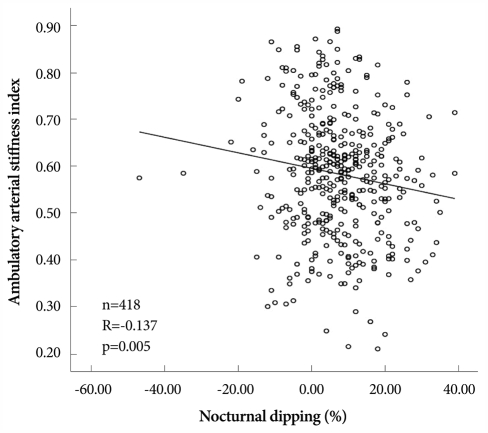
AASI was significantly higher in non-dippers than in dippers (0.602±0.133 vs. 0.552±0.128; p<0.001). Bivariate correlations of AASI with 24 hour ABPM variables are listed in Table 4. AASI had significant correlation with average SBP (R=0.232, p<0.001), average PP (R=0.363, p<0.001), SD of DBP (R=-0.352, p<0.001), SD of PP (R=0.330, p<0.001), SD of HR (R=-0.268, p<0.001), CV for SBP (R=-0.135, p=0.006), CV for DBP (R=-0.318, p<0.001), and nocturnal dipping (R=-0.137, p=0.005) (Fig. 2).
Table 4.
Bivariate correlation of AASI with 24-hour ABPM parameters
AASI: ambulatory arterial stiffness index, ABPM: ambulatory blood pressure monitoring, SBP: systolic blood pressure, DBP: diastolic blood pressure, PP: pulse pressure, MAP: mean arterial pressure, HR: heart rate, BPM: beats per minute, SD: standard deviation, CV: coefficient of variation
Fig. 2.
Correlation of ambulatory arterial stiffness index with nocturnal dipping.
Independent predictors of higher ambulatory arterial stiffness index
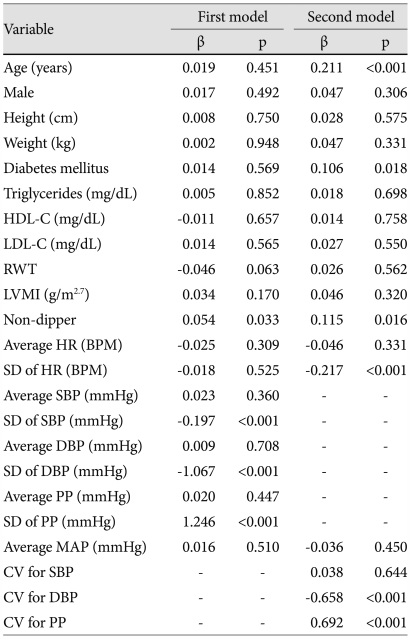
In the first model including age, sex, height, weight, LVMI, RWT, diabetes mellitus, lipid profile, average MAP, average PP, SD of PP, average SBP, SD of SBP, average DBP, SD of DBP, average HR, SD of HR, and dipping status, statistically significant predictors of higher AASI were SD of PP (β=1.246, p<0.001), SD of DBP (β=-1.067, p<0.001), SD of SBP (β=-0.197, p<0.001), and non-dipper status (β=0.054, p=0.033) (Table 5). In the second model including age, sex, height, weight, LVMI, RWT, diabetes mellitus, lipid profile, average MAP, CV for SBP, CV for DBP, CV for PP, average HR, SD of HR, and dipping status, statistically significant predictors of higher AASI included CV for PP (β=0.692, p<0.001), CV for DBP (β=-0.658, p<0.001), age (β=0.211, p<0.001), SD of HR (β=-0.217, p<0.001), non-dipper status (β=0.115, p=0.016), and diabetes mellitus (β=0.106, p=0.018) (Table 5).
Table 5.
Independent predictors of higher AASI in stepwise multiple linear regression analysis models
AASI: ambulatory arterial stiffness index, HDL-C: high density lipoprotein-cholesterol, LDL-C: low density lipoprotein-cholesterol, RWT: relative wall thickness, LVMI: left ventricular mass index, HR: heart rate, BPM: beats per minute, PP: pulse pressure, SD: standard deviation, MAP: mean arterial pressure, SBP: systolic blood pressure, DBP: diastolic blood pressure, CV: coefficient of variation
Discussion
This study demonstrated that AASI is closely related with BP variability. Furthermore, AASI has a much stronger relationship with BP variability than with BP itself. BP variability and BP is known to be relevant to the adverse consequence of hypertension.4-7) These findings suggest that AASI reflects BP variability and arterial stiffness.
Mathematically, DBP variability is inversely correlated with AASI. After adjusting the associated variables, our results are consistent with this principle. Increased BP variability could potentially be caused by increased average BP. However, after adjusting the average BP, BP variability was still an independent risk factor for higher AASI. This result suggests that AASI is closely related to BP variability.
In the first regression model, PP variability was a positive determinant of AASI after adjusting the PP. PP is one of the most important parameters for arterial stiffness. This result suggests that SD of PP may be a predictor for prognosis. We also documented that BP variability factors are stronger determinants of AASI than average BP. In the first model, SD of PP was the strongest independent predictor of higher AASI.
Considering that variability size is dependent on the mean value, CV appears to be a better indicator. In the second model, CV for PP and DBP, age, SD of HR, non-dipper, and diabetes mellitus were independent predictors. From these independent predictors, CV for PP was the strongest independent predictor of AASI. Thus, CV for PP or the SD of PP, which represents PP variability, is better related to AASI than PP itself, which is a well-known parameter of arterial stiffness.
In this study, AASI is significantly higher in non-dipper than in dipper, as demonstrated in a previous study. Non-dipper is known to be associated with several conditions, including autonomic dysregulation and target organ damage.19),20) In addition, HR variability was independently and inversely correlated with AASI. HR variability is decreased in patients with autonomic dysfunction.20) Furthermore, a previous study had documented that reduced HR variability estimated by ABPM has prognostic significance for cardiovascular mortality.6) According to our study, there is a possibility that non-dipper or autonomic dysregulation may confound AASI in predicting cardiovascular outcome.
On the contrary, LVH is associated with arterial stiffness10-12) and with increased PP.21-23) In our study, LVMI was positively correlated with AASI in the crude relationship. However, in regression analysis models, the relationship between AASI and LVMI did not survived. Concentric left ventricular remodeling is also associated with arterial stiffeness13),14) and with left ventricular pressure overload.24) RWT was positively correlated with AASI in the crude relationship. However, in the regression analysis model, RWT was not an independent predictor of AASI. These results imply that AASI have weak relationship with increased afterload resulted from arterial stiffness.
The finding that AASI has a weak correlation with PP and with SBP in this study may be interpreted as AASI reflects different aspects of arterial stiffness as measured by PP. It is consistent with the outcomes of a previous study that AASI was a significant predictor of stroke in a general population, after having adjusted for PP.25)
PP is mainly affected by stroke volume and arterial elasticity, which determines both arterial compliance and wave reflection.26) When symphathetic tone increases and parasymphathetic nervous activity decreases, the variability of stroke volume rises.27) On the other hand, as arterial distending pressure increases, the elasticity decreases.28) Thus, increased stroke volume might cause decreased elasticity, which causes both reduced arterial compliance and increased pulse wave velocity. Therefore, autonomic dysfunction, which increases stroke volume variability, may give rise to increased PP variability.
We have demonstrated that AASI has much stronger relationship with PP variability than with PP itself. Thus, we might speculate that AASI which is regarded as a parameter of arterial stiffness may reflect PP variability, autonomic nervous dysfunction, or increased sympathetic tone.
There are limitations in this study. First, we have not demonstrated a direct relationship between PP variability and autonomic cardiovascular regulation. Therefore, the relationship between PP variability and autonomic cardiovascular regulation remains conjectural. However, there is other evidence that AASI is affected by autonomic cardiovascular regulation. According to our results, AASI is inversely correlated with the SD of HR. HR variability decreases in patients with autonomic dysfunction.20) Furthermore, non-dipper was an independent predictor of higher AASI after adjusting clinical variables. These results support that AASI is affected by autonomic dysregulation. Second, pulse wave velocity, which is regarded as standard method to measure arterial stiffness, was not available in this study. Therefore, there is some limitation to demonstrate the relationship between AASI and arterial stiffness. However, we have suggested average PP, LVMI and RWT as parameters of arterial stiffness. Average PP, LVMI10-12) and RWT13),14) also represent arterial stiffness. None of these parameters were independently associated with AASI. These results suggest that AASI have a weak relationship with arterial stiffness.
In conclusion, AASI is closely related to BP variability. This suggests that AASI is not only a parameter for arterial stiffness, but also a parameter for BP variability.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Li Y, Wang JG, Dolan E, et al. Ambulatory arterial stiffness index derived from 24-hour ambulatory blood pressure monitoring. Hypertension. 2006;47:359–364. doi: 10.1161/01.HYP.0000200695.34024.4c. [DOI] [PubMed] [Google Scholar]
- 2.Dolan E, Thijs L, Li Y, et al. Ambulatory arterial stiffness index as a predictor of cardiovascular mortality in the Dublin Outcome Study. Hypertension. 2006;47:365–370. doi: 10.1161/01.HYP.0000200699.74641.c5. [DOI] [PubMed] [Google Scholar]
- 3.Dolan E, Li Y, Thijs L, et al. Ambulatory arterial stiffness index: rationale and methodology. Blood Press Monit. 2006;11:103–105. doi: 10.1097/01.mbp.0000200478.19046.dd. [DOI] [PubMed] [Google Scholar]
- 4.Kikuya M, Ohkubo T, Metoki H, et al. Day-by-day variability of blood pressure and heart rate at home as a novel predictor of prognosis: the Ohasama study. Hypertension. 2008;52:1045–1050. doi: 10.1161/HYPERTENSIONAHA.107.104620. [DOI] [PubMed] [Google Scholar]
- 5.Mancia G, Parati G, Hennig M, et al. Relation between blood pressure variability and carotid artery damage in hypertension: baseline data from the European Lacidipine Study on Atherosclerosis (ELSA) J Hypertens. 2001;19:1981–1989. doi: 10.1097/00004872-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Kikuya M, Hozawa A, Ohokubo T, et al. Prognostic significance of blood pressure and heart rate variabilities: the Ohasama study. Hypertension. 2000;36:901–906. doi: 10.1161/01.hyp.36.5.901. [DOI] [PubMed] [Google Scholar]
- 7.Frattola A, Parati G, Cuspidi C, Albini F, Mancia G. Prognostic value of 24-hour blood pressure variability. J Hypertens. 1993;11:1133–1137. doi: 10.1097/00004872-199310000-00019. [DOI] [PubMed] [Google Scholar]
- 8.Schillaci G, Parati G, Pirro M, et al. Ambulatory arterial stiffness index is not a specific marker of reduced arterial compliance. Hypertension. 2007;49:986–991. doi: 10.1161/HYPERTENSIONAHA.106.082248. [DOI] [PubMed] [Google Scholar]
- 9.Baumann M, Dan L, Nurnberger J, Heemann U, Witzke O. Association of ambulatory arterial stiffness index and brachial pulse pressure is restricted to dippers. J Hypertens. 2008;26:210–214. doi: 10.1097/HJH.0b013e3282f25b6e. [DOI] [PubMed] [Google Scholar]
- 10.Darne B, Girerd X, Safar M, Cambien F, Guize L. Pulsatile versus steady component of blood pressure: a cross-sectional analysis and a prospective analysis on cardiovascular mortality. Hypertension. 1989;13:392–400. doi: 10.1161/01.hyp.13.4.392. [DOI] [PubMed] [Google Scholar]
- 11.Girerd X, Laurent S, Pannier B, Asmar R, Safar M. Arterial distensibility and left ventricular hypertrophy in patients with sustained essential hypertension. Am Heart J. 1991;122:1210–1214. doi: 10.1016/0002-8703(91)90941-a. [DOI] [PubMed] [Google Scholar]
- 12.Rhee B, Park J, Kim H, et al. Increased aortic stiffness is associated with increased left ventricular mass and diastolic dysfunction. Korean Circ J. 2005;35:525–532. [Google Scholar]
- 13.Shin J, Lee J, Lim H, Lee B, Kim M, Choi B. The relationship between the pulse wave velocity (PWV) and the left ventricular geometry: a community-based cross-sectional study. Korean Circ J. 2005;35:683–689. [Google Scholar]
- 14.Roman MJ, Ganau A, Saba PS, Pini R, Pickering TG, Devereux RB. Impact of arterial stiffening on left ventricular structure. Hypertension. 2000;36:489–494. doi: 10.1161/01.hyp.36.4.489. [DOI] [PubMed] [Google Scholar]
- 15.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 16.Daniels SR, Kimball TR, Morrison JA, Khoury P, Meyer RA. Indexing left ventricular mass to account for differences in body size in children and adolescents without cardiovascular disease. Am J Cardiol. 1995;76:699–701. doi: 10.1016/s0002-9149(99)80200-8. [DOI] [PubMed] [Google Scholar]
- 17.de Simone G, Daniels SR, Devereux RB, et al. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20:1251–1260. doi: 10.1016/0735-1097(92)90385-z. [DOI] [PubMed] [Google Scholar]
- 18.Muscholl MW, Hense HW, Brockel U, Doring A, Riegger GA, Schunkert H. Changes in left ventricular structure and function in patients with white coat hypertension: cross sectional survey. BMJ. 1998;317:565–570. doi: 10.1136/bmj.317.7158.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abate G, D'Andrea L, Battestini M, Zito M, Di Iorio A. Autonomic nervous activity in elderly dipper and non-dipper patients with essential hypertension. Aging (Milano) 1997;9:408–414. doi: 10.1007/BF03339622. [DOI] [PubMed] [Google Scholar]
- 20.Kohara K, Nishida W, Maguchi M, Hiwada K. Autonomic nervous function in non-dipper essential hypertensive subjects: evaluation by power spectral analysis of heart rate variability. Hypertension. 1995;26:808–814. doi: 10.1161/01.hyp.26.5.808. [DOI] [PubMed] [Google Scholar]
- 21.Park JS, Park CG, Park MY, et al. Relation of blood pressure components to left ventricular hypertrophy and coronary heart disease with aging. Korean Circ J. 2004;34:142–150. [Google Scholar]
- 22.Khattar RS, Acharya DU, Kinsey C, Senior R, Lahiri A. Longitudinal association of ambulatory pulse pressure with left ventricular mass and vascular hypertrophy in essential hypertension. J Hypertens. 1997;15:737–743. doi: 10.1097/00004872-199715070-00005. [DOI] [PubMed] [Google Scholar]
- 23.Pannier B, Brunel P, el Aroussy W, Lacolley P, Safar ME. Pulse pressure and echocardiographic findings in essential hypertension. J Hypertens. 1989;7:127–132. [PubMed] [Google Scholar]
- 24.Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest. 1975;56:56–64. doi: 10.1172/JCI108079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansen TW, Staessen JA, Torp-Pedersen C, et al. Ambulatory arterial stiffness index predicts stroke in a general population. J Hypertens. 2006;24:2247–2253. doi: 10.1097/01.hjh.0000249703.57478.78. [DOI] [PubMed] [Google Scholar]
- 26.Dart AM, Kingwell BA. Pulse pressure: a review of mechanisms and clinical relevance. J Am Coll Cardiol. 2001;37:975–984. doi: 10.1016/s0735-1097(01)01108-1. [DOI] [PubMed] [Google Scholar]
- 27.Akselrod S, Amitayt Y, Lang RM, Mor-Avi V, Keselbrener L. Spectral analysis of left ventricular area variability as a tool to improve the understanding of cardiac autonomic control. Physiol Meas. 2000;21:319–331. doi: 10.1088/0967-3334/21/2/311. [DOI] [PubMed] [Google Scholar]
- 28.Gkaliagkousi E, Douma S. The pathogenesis of arterial stiffness and its prognostic value in essential hypertension and cardiovascular diseases. Hippokratia. 2009;13:70–75. [PMC free article] [PubMed] [Google Scholar]