Abstract
Background
Both maternal obesity and inflammatory bowel diseases (IBDs) are increasing. It was hypothesized that maternal obesity induces an inflammatory response in the fetal large intestine, predisposing offspring to IBDs.
Methods
Nonpregnant ewes were assigned to a control (Con, 100% of National Research Council [NRC] recommendations) or obesogenic (OB, 150% of NRC) diet from 60 days before conception. The large intestine was sampled from fetuses at 135 days (term 150 days) after conception and from offspring lambs at 22.5 ± 0.5 months of age.
Results
Maternal obesity enhanced mRNA expression tumor necrosis factor (TNF)α, interleukin (IL)1α, IL1β, IL6, IL8, and monocyte/macrophage chemotactic protein-1 (MCP1), as well as macrophage markers, CD11b, CD14, and CD68 in fetal gut. mRNA expression of Toll-like receptor (TLR) 2 and TLR4 was increased in OB versus Con fetuses; correspondingly, inflammatory NF-κB and JNK signaling pathways were also upregulated. Both mRNA expression and protein content of transforming growth factor (TGF) β was increased. The IL-17A mRNA expression and protein content was higher in OB compared to Con samples, which was associated with fibrosis in the large intestine of OB fetuses. Similar inflammatory responses and enhanced fibrosis were detected in OB compared to Con offspring.
Conclusions
Maternal obesity induced inflammation and enhanced expression of proinflammatory cytokines in fetal and offspring large intestine, which correlated with increased TGFβ and IL17 expression. These data show that maternal obesity may predispose offspring gut to IBDs.
Keywords: inflammatory bowel disease, gut, sheep, fetus, inflammation, obesity, offspring
Obesity has become a serious problem in the United States, affecting around one-third of the total population.1 According to the National Health and Nutrition Examination Survey (1999–2002), 29% of nonpregnant women between 20–39 years of age are obese and there is a continuing rapid increase in obesity. Maternal obesity (MO) negatively affects maternal health and fetal development that can result in harmful, persistent effects in offspring.2–4 Inflammatory disorders of intestine, including pediatric Crohn’s disease (CD), is also increasing.5–8 MO is associated with low-grade fetal inflammation.9 Since the gastrointestinal (GI) tract is a major immune organ, we hypothesize that MO leads to inflammation in fetal GI, which has a persistent effect on offspring gut raising the possibility of inflammatory bowel diseases (IBDs).
Sheep have been extensively used as a model for human pregnancy studies.10–15 As a precocial species such as humans, sheep pregnancy studies provide power for translation to human pregnancy, which is in contrast to altricial species such as rodents.16,17 The intestinal mucosal immune system develops around midgestation in sheep and humans.18 Lymphoid follicles are lymphoid tissues distributed along the large intestine. In the small intestine, lymphoid follicles aggregate to form Peyer’s patches. Lymphoid follicles and Peyer’s patches are key sites for the mucosal immune response in the GI tract. T-lymphocytes are activated in lymphoid follicles and then differentiate into T-helper (Th) 1 cells responsible for the cell-mediated response, or Th2 cells which mediate humoral immune responses.19 At the same time in gestation, a group of T cells differentiate into regulatory T cells (Tregs), which mediate overall immune responses.20 Recently, a new population of T cells, proinflammatory Th17 cells, has been identified, which are involved in intestinal inflammation and are associated with the development of CD.21 Transforming growth factor (TGF) β promotes differentiation of Th17 cells.22 Therefore, we hypothesize that MO induces an inflammatory response in the fetal large intestine which activates TGF-β and Th17 differentiation, predisposing offspring gut to IBDs. Using the pregnant sheep model, we investigated the impact of MO on the inflammation in the fetal large intestine in late gestation and offspring gut. Our data showed that MO upregulates the expression of inflammation cytokines and Toll-like receptors (TLR), and nuclear factor kappa B (NF-κB) and c-Jun N-terminal kinase (JNK) pathways, as well as the TGFβ pathway and Th17 differentiation in the large intestine of fetuses of obese mothers, which elicited persistent effects on offspring gut.
MATERIALS AND METHODS
Care and Use of Animals
All animal procedures were approved by the University of Wyoming Animal Care and Use Committee. Multiparous Rambouillet/Columbia ewes were studied. Ewes were all mated to a single ram. From 60 days before conception to day 135 of gestation (first day of mating = d0), ewes were individually fed either a highly palatable diet at 100% (Con) of National Research Council (NRC) recommendations for energy23 (n = 20), or 150% (OB) of recommended energy requirements for early gestation (n = 20), as previously reported.24,25 Ewes were housed in individual pens in a temperature-controlled room (≈20°C). All ewes were weighed at weekly intervals and rations were adjusted for weekly changes in metabolic body weight (BW0.75).26,27 Body condition was scored at monthly intervals to evaluate changes in fatness. A body condition score of 1 (emaciated) to 9 (obese) was assigned by two trained observers after palpation of the transverse and vertical processes of the lumbar vertebrae (L2 through L5) and the region around the tail head.28 Ewes carrying twin fetuses were utilized in the d135 fetal study. Ewes carrying singleton fetuses were allowed to lamb and male offspring from Con ewes (n = 6) and OB ewes (n = 6) were randomly selected for further study. Lambs were given free access to a standard commercially available creep feed (Lamb Creep B30 w/Bovatec; Ranch-way Feeds, Ft. Collins, CO) from birth to weaning. At 120 ± 3 days of age lambs were weaned, maintained together, and fed a diet of high-quality alfalfa hay and corn to NRC requirements till 19.5 ± 0.5 months of age, followed by a 12-week ad libitum feeding before necropsy at 22.5 ± 0.5 months of age. The detailed feeding regimen has been described previously.29
Tissue Collection
Immediately before necropsy on d135 of gestation (Term 150 days), ewes were weighed and sedated with intravenous ketamine (22.2 mg/kg) and anesthesia was maintained by isoflurane inhalation (0.5%–2.5%). Ewes were exsanguinated via heart puncture while under general anesthesia and fetuses quickly removed. The fetus was then weighed, eviscerated, and weighed again. Four twin pregnancies in each group were selected. Fetal gender was balanced between treatment groups (7 male fetuses and 1 female fetus in each group). Fetal large intestine (the complete spiral region ≈15 cm long) was collected. The middle segment (≈2 cm) of the spiral region was placed in a tissue cassette (Tissue Tek, Miles Labs, Elkhart, IN) and fixed with 4% (w/v) paraformaldehyde in a phosphate buffer (0.12 M PBS; pH 7.4) and paraffin-embedded for histochemical analyses. The rest of segments were cut open to remove the contents, washed in PBS buffer, and frozen in liquid nitrogen for Western blotting and real-time quantitative polymerase chain reaction (PCR) analyses.
At 22.5 ± 0.5 months of age, male offspring were euthanized and the large intestine (the middle portion of spiral region ≈8 cm long) was sampled and utilized for biochemical analyses.
Antibodies
Antibodies against phos-SAPK/JNK (Thr183/Tyr185) (Cat. No. 9251), SAPK/JNK (Cat. No. 9252), phos-c-Jun (Ser63) (Cat. No. 9261), phos-c-Jun (Ser73) (Cat. No. 9164), c-Jun (60A8) (Cat. No. 9165), phos-IKKα/β (Ser176/180) (Cat. No. 2697), IκBα (Cat. No. 4814), phos-IκBα (Cat. No. 2859), phos-NF-kB p65 (Ser536) (Cat. No. 3033) and NF-kB p65 (Cat. No. 4764), TGFβ (Cat. No. 3709), and TNFα (Cat. No. 3707) were purchased from Cell Signaling (Danvers, MA). Anti-IL17 (Cat. No. sc-6076) antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-β-tubulin (Cat. No. T4026) antibody was purchased from Sigma (St. Louis, MO). IRDye 800CW goat antirabbit secondary antibody and IRDye 680 goat antimouse secondary antibody were purchased from LI-COR Biosciences (Lincoln, NE).
Histochemical Analyses
The middle portion of the spiral region of the fetal large intestine was fixed in 4% (w/v) paraformaldehyde in phosphate buffer (0.12 M; pH 7.4), embedded in paraffin, and sectioned at 5 μm. Twelve 5-μm sections evenly spaced over a 450-μm area of each large intestine were evaluated for collagen content. Sections were rehydrated by a series of incubations in xylene and ethanol solutions, then stained with Masson Trichrome stain30 which stains smooth muscle cells red, nuclei black, and collagen blue. Six fields per sample were randomly selected for quantification of collagen/total area ratio using the ImageJ 1.30v software (National Institutes of Health, Bethesda, MD). In addition, the thickness of the intestinal wall was also measured using the ImageJ 1.30v software and expressed as the relative thickness versus Con group.
Collagen Content Analyses
Intestine samples were ground and dried in a convection oven at 60°C and the samples were weighed and hydrolyzed in 6 N HCl at 105°C for 16 hours. An aliquot was removed for hydroxyproline determination using the method of Woessner.31 Collagen concentration (mg/mg dry sample weight) was calculated assuming collagen weighs 7.25 times the measured weight of hydroxyproline.
Real-Time Quantitative PCR (qRT-PCR)
Total RNA was extracted using Trizol Reagent (Sigma), treated with DNase I (Qiagen, Valencia, CA), and purified with the RNeasy Mini kit (Qiagen). cDNA was synthesized with the SuperScript III first-strand synthesis kit (Invitrogen, Carlsbad, CA). Real-time qRT-PCR was conducted on a Bio-Rad Laboratories (Hercules, CA) iQ5 machine. Primers used were synthesized by Invitrogen. β-Tubulin was used as the housekeeping gene. Primer sequences are listed in Table 1. Sybr Green Master Mix (Bio-Rad) was used in all PCR reactions (20 μL total volume). The final primer concentration was 200 nM for each gene. The amplification efficiency was 0.90–0.99. The qRT-PCR conditions were 95°C, 3 minutes; 35 cycles of 95°C for 10 seconds, 56°C for 20 seconds, and 72°C for 20 seconds. At the end of each run, dissociation melt curves were obtained to confirm the purity of PCR product.
TABLE 1.
Primer Sets Used for Real-time RT-PCR
| Gene Name | Product Size | Direction | Sequence |
|---|---|---|---|
| CD11b | 80bp | Forward: Reverse: |
GTCATTGGGGTGGGAGATG TCAGCAGGGGGCTTAGATG |
| CD14 | 98bp | Forward: Reverse: |
CTCAGCGTGCTTGATCTCAG AAGGGATTTCCGTCCAGAGT |
| CD68 | 144bp | Forward: Reverse: |
CAGGGGACAGGGAATGACT CCAAGTGGTGGTTCTGTGG |
| IL1α | 93bp | Forward: Reverse: |
GTGCTCAAAATGAAGACGAACC CCCAGAAGAAGAGGAGATTGGT |
| IL1β | 85bp | Forward: Reverse: |
CGTCTTCCTGGGACGTTTTAG CTGCGTATGGCTTCTTTAGGG |
| IL6 | 116bp | Forward: Reverse: |
TCATCCTGAGAAGCCTTGAGA TTTCTGACCAGAGGAGGGAAT |
| IL8 | 132bp | Forward: Reverse: |
GCTGGCTGTTGCTCTCTTG AATTTGGGGTGGAAAGGTG |
| IL17A | 111bp | Forward: Reverse: |
TGCTACTGCTTCTGAGTCTGGTGGC TGACCCTCACATGCTGTGGGAAGTT |
| MCP1 | 133bp | Forward: Reverse: |
CCAGCAGCAAGTGTCCTAAAG GGCTTTGGAGTTTGGTTTTTC |
| TGFβ | 149bp | Forward: Reverse: |
AAAAGAACTGCTGTGTTCGTCA GACCTTGCTGTACTGTGTGTCC |
| TLR2 | 102bp | Forward: Reverse: |
CAAGAGGAAGCCCAGGAAG TGGACCATGAGGTTCTCCA |
| TLR4 | 149bp | Forward: Reverse: |
TGCTGGCTGCAAAAAGTATG CCCTGTAGTGAAGGCAGAGC |
| TNFα | 103bp | Forward: Reverse: |
ACACCATGAGCACCAAAAGC AGGCACAAGCAACTTCTGGA |
| β-tubulin | 141bp | Forward: Reverse: |
CGAGAGCTGTGACTGTCTGC GGCATGACGCTAAAGGTGTT |
Immunoblotting Analysis
Immunoblot analysis was conducted according to the procedures previously described.24,32 Membranes were visualized by Odyssey Infrared Imaging System (LI-COR Biosciences). Density of bands was quantified and then normalized with reference to the β-tubulin content.
Statistical Analysis
Statistical analyses were conducted as previously described in this model.24,32,33 Briefly, each fetus was considered an experimental unit. Data were analyzed as a complete randomized design using GLM (General Linear Model of Statistical Analysis System, SAS, Cary, NC, 2000). Mean ± standard errors of mean (SEM) are reported. Statistical significance is considered at P < 0.05.
RESULTS
Animal Weight
Before assignment to dietary treatments, there was no difference in body weight or body condition score among the ewes, while at the end of the treatment period both maternal body weight and body condition score were higher in OB than Con groups (P < 0.05), indicating that excessive nutrition had resulted in MO (Table 2).
TABLE 2.
Maternal and Fetal Body Weight of Control and Obese Sheep
| Category | Con | OB | Significance |
|---|---|---|---|
| Maternal | |||
| At the beginning of treatments | |||
| Body condition score | 5.5 ± 0.3 | 5.9 ± 0.3 | NS |
| Body weight (kg) | 75.7 ± 7.3 | 65.1 ± 4.7 | NS |
| At the end of treatments | |||
| Body condition score | 6.1 ± 0.4 | 8.5 ± 0.4 | P < 0.05 |
| Body weight (kg) | 90.4 ± 7.7 | 107.6 ± 7.1 | P < 0.05 |
| Fetal | |||
| Body weight (g) | 5180.3 ± 232.4 | 4977.1 ± 268.5 | NS |
Means ± SEM. n = 8. control (Con) and obese (OB) ewes.
Inflammatory Cytokine and Chemokine Expression in Fetal Intestine
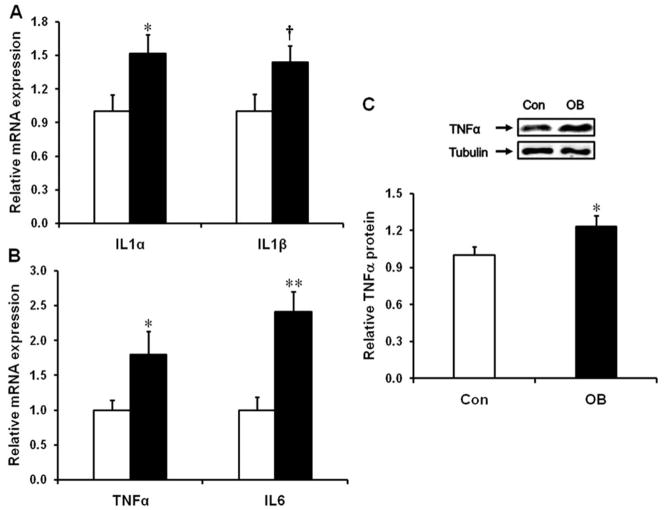
RT-PCR results showed a higher expression of interleukin (IL)-1α mRNA (51.9 ± 16.4%, P < 0.05) and a trend of increase for IL-1β mRNA (44.0 ± 14.5%, P = 0.06) (Fig. 1A). mRNA expression of TNF-α increased by 79.5 ± 33.3% (P < 0.05) (Fig. 1B), and the protein content of TNF-α increased by 23.0 ± 8.9% (P < 0.05) (Fig. 1C). IL-6 mRNA was also markedly increased (140.9 ± 29.3%, P < 0.01) in the large intestine of fetuses from OB compared to Con mothers (Fig. 1B).
FIGURE 1.
mRNA expression of inflammatory cytokines in the d135 fetal large intestine of control (Con□) and obese (OB■) sheep. (A) mRNA expression of IL1α and IL1β. (B) mRNA expression of TNFα and IL6. (C) Protein content of TNFα (mean ± SEM; *P<0.05; **P<0.01; †: P < 0.10; n = 8 in each group).
In addition, mRNA for monocyte activation marker cluster of differentiation (CD)11b (98.5 ± 41.7% higher, P < 0.05) and macrophage differentiation and maturation markers CD14 (68.8 ± 16.9% higher, P < 0.01) and CD68 (227.0 ± 52.3% higher, P < 0.01) were all increased in the fetal large intestine of OB sheep (Supporting Fig. 1A). Consistently, the mRNA expression of IL-8 was dramatically increased in the fetal large intestine of OB sheep (90.5 ± 22.4% higher, P < 0.01), while mRNA level of monocyte chemotactic protein-1 (MCP-1) tended to increase (37.7 ± 12.7%, P = 0.06) (Supporting Information Fig. 1B).
Inflammation Signaling in Fetal Large Intestine
Expression of mRNA for the pattern recognition receptors (PRRs), TLR2 and TLR4 was increased by 62.7 ± 23.4% (P < 0.05) and 45.5 ± 6.9% (P < 0.01), respectively, in OB compared to Con fetuses (Fig. 2). Western blotting further indicated that IKKβ phosphorylation was increased in the OB compared to Con fetal intestine (51.6 ± 12.6%, P < 0.05) (Fig. 3A). The phosphorylation of NF-κB subunit p65 increased by 37.2 ± 8.7% (P < 0.05) (Fig. 3C). There was a trend of decrease in IκB α total protein content (17.1 ± 5.5%, P = 0.05), but no significant increase in the phosphorylation of IκBα (Fig. 3B) in the OB fetal large intestine.
FIGURE 2.
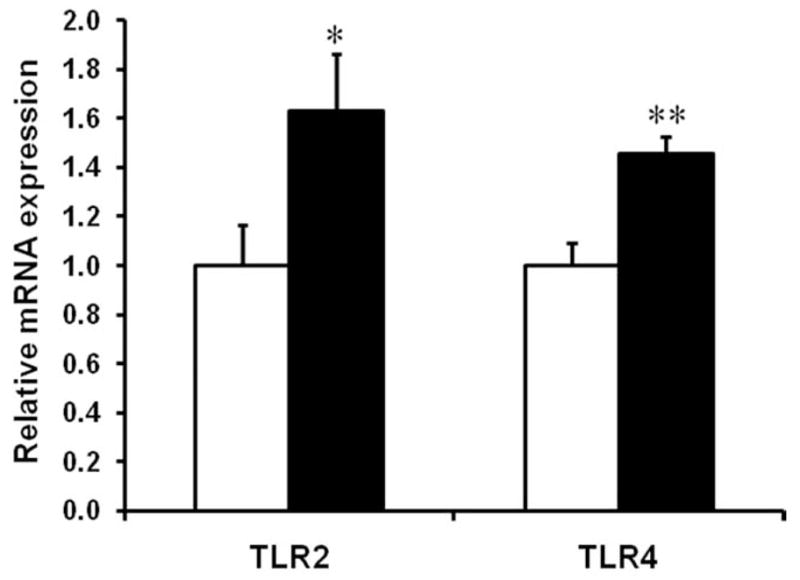
TLR2 and TLR4 mRNA content in the d135 fetal large intestine of Con (□) and OB (■) sheep (mean ± SEM; *P < 0.05; **P < 0.01; n = 8 in each group).
FIGURE 3.
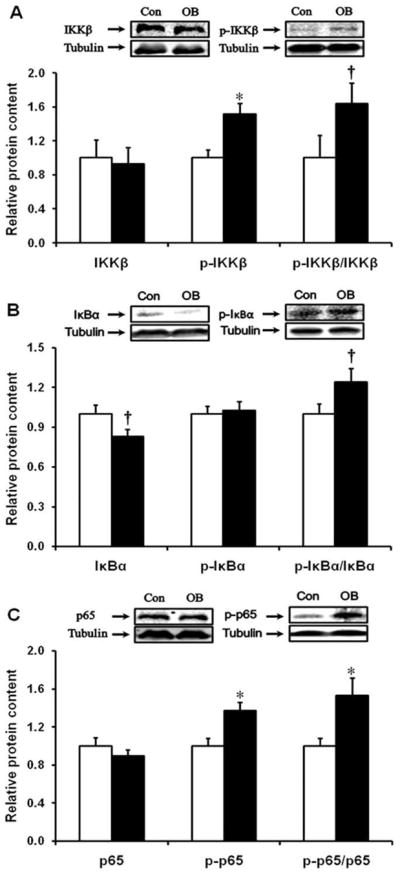
NF-κB inflammatory signaling pathway in d135 fetal large intestine of Con (□) and OB (■) sheep. (A) IKKβ and phospho-IKKβ content. (B) Inhibitor of κB and the phosphorylation of Inhibitor of κB. (C) NF-κB subunit p65 and phospho-p65 content (mean ± SEM; *P < 0.05; †: P < 0.10; n = 8 in each group).
Phosphorylation of JNK (Thr183/Tyr185) was increased by 80.7 ± 24.6% (P < 0.05) (Supporting Fig. 2A), and its downstream target phos-c-Jun at Ser63 (P < 0.05) and Ser73 (P = 0.05) sites was increased by 41.1 ± 18.4% and 47.9 ± 14.3%, respectively, in the OB fetal large intestine (Supporting Information Fig. 2B).
TGF-β and Fibrosis in Fetal Large Intestine
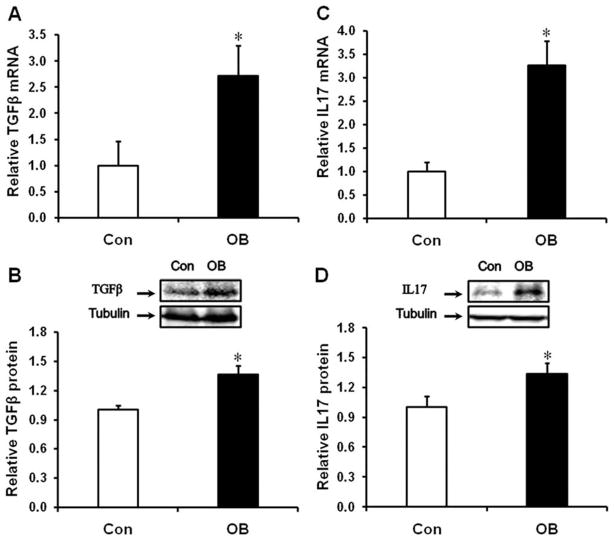
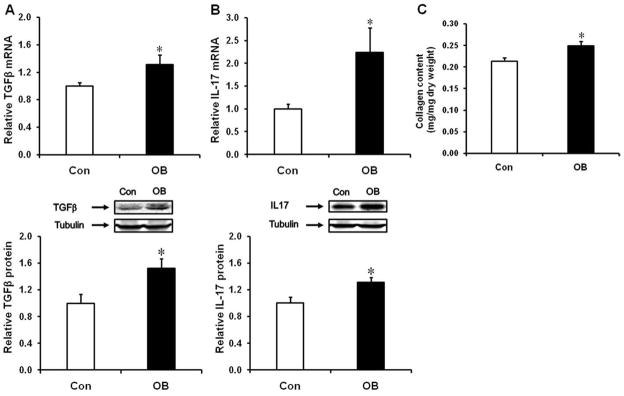
Both mRNA (Fig. 4A, P < 0.05) and protein (Fig. 4B, P < 0.05) content of TGF-β were increased in OB compared to Con fetal intestine, and the mRNA expression and protein content of IL-17, a cytokine marker of Th17 cells, was higher in OB compared to Con fetal large intestine (Fig. 4C, D).
FIGURE 4.
TGFβ and IL17 mRNA and protein content in d135 fetal large intestine of Con (□) and OB (■) sheep. (A) TGFβ mRNA expression. (B) TGFβ protein content. (C) IL17 mRNA expression. (D: IL17 protein content (Mean ± SEM; *P < 0.05; n = 8 in each group).
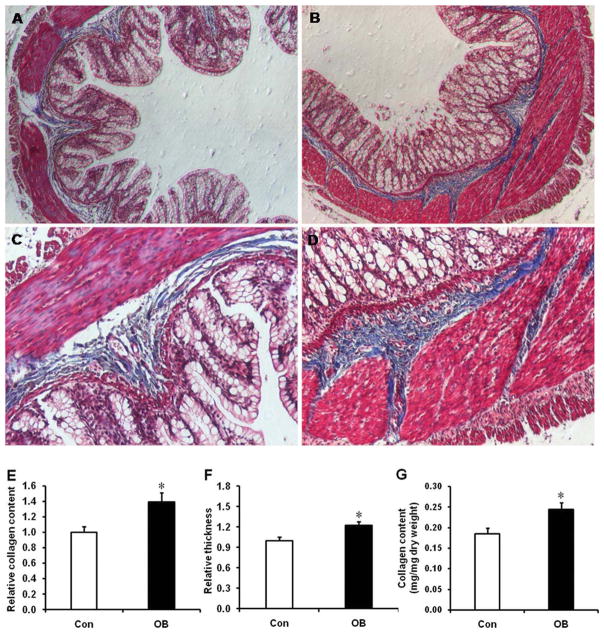
TGFβ is known to induce fibrogenesis. Using Trichrome staining, a higher collagen content and infiltration was observed in the OB group (Fig. 5). A greater collagen/total area ratio was observed in OB (38.9 ± 19.0%, P < 0.05) compared to Con fetal intestine (Fig. 5E). In addition, the intestinal wall was thicker in the fetal intestine of OB compared to that of Con ewes (Fig. 5F). The collagen content was also higher in OB compared to Con fetuses (Fig. 5G).
FIGURE 5.
Masson Trichrome Staining for the d135 fetal large intestine of Con (□) and OB (■) sheep. (A) Large intestine of Con sheep at 40 × magnification. (B) Large intestine of OB sheep at 40 × magnification. (C) Large intestine of Con sheep at 100 × magnification. (D) Large intestine of OB sheep at 100 × magnification. (E) Relative collage content measured as the ratio of collagen/total area. (F) Relative wall thickness of intestine. (G) The content of collagen (mean ± SEM; *P < 0.05; n = 8 in each group). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
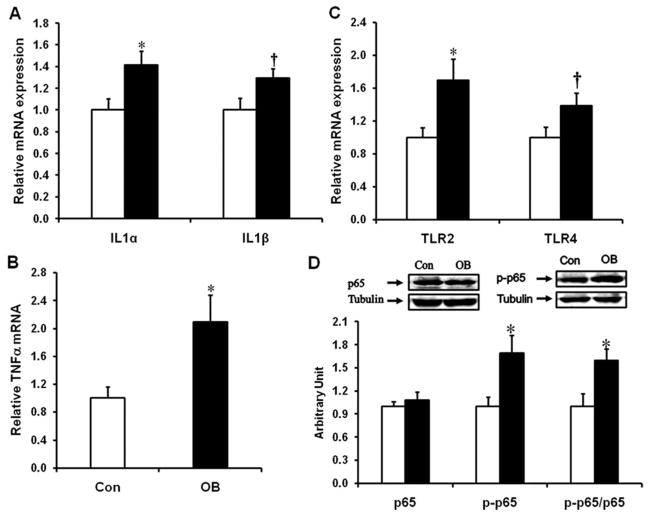
Inflammation and Fibrosis Were Detected in the Large Intestine of OB Offspring
The mRNA expression of inflammatory cytokines, IL1α, IL1β, and TNFα, was enhanced in the large intestine of OB offspring (Fig. 6A,B); the mRNA expression of TLR2 and TLR4 was also enhanced (Fig. 6C). Consistently, phosphorylation of NF-κB subunit p65 was also higher in OB compared to Con offspring gut, showing an inflammatory response (Fig. 6D).
FIGURE 6.
The expression of inflammatory cytokines, TLR2 and TLR4, and activation of inflammatory signaling in the offspring large intestine of Con (□) and OB (■) sheep. (A) IL1 mRNA expression. (B) TNFα mRNA expression. (C) TLR2 and TLR4 mRNA expression. (D) p65 and phospho-p65 content (mean ± SEM; *P < 0.05; †P < 0.1; n = 6 in each group).
As observed in the fetal intestine, the mRNA expression and protein content of both TGFβ and IL17 were also enhanced in OB compared to Con offspring gut (Fig. 7A,B). Further, the content of collagen was higher in OB offspring gut (Fig. 7C).
FIGURE 7.
TGFβ and IL17 expression, and collagen content in offspring large intestine of Con (□) and OB (■) sheep. (A) TGFβ mRNA expression and protein content. (B) IL17 mRNA expression and protein content. (C) Collagen content (mean ± SEM; *P < 0.05; n = 6 in each group).
DISCUSSION
Importance of the Fetal Stages of Mucosal Immune System Development
The intestinal immune system develops during fetal life and matures following exposure to microflora after birth.18 The lymphoid tissues developed in the fetal intestine include lymphoid follicles along the large intestine and Peyer’s patches in the small intestine. T-lymphocytes are crucial for immune responses and their differentiation from naïve T-lymphocytes is actively proceeding in the fetal intestine. There are four major populations of differentiated T-lymphocytes, Th1, Th2, and Treg, as well as the recently identified Th17 cells.22 Th1 cells are mainly involved in cell-mediated responses while Th2 cells mediate the humoral immune response.19 Differentiation of Th1 and Th2 is largely mediated by the presence/absence of proinflammatory cytokines.34–36 Both Th1 and Th2 activation have been linked to IBDs.37,38 Recent studies indicate the importance of Treg and Th17 in the development of IBDs. Treg suppresses both Th1 and Th2 activation, thereby exerting a regulatory effect on immune responses.39 Th17 cells are a recently identified distinct population of inflammatory T-cells proven to be crucial for the development of IBDs.40 The differentiation of Th17 cells is driven by TGFβ and the inflammatory cytokine, IL6.20 In addition, Treg cells convert into Th17 cells in the presence of inflammation.41 Therefore, the presence of an inflammatory response can influence differentiation of T-cells, resulting in Th17 dominance with the potential predisposition of the intestine to IBDs. This sequence of changes suggests a possible association of the inflammatory response we have seen in fetal and offspring gut of obese mothers, and the surge in IBDs in recent years.6–8
Inflammation in the Fetal Large Intestine of Obese Mothers
Toll-like receptors belong to a family of pattern recognition receptors that play an important role in inducing inflammation.42 NF-κB is an important downstream mediator of TLR2 and TLR4-induced inflammatory response.43 In the quiescent state, NF-κB binds to IκB. When signals through TLRs activate the IKKs, IKKα, and IKKβ, they phosphorylate IκB resulting in degradation of these inhibitors. This process releases NF-κB from IκB and allows the translocation of NF-κB to the nucleus where it activates transcription of specific genes.44 In this study, mRNA expression of TLR2 and TLR4, and the NF-κB pathway was enhanced in the OB fetuses and offspring, indicating the presence of an inflammatory response.
Inflammation is accompanied by increased expression of inflammatory cytokines. We observed a higher expression of inflammatory cytokines in the large intestine of fetuses and offspring of obese sheep, including a dramatic increase in IL6, which is necessary for the Th17 differentiation.20 Macrophages, upon activation, are the main source of proinflammatory cytokines.45 Therefore, we further analyzed the mRNA expression of macrophage markers, CD11b, CD14, and CD68, which were all upregulated in the OB group. In addition, IL8, a chemokine responsible for recruiting macrophages to a site of infection, was also more abundant in the OB fetal large intestine compared to Con intestine. Altogether, these results suggest that MO induced inflammatory response in the fetal large intestine of OB sheep which demonstrated persistent effects on OB offspring gut.
Enhancement of TGFβ and fibrosis in OB Fetal Large Intestine
TGFβ regulates the differentiation of naïve T-cells via promotion of Th17 differentiation.46 In this study we observed that TGFβ protein content and mRNA expression were both increased in OB fetal large intestine, in combination with a dramatically higher level of IL6 and inflammation which should lead to increased Th17 differentiation. Indeed, both mRNA expression and protein content of the cytokine marker of Th17, IL17, was much higher in the OB fetal intestine. We also observed the increased IL17 mRNA and protein content in offspring OB gut. These data demonstrated that MO enhances Th17 differentiation, which might partially explain the surge in IBDs in recent years in developed countries in which there has been a surge in maternal obesity.6–8
Intestinal strictures are commonly found in IBDs, such as CD, which is associated with deposition of collagen.47 TGFβ induces fibrogenesis and accumulation of collagen in the deeper layers of the gut.48 As reported above, we observed collagen infiltration and accumulation in the fetal and offspring large intestine of OB sheep.
In conclusion, our findings demonstrate that MO stimulates production of inflammatory cytokines and enhanced inflammation signaling in the late gestation fetal large intestine, accompanied by increased TGFβ expression and fibrosis in the fetal gut. TGFβ in combination with inflammatory cytokines such as IL6 drives naïve T-cell differentiation to Th17 cells, which further promotes inflammation. In addition, we observed that inflammation persists in the OB offspring gut, which may predispose offspring gut to IBDs.
Supplementary Material
Acknowledgments
The authors thank Adam Uthlaut and Dr. Nathan Long for assistance with animal care and tissue collection.
Supported by USDA-NRI 2008-35203-19084 and NIH INBRE P20RR016474.
Footnotes
Additional supporting information may be found in the online version of this article.
References
- 1.Hedley AA, Ogden CL, Johnson CL, et al. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 2.Fowden AL, Ward JW, Wooding FP, et al. Programming placental nutrient transport capacity. J Physiol. 2006;572:5–15. doi: 10.1113/jphysiol.2005.104141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathanielsz PW. Animal models that elucidate basic principles of the developmental origins of adult diseases. Ilar J. 2006;47:73–82. doi: 10.1093/ilar.47.1.73. [DOI] [PubMed] [Google Scholar]
- 4.Barker DJ. Fetal programming of coronary heart disease. Trends Endocrinol Metab. 2002;13:364–368. doi: 10.1016/s1043-2760(02)00689-6. [DOI] [PubMed] [Google Scholar]
- 5.Meinzer U, Idestrom M, Alberti C, et al. Ileal involvement is age dependent in pediatric Crohn’s disease. Inflamm Bowel Dis. 2005;11:639–644. doi: 10.1097/01.mib.0000165114.10687.bf. [DOI] [PubMed] [Google Scholar]
- 6.Perminow G, Brackmann S, Lyckander LG, et al. A characterization in childhood inflammatory bowel disease, a new population-based inception cohort from south-eastern Norway, 2005–07, showing increased incidence in Crohn’s disease. Scand J Gastroenterol. 2009;44:446–456. doi: 10.1080/00365520802647434. [DOI] [PubMed] [Google Scholar]
- 7.Grieci T, Butter A. The incidence of inflammatory bowel disease in the pediatric population of southwestern Ontario. J Pediatr Surg. 2009;44:977–980. doi: 10.1016/j.jpedsurg.2009.01.038. [DOI] [PubMed] [Google Scholar]
- 8.Rufo PA, Bousvaros A. Challenges and progress in pediatric inflammatory bowel disease. Curr Opin Gastroenterol. 2007;23:406–412. doi: 10.1097/MOG.0b013e3281b115c2. [DOI] [PubMed] [Google Scholar]
- 9.Yan X, Zhu MJ, Xu W, et al. Up-regulation of Toll-like receptor 4/nuclear factor-{kappa}B signaling is associated with enhanced adipogenesis and insulin resistance in fetal skeletal muscle of obese sheep at late gestation. Endocrinology. 2010;151:380–387. doi: 10.1210/en.2009-0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson MS, Thamotharan M, Kao D, et al. Effects of acute hyper-insulinemia on insulin signal transduction and glucose transporters in ovine fetal skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2005;288:R473–481. doi: 10.1152/ajpregu.00405.2004. [DOI] [PubMed] [Google Scholar]
- 11.Capper JL, Wilkinson RG, Mackenzie AM, et al. Polyunsaturated fatty acid supplementation during pregnancy alters neonatal behavior in sheep. J Nutr. 2006;136:397–403. doi: 10.1093/jn/136.2.397. [DOI] [PubMed] [Google Scholar]
- 12.Cleal JK, Poore KR, Boullin JP, et al. Mismatched pre- and postnatal nutrition leads to cardiovascular dysfunction and altered renal function in adulthood. Proc Natl Acad Sci U S A. 2007;104:9529–9533. doi: 10.1073/pnas.0610373104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kramer BW, Kallapur SG, Moss TJ, et al. Intra-amniotic LPS modulation of TLR signaling in lung and blood monocytes of fetal sheep. Innate Immun. 2009;15:101–107. doi: 10.1177/1753425908100455. [DOI] [PubMed] [Google Scholar]
- 14.Skopal-Chase JL, Pixley JS, Torabi A, et al. Immune ontogeny and engraftment receptivity in the sheep fetus. Fetal Diagn Ther. 2009;25:102–110. doi: 10.1159/000203399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sozo F, O’Day L, Maritz G, et al. Repeated ethanol exposure during late gestation alters the maturation and innate immune status of the ovine fetal lung. Am J Physiol Lung Cell Mol Physiol. 2009;296:L510–518. doi: 10.1152/ajplung.90532.2008. [DOI] [PubMed] [Google Scholar]
- 16.Regnault TR, Friedman JE, Wilkening RB, et al. Fetoplacental transport and utilization of amino acids in IUGR—a review. Placenta. 2005;26 (Suppl A):S52–62. doi: 10.1016/j.placenta.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Anthony RV, Scheaffer AN, Wright CD, et al. Ruminant models of prenatal growth restriction. Reprod Suppl. 2003;61:183–194. [PubMed] [Google Scholar]
- 18.Griebel P, Hein WR, Dudler L, et al. Phenotype and function of stromal cells cloned from the ileal Peyer’s patch of sheep. Stem Cells. 1993;11:130–143. doi: 10.1002/stem.5530110208. [DOI] [PubMed] [Google Scholar]
- 19.Constant SL, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu Rev Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 20.Mucida D, Park Y, Kim G, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 21.Brand S. Crohn’s disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn’s disease. Gut. 2009;58:1152–1167. doi: 10.1136/gut.2008.163667. [DOI] [PubMed] [Google Scholar]
- 22.Qin H, Wang L, Feng T, et al. TGF-beta promotes Th17 cell development through inhibition of SOCS3. J Immunol. 2009;183:97–105. doi: 10.4049/jimmunol.0801986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Research Council. Nutrient requirements of sheep. 6. Washington, DC: National Academy Press; 1985. [Google Scholar]
- 24.Zhu MJ, Han B, Tong J, et al. AMP-activated protein kinase signalling pathways are down regulated and skeletal muscle development impaired in fetuses of obese, over-nourished sheep. J Physiol. 2008;586:2651–2664. doi: 10.1113/jphysiol.2007.149633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tong JF, Yan X, Zhu MJ, et al. Maternal obesity downregulates myogenesis and beta-catenin signaling in fetal skeletal muscle. Am J Physiol Endocrinol Metab. 2009;296:E917–924. doi: 10.1152/ajpendo.90924.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corbett SW, Keesey RE. Energy balance of rats with lateral hypothalamic lesions. Am J Physiol. 1982;242:E273–279. doi: 10.1152/ajpendo.1982.242.4.E273. [DOI] [PubMed] [Google Scholar]
- 27.Ford SP, Zhang L, Zhu MJ, et al. Maternal obesity accelerates fetal pancreatic beta-cell but not alpha-cell development in the sheep: prenatal and postnatal consequences. Am J Physiol Regul Integr Comp Physiol. 2009;297:R835–843. doi: 10.1152/ajpregu.00072.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanson DW, West TR, Tatman WR, et al. Relationship of body composition of mature ewes with condition score and body weight. J Anim Sci. 1993;71:1112–1116. doi: 10.2527/1993.7151112x. [DOI] [PubMed] [Google Scholar]
- 29.Long NM, George LA, Uthlaut AB, et al. Maternal obesity and high nutrient intake before and during gestation in the ewe results in altered growth, adiposity, and glucose tolerance in adult offspring. J Anim Sci. 2010;88:3546–3553. doi: 10.2527/jas.2010-3083. [DOI] [PubMed] [Google Scholar]
- 30.Carson FL. Histotechnology. 2. American Society for Clinical Pathology; 2007. [Google Scholar]
- 31.Woessner JF. The determination of hydroxyproline in tissue and protein samples containing small proportions of this amino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 32.Zhu MJ, Ford SP, Means WJ, et al. Maternal nutrient restriction affects properties of skeletal muscle in offspring. J Physiol. 2006;575:241–250. doi: 10.1113/jphysiol.2006.112110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu MJ, Ford SP, Nathanielsz PW, et al. Effect of maternal nutrient restriction in sheep on the development of fetal skeletal muscle. Biol Reprod. 2004;71:1968–1973. doi: 10.1095/biolreprod.104.034561. [DOI] [PubMed] [Google Scholar]
- 34.Ridyard AE, Nuttall TJ, Else RW, et al. Evaluation of Th1, Th2 and immunosuppressive cytokine mRNA expression within the colonic mucosa of dogs with idiopathic lymphocytic-plasmacytic colitis. Vet Immunol Immunopathol. 2002;86:205–214. doi: 10.1016/s0165-2427(02)00039-9. [DOI] [PubMed] [Google Scholar]
- 35.Beckett CG, Dell’Olio D, Kontakou M, et al. Analysis of interleukin-4 and interleukin-10 and their association with the lymphocytic infiltrate in the small intestine of patients with coeliac disease. Gut. 1996;39:818–823. doi: 10.1136/gut.39.6.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lorentz A, Bischoff SC. Regulation of human intestinal mast cells by stem cell factor and IL-4. Immunol Rev. 2001;179:57–60. doi: 10.1034/j.1600-065x.2001.790106.x. [DOI] [PubMed] [Google Scholar]
- 37.Fuss IJ, Neurath M, Boirivant M, et al. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996;157:1261–1270. [PubMed] [Google Scholar]
- 38.Eri R, Kodumudi KN, Summerlin DJ, et al. Suppression of colon inflammation by CD80 blockade: evaluation in two murine models of inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:458–470. doi: 10.1002/ibd.20344. [DOI] [PubMed] [Google Scholar]
- 39.Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol. 2009;27:313–338. doi: 10.1146/annurev.immunol.021908.132657. [DOI] [PubMed] [Google Scholar]
- 40.Boniface K, Blom B, Liu YJ, et al. From interleukin-23 to T-helper 17 cells: human T-helper cell differentiation revisited. Immunol Rev. 2008;226:132–146. doi: 10.1111/j.1600-065X.2008.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kitani A, Xu L. Regulatory T cells and the induction of IL-17. Mucosal Immunol. 2008;1(suppl 1):S43–46. doi: 10.1038/mi.2008.51. [DOI] [PubMed] [Google Scholar]
- 42.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 43.Yuan M, Konstantopoulos N, Lee J, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 44.Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107:135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Batista ML, Jr, Santos RV, Cunha LM, et al. Changes in the proinflammatory cytokine production and peritoneal macrophage function in rats with chronic heart failure. Cytokine. 2006;34:284–290. doi: 10.1016/j.cyto.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 46.Monteleone G, Boirivant M, Pallone F, et al. TGF-beta1 and Smad7 in the regulation of IBD. Mucosal Immunol. 2008;1(suppl 1):S50–53. doi: 10.1038/mi.2008.55. [DOI] [PubMed] [Google Scholar]
- 47.Burke JP, Mulsow JJ, O’Keane C, et al. Fibrogenesis in Crohn’s disease. Am J Gastroenterol. 2007;102:439–448. doi: 10.1111/j.1572-0241.2006.01010.x. [DOI] [PubMed] [Google Scholar]
- 48.Van Assche G, Geboes K, Rutgeerts P. Medical therapy for Crohn’s disease strictures. Inflamm Bowel Dis. 2004;10:55–60. doi: 10.1097/00054725-200401000-00009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.