Summary
Bacterial core RNA polymerase can initiate transcription at promoters only if guided by a σ subunit that directs the core enzyme to a subset of σ-specific promoters. Specific and stable interactions between the promoter DNA elements and σ are required for efficient promoter recognition. At the same time, persistent σ-DNA contacts can hinder RNA polymerase escape from a promoter or halt the enzyme downstream from the transcription start site, thereby reducing transcription of the affected genes. This microcommentary reviews recent data arguing that σ-dependent stalled transcription complexes form frequently in vivo, where they likely play important and diverse regulatory roles.
Bacterial core RNA polymerase (RNAP; subunit composition α2ββ′ω) relies on the specificity σ subunit to initiate RNA synthesis at promoters. Competition among different σ factors (as many as 65 species can coexist in a bacterial cell) for the core RNAP determines the pattern of gene expression. In a classical view of the transcription cycle, the only role of the σ subunit is to program the pool of core RNAP molecules to recognize a subset of σ-specific promoters, thereby turning on genes that control heat-shock, sporulation, nitrogen assimilation, and other regulatory pathways in response to changes in environment. Once RNAP productively initiates RNA synthesis, σ is thought to be dispensable. In the simplest scenario, σ is released to enter the next round of competition, whereas the core enzyme becomes a target for elongation factors such as NusA. This view was supported by the early in vitro studies demonstrating that the rapid and obligatory σ release is triggered by extension of the nascent RNA to 8–9 nucleotides (see Mooney et al., 2005 and references therein). However, σ/core contacts are extensive (10 000 Å2 total interaction surface) and breaking them in a single step might turn out to be energetically costly (or even unnecessary). Indeed, the Ebright and Nudler groups (Bar-Nahum and Nudler, 2001; Mukhopadhyay et al., 2001) argued that σ might stay bound to the core throughout elongation. In contrast, Chip–chip analyses indicate that in vivo the vast majority of σ70 is rapidly released from the elongating RNAP (Raffaelle et al., 2005). It is important to note that the key question is not when the σ subunit is released from the core but whether it plays any regulatory role beyond promoter recognition or remains functionally silent even if not released upon escape.
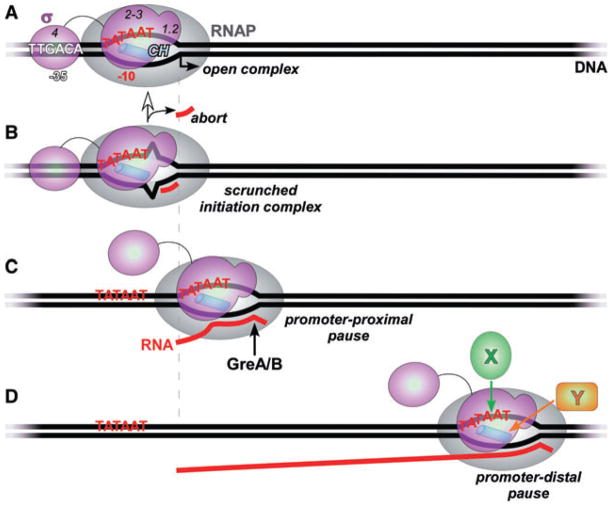
Each σ factor recognizes promoter DNA elements and mediates melting of the double-stranded DNA to form the transcriptionally competent open complex (Fig. 1A). σ70 specifically binds to the −10 element located in the non-template DNA strand (Roberts and Roberts, 1996); the template strand becomes available for base pairing with the incoming substrates. The inherent affinity of σ for its DNA target is essential for its function. However, tight binding of σ to DNA can inhibit transcription: a perfect recognition sequence might ‘lock’ RNAP in abortive cycling at the promoter (Fig. 1B) or induce RNAP pausing when located downstream of the promoter (Fig. 1C and D). Both types of inhibitory events have been reported (see Hatoum and Roberts, 2008 for references) but their ubiquity in vivo has not been assessed.
Fig. 1.
Regulatory targets of the σ subunit.
A. The ‘classical’ σ-subunit target, the open promoter complex. σ specifically recognizes core promoter sequences (shown here for the primary σ70 from E. coli) and, most importantly for all members of this family, mediates the melting of the DNA to expose the transcription start site (a bent arrow) on the template DNA strand to which the incoming initiating NTP substrate would base pair. At a typical promoter, σ70 region 4 binds to the −35 hexamer, σ1.2 – to the discriminator DNA, and σ2–3 – to the −10 hexamer and the β′ subunit clamp helices (CH; blue cylinder), the principal σ-core interaction site (Haugen et al., 2006; Mooney et al., 2005). The interactions between σ1.2–3 and the transcription complex can be maintained during elongation (Mooney et al., 2005).
B. In the initiation complex, the transcription bubble is enlarged upon RNA synthesis but the RNAP remains stationary because the σ-DNA contacts persist. The ‘excess’ DNA is scrunched (Kapanidis et al., 2006; Revyakin et al., 2006) to allow for translocation of the active site along the template. The accumulated stress can be relieved in two ways: (i) the enzyme reiteratively makes and releases (aborts) short, typically 2–8 nt long transcripts, thus reverting to the open complex state; or (ii) the σ-DNA bonds are broken; the core RNAP leaves the promoter (escapes) and enters the elongation phase.
C. The promoter-proximal pause. The (not yet released from the core RNAP) σ subunit recognizes the second −10 element located downstream from the start site. This interaction depends on the same set of contacts (regions 2–3 and perhaps 1.2) and induces a block to RNAP translocation (pause). An intermediate scrunched state induced by σ-DNA contacts (Marr and Roberts, 2000) is relaxed when RNAP moves back and extrudes the nascent RNA, disengaging the 3′ end from the active site. This backtracked complex is a target for Gre proteins (Marr and Roberts, 2000) that facilitate the endonucleolytic removal of the extruded RNA to allow for the next round of nucleotide addition.
D. The promoter-distal pause triggered by (most likely) de novo recruitment of the σ subunit to RNAP that transcribes through a −10 element located far from the start site (Brodolin et al., 2004). This complex likely undergoes the same structural rearrangements as the proximal pause. Auxiliary elongation factors that bind to the non-template DNA strand (X) and/or to the β′ CH (Y) would insulate RNAP from σ-induced pausing (Sevostyanova et al., 2008).
In this issue of Molecular Microbiology, Hatoum and Roberts argue that RNAP stalling after open complex formation is widespread in bacteria, and might present a target for regulation. This elegant study extends the pioneering work from the Roberts lab demonstrating that σ can regulate transcription outside of the promoter context (Ring et al., 1996). Ring et al. have shown that not-yet-released σ70 induces RNAP pausing at a −10-like element located just downstream from the start site of the PR′ promoter in the λ late operon. This pause is required to mediate recruitment of the λ Q protein to RNAP. After recruitment, Q becomes a ‘subunit’ of the transcription elongation complex and instructs RNAP to read through many consecutive termination signals, thereby ensuring the completion of the λ lytic cycle. For years, the PR′ promoter remained the only known target of σ during elongation, until a similar promoter-proximal pause was characterized in vitro in the lacUV5 mutant variant of the Escherichia coli lac promoter (Brodolin et al., 2004; Nickels et al., 2004).
To ask whether σ-induced stalling occurs in vivo at natural E. coli promoters, Hatoum and Roberts (2008) surveyed randomly selected transcription units. They used KMnO4 probing of the chromosomal DNA to detect transcription complexes stalled after initiation; in such complexes, the transcription bubble should be displaced downstream (by ~15 nt) relative to the region melted in the open promoter complex. KMnO4 probing can only be used to assay those promoters that both form stable open complexes and are relatively strong – excluding, for example, the ribosomal rrn promoters that are highly active but form very short-lived open complexes. However, 34 out of 118 promoters examined were amenable to this analysis. Among these, a remarkably large fraction (seven promoters, or ~20%) gave rise to transcription intermediates stalled near the promoter! In addition, complexes stalled far downstream (~100 nt) from the start site were also detected in this study; σ-dependent pausing at a distal site has been observed in vitro (Mooney and Landick, 2003).
Hatoum and Roberts show that, just as in the PR′ case, stalling next to all seven promoters is σ dependent: an amino acid substitution at the binding interface with β′ (σLeu402Phe) that diminishes the promoter-proximal pausing at PR′ also reduces the in vivo idling near promoters. These data support the hypothesis that the σ/β′ CH interaction (Fig. 1A) is critically important for the σ function, particularly after promoter escape when other σ/core contacts are lost (Mooney et al., 2005; Sevostyanova et al., 2008). Promoter-proximal pausing also relies on base-specific contacts between σ and the −10 element (Marr and Roberts, 2000; Brodolin et al., 2004; Nickels et al., 2004). To elucidate the mechanism of stalling, Hatoum and Roberts ‘inactivated’ the −10 element, either within the core promoter or downstream from the start site, and tested for the retention of stalled complexes. This mutational analysis indicates that some of these complexes (lacZ, cspD, tnaA) are likely paused after escape, whereas others (rplK, rpsA) are caught in reiterative abortive synthesis.
This study clearly demonstrates that the ‘initiation’ σ factor does not relinquish its effects on transcription even after it breaks the contacts with the promoter DNA and completes its ‘primary’ initiation job. Further, even though some classes of promoters (weak or forming unstable complexes) are absent from this set, these data strongly argue that RNAP stalling after initiation does not happen just at a couple of model promoters but is a frequent occurrence in E. coli. These complexes are not silent off-pathway intermediates. First, similarly to its role in the λ lytic cycle, stalling may be required for recruitment to the elongating RNAP of yet-unknown cellular proteins with diverse regulatory functions. Second, stalling limits transcription and might be modulated by accessory proteins such as GreA, which decreases promoter-proximal stalling up to 19-fold at rpsU (Hatoum and Roberts, 2008). Both promoter-proximal and distal stall sites could respond to transcription factors: although Hatoum and Roberts did not observe GreA effects at distal sites, another family of proteins that includes RfaH and its paralogues (NusG, ActX) would be expected to inhibit σ-dependent pausing during elongation. RfaH binds simultaneously to the non-template DNA strand and the β′ CH and abrogates σ-dependent pausing, at least in vitro (Sevostyanova et al., 2008). Future studies will undoubtedly uncover many specific examples of transcription regulation aimed at the stalled transcription intermediates.
Acknowledgments
I am grateful to Ruth Saecker for discussions. The work in IA laboratory is supported by GM67153 grant from National Institutes of Health.
References
- Bar-Nahum G, Nudler E. Isolation and characterization of sigma (70)-retaining transcription elongation complexes from Escherichia coli. Cell. 2001;106:443–451. doi: 10.1016/s0092-8674(01)00461-5. [DOI] [PubMed] [Google Scholar]
- Brodolin K, Zenkin N, Mustaev A, Mamaeva D, Heumann H. The sigma 70 subunit of RNA polymerase induces lacUV5 promoter-proximal pausing of transcription. Nat Struct Mol Biol. 2004;11:551–557. doi: 10.1038/nsmb768. [DOI] [PubMed] [Google Scholar]
- Hatoum A, Roberts JW. Prevalence of RNA polymerase stalling at E. coli promoters after open complex formation. Mol Microbiol. 2008 doi: 10.1111/j.1365-2958.2008.06138.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen SP, Berkmen MB, Ross W, Gaal T, Ward C, Gourse RL. rRNA promoter regulation by nonoptimal binding of sigma region 1.2: an additional recognition element for RNA polymerase. Cell. 2006;125:1069–1082. doi: 10.1016/j.cell.2006.04.034. [DOI] [PubMed] [Google Scholar]
- Kapanidis AN, Margeat E, Ho SO, Kortkhonjia E, Weiss S, Ebright RH. Initial transcription by RNA polymerase proceeds through a DNA-scrunching mechanism. Science. 2006;314:1144–1147. doi: 10.1126/science.1131399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr MT, Roberts JW. Function of transcription cleavage factors GreA and GreB at a regulatory pause site. Mol Cell. 2000;6:1275–1285. doi: 10.1016/s1097-2765(00)00126-x. [DOI] [PubMed] [Google Scholar]
- Mooney RA, Landick R. Tethering sigma70 to RNA polymerase reveals high in vivo activity of sigma factors and sigma70-dependent pausing at promoter-distal locations. Genes Dev. 2003;17:2839–2851. doi: 10.1101/gad.1142203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney RA, Darst SA, Landick R. Sigma and RNA polymerase: an on-again, off-again relationship? Mol Cell. 2005;20:335–345. doi: 10.1016/j.molcel.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay J, Kapanidis AN, Mekler V, Kortkhonjia E, Ebright YW, Ebright RH. Translocation of sigma (70) with RNA polymerase during transcription: fluorescence resonance energy transfer assay for movement relative to DNA. Cell. 2001;106:453–463. doi: 10.1016/s0092-8674(01)00464-0. [DOI] [PubMed] [Google Scholar]
- Nickels BE, Mukhopadhyay J, Garrity SJ, Ebright RH, Hochschild A. The sigma 70 subunit of RNA polymerase mediates a promoter-proximal pause at the lac promoter. Nat Struct Mol Biol. 2004;11:544–550. doi: 10.1038/nsmb757. [DOI] [PubMed] [Google Scholar]
- Raffaelle M, Kanin EI, Vogt J, Burgess RR, Ansari AZ. Holoenzyme switching and stochastic release of sigma factors from RNA polymerase in vivo. Mol Cell. 2005;20:357–366. doi: 10.1016/j.molcel.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Revyakin A, Liu C, Ebright RH, Strick TR. Abortive initiation and productive initiation by RNA polymerase involve DNA scrunching. Science. 2006;314:1139–1143. doi: 10.1126/science.1131398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring BZ, Yarnell WS, Roberts JW. Function of E. coli RNA polymerase σ factor σ70 in promoter-proximal pausing. Cell. 1996;86:485–493. doi: 10.1016/s0092-8674(00)80121-x. [DOI] [PubMed] [Google Scholar]
- Roberts C, Roberts J. Base-specific recognition of the nontemplate strand of promoter DNA by E. coli RNA polymerase. Cell. 1996;86:495–501. doi: 10.1016/s0092-8674(00)80122-1. [DOI] [PubMed] [Google Scholar]
- Sevostyanova A, Svetlov V, Vassylyev DG, Artsimovitch I. The elongation factor RfaH and the initiation factor σ bind to the same site on the transcription elongation complex. Proc Natl Acad Sci USA. 2008;105:865–870. doi: 10.1073/pnas.0708432105. [DOI] [PMC free article] [PubMed] [Google Scholar]