Abstract
Inhibition of cytochrome P450 (CYP) is a major cause of herb–drug interactions. The CYP1A2 enzyme plays a major role in the metabolism of drugs in humans. Its broad substrate specificity, as well as its inhibition by a vast array of structurally diverse herbal active ingredients, has indicated the possibility of metabolic herb–drug interactions. Therefore nowadays searching inhibitors for CYP1A2 from herbal medicines are drawing much more attention by biological, chemical and pharmological scientists. In our work, a pharmacophore model as well as the docking technology is proposed to screen inhibitors from herbal ingredients data. Firstly different pharmaphore models were constructed and then validated and modified by 202 herbal ingredients. Secondly the best pharmaphore model was chosen to virtually screen the herbal data (a curated database of 989 herbal compounds). Then the hits (147 herbal compounds) were continued to be filtered by a docking process, and were tested in vitro successively. Finally, five of eighteen candidate compounds (272, 284, 300, 616 and 817) were found to have inhibition of CYP1A2 activity. The model developed in our study is efficient for in silico screening of large herbal databases in the identification of CYP1A2 inhibitors. It will play an important role to prevent the risk of herb–drug interactions at an early stage of the drug development process.
Keywords: CYP1A2, pharmacophore, docking, herb–drug interaction
1. Introduction
During the last decade, North America and Europe have witnessed a drastic growth in the use of herbal medicine. In a comprehensive review on the usage of complementary and alternative medicine, 16% of prescription drug users are reported to consume herbal supplements [1], two-thirds of women used herbs for perimenopausal symptoms, 45% of parents gave their children herbal treatments and 45% of pregnant women tried herbal remedy [2]. Meanwhile, some reports have indicated that more than 60% of patients use their herbal medicine with conventional medicines as usual [3]. Because some of constituents in herbal products may be substrates, inhibitors and/or inducers of the cytochrome P450 enzymes (CYPs) [4,5], thus could have an impact on the pharmacokinetics of a co-administered drug metabolized by the CYP enzymes, which will probably lead to the herb–drug interaction. The most well-known example of an herb–drug interaction is St John’s wort, which could cause the clearance of CYP1A2 substrates such as theophylline [6]. Clinically significant interactions were also reported from the use of grapefruit juices in calcium antagonists, antihistamine and benzodiazepines treatment [7–9]. Nowadays, the interaction of drugs with herbal medicines has become a significant safety concern, especially for drugs with narrow therapeutic indices.
Among all of these cytochrome P450 enzymes, CYP1A2 should attract more attention since it is one of the key enzymes with the important role in the metabolic clearance of 5% of currently marketed drugs [10]. Also it is involved in a number of clinical herb–drug interactions [11–13]. When herbal medicines are used with western medicines metabolized by CYP1A2 at the same time, it is necessary to know whether this herb medicine will inhibit CYP1A2 or not. This means that the prediction of herb–drug interactions in vitro is important and thus many herbal medicines were tested in vitro by scientists [14–16]. However, the number of herbal medicines is large. Traditional screening technologies such as testing each herbal medicine to enzyme in vitro or in vivo would not only be costly, but also inefficient. Recently, several attempts in the application of computational models for CYP1A2 ligand binding have been reported, reflecting the desire of early identification of CYP1A2 inhibitors [17–22]. Taesung Moon et al. [23] pioneered investigations on the quantitative structure-activity relationship (QSAR) of flavonoids and their derivatives. However, their samples were only flavonoids, which were not representative of all herb ingredients and fail to accurately predict the inhibitory potencies of structurally unrelated herb ingredients.
In order to better predict herb–drug interaction as well as select rapid inhibitors from herbal ingredients, a combinational pharmacophore model were presented to virtually screen the herb data before those candidate compounds were tested in vitro to determine their inhibitory effect on CYP1A2. The model developed here is efficient for virtual screening of large herbal databases for identification of CYP1A2 inhibitors, and it will play an important role to prevent the risk of herb–drug interactions at an early stage of the drug development process.
2. Results and Discussion
2.1. Pharmacophore Models
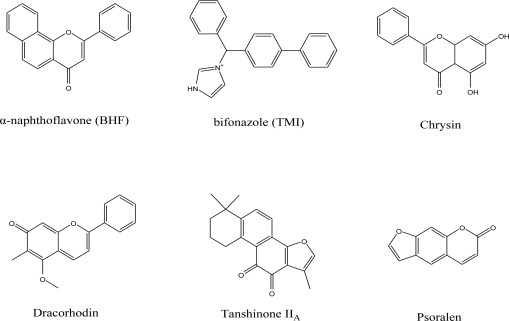
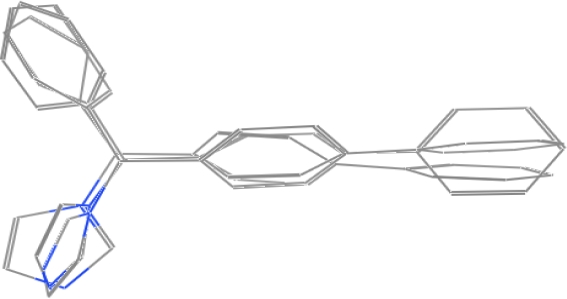
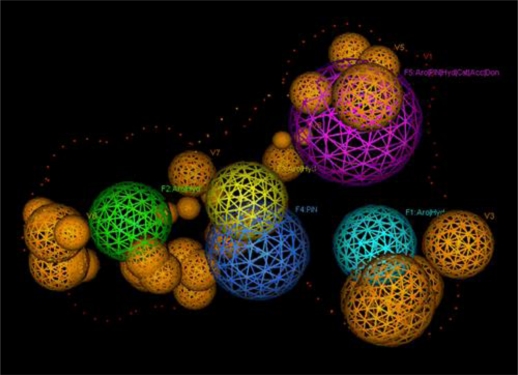
For the pharmacophore screening, the key step was to choose a good template molecule. In this study, several template molecules (Figure 1) could be obtained to generate the pharmacophore: (1) the substrates extracted from complex structures of CYP1A2 and its homologous enzymes; and (2) inhibitors reported in the literature [24]. Different template molecules based on individual or integrated information above were used to generate the pharmacophores. Then up to 202 different herb integrants tested in vitro by our group were used as the test dataset (supplement Table 2). The molecular structure of selected template was shown in Figure 2. Finally, the pharmacophore model was obtained (Figure 3). The true positive rate and true negative rate of the best pharmacophore model were 84.6% (11/13) and 86.8% (164/189), respectively. Other results of different pharmacophore models are also shown in Table 1 as a comparison.
Figure 1.
Molecular structure of the template molecules used in this work.
Figure 2.
The molecular structure of selected template by superposing three bifonazole in three different conformations.
Figure 3.
The final pharmacophore of CYP1A2. F1–F3: Aro|Hyd; F4: PiN; F5: Aro|PiN|Hyd|Cat|Acc|Don; V1: Exterior Volume; V2–V8: Excluded Volume.
Table 1.
The results of different pharmacophore models.
| Compounds Used as a Template | True Positive Rate | True Negative Rate |
|---|---|---|
| BHF, TMI | 61.5% | 85.2% |
| BHF, Chrysin and Tanshinone IIA | 69.2% | 96.8% |
| Chrysin, Psoralen and Dracorhodin | 61.5% | 89.9% |
| Three TMI in different conformations without optimization | 84.6% | 77.2% |
| Three TMI in different conformations with sophisticated optimization | 84.6% | 86.8% |
There were still disputes on how to superpose these template molecules used to build the pharmacophore [25]. It is known that there are two opinions on the active conformation [26–28]; some people prefer to optimize the energy of these template molecules and obtain the conformation with minimum energy. While another group of people believe that the docking conformation is the active conformation and these template molecules extracted from the crystal structure should be superposed directly. Our results have shown that the latter opinion is more reasonable compared with the former one [29].
By observing the complex crystal structure of CYP1A2 and its homologous enzymes, we found that not only different substrates could exist in the same acceptor, but also different conformations of one substrate could exist within one complex crystal structure. It is obvious that superposing the different conformations of one substrate appearing in one complex crystal structure will be the best option, since these conformations were not only the true active conformation, but also reduces errors commonly caused by trying different crystal structures, measurements and analysis conditions. As a validation, the results shown in Table 1 were proved by Zhao et al. recently [24].
In addition, our work also indicated that it was important to collect some negative data in the building of pharmacophore, since excluded volume of the pharmacophore was built on the negative data. Also the building of excluded volume is the key to increase the true negative rate. However, this step was often ignored by former research groups.
Finally, 147 hits were filtered out by the selected pharmacophore model from 989 compounds, which were separated from various herbs collected in our group. Formerly, compounds in Chinese Nature Products Database (CNPD v.2004.1) [30] were also screened by using this pharmacophore model. Unfortunately, this research had to be abandoned because hits in CNPD were unavailable.
2.2. Docking Results
Admittedly, two challenges in the field of molecular docking still exist: (1) ligand placement in active site, and (2) scoring of docked poses [31,32]. However, compared with the semi-quantitative method of the pharmacophore model, molecular docking, as one of the quantitative methods, is better for prioritizing the hits with the help of deriving stable docking parameters and combing. Recently, the work of Yu Chen and Brian K. Shoichet [33] reinforced more confidence to docking results.
The purpose of the dock application is to search for favorable binding configurations between small to medium-sized ligands and a not too flexible macromolecular target, which is usually a protein. For each ligand, a number of configurations called poses are generated and scored in an effort to determine favorable binding modes. Optionally, poses can be constrained to fit a pharmacophore query. The top scoring poses are written to a database for further analysis.
In addition, 147 compounds formerly filtered from the pharmacophore model were docked into the active site of CYP1A2. For comparison, docking was run twice and results are presented in supplement Table 1. It can be seen that this set of docking parameters almost produced stable docking results.
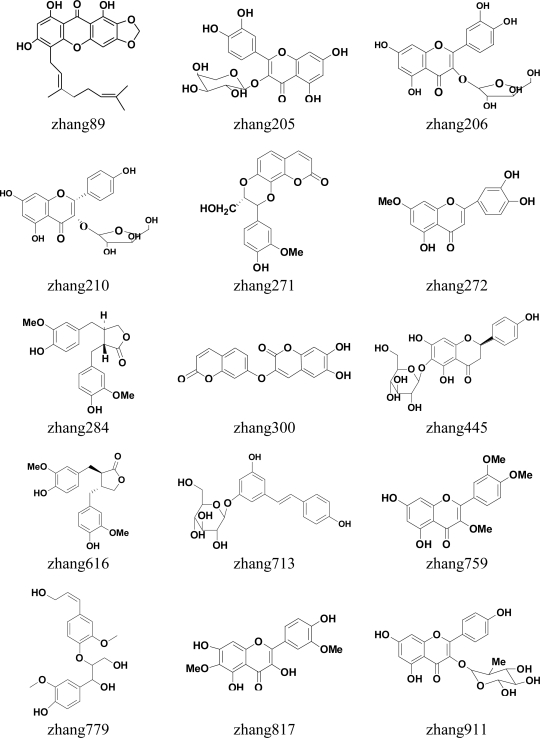
Finally, the same hits appearing in the top 20 of both results were chosen. Thus, up to 18 hits (Figure 4) were filtered into the next in vitro screening.
Figure 4.
Molecular structure of 18 compounds tested in vitro.
2.3. In Vitro Testing
A total of 18 candidate compounds selected by pharmacophore and docking were examined for their potential to inhibit rhCYP1A2 phenacetin O-deethylation activity at a test concentration of 1 μM. The results of in vitro testing were listed in Table 2. Five of 18 test compounds (272, 284, 300, 616 and 817) were confirmed to have inhibitory activities to CYP1A2. The model was validated by the in vitro experiment where accuracy was 27.8% (5/18).
Table 2.
Results of in vitro testing.
| Number | Active Ingredients | % of Control Indication Activity |
|---|---|---|
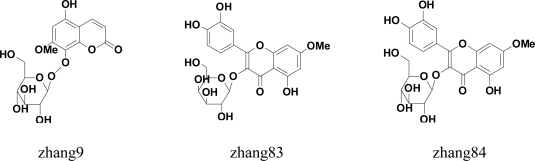
| zhang 9 | 5-hydroxy-7-methoxycoumarin-8-O-d-glucopyranoside | >90% |
| zhang 83 | rhamnetin 3-O-β-d-galactopyranoside | >90% |
| zhang 84 | rhamnetin 3-O-β-d-glucopyranoside | >90% |
| zhang 89 | 1,6,8-trihydroxy-2,3-methylenedioxy-5-geranylxanthone | >90% |
| zhang 205 | quercetin-3-O-β-d-arabinopyranoside | >90% |
| zhang 206 | quercetin-3-O-α-d-arabinofuranose | >90% |
| zhang 210 | dihydrokaempferol-3-O-α-d-arabinofuranose | >90% |
| zhang 271 | 5′-demethoxy Daphneticin | >90% |
| zhang 272 | 3′-hydroxy-Genkwanin | <90% |
| zhang 284 | (−)-Matairesinol | <90% |
| zhang 300 | Edgeworthin | <90% |
| zhang 445 | Hemiphloin | >90% |
| zhang 616 | (+)-Matairesinol | <90% |
| zhang 713 | Piceid | >90% |
| zhang 759 | 3,3′,4′-Tri-Me ether-3,3′,4′,5,7-Pentahydroxyflavone | >90% |
| zhang 779 | (−)-Threo-guaiacylglycerol-8-O-4′-(coniferyl alcohol) ether | >90% |
| zhang 817 | Spinacetin | <90% |
| zhang 911 | Kaempferol-3-O-α-l-rhamnopyranoside | >90% |
Note: Values represent the average of duplicate determinations. Values in italics represent those exhibiting 10% inhibition or greater.
In this study, 971 herbal compounds were excluded by virtual screen and the exclusion rate was 98.2%. The high exclusion rate for screening larger database was very helpful, since it is not only time-saving and economic, but also helps to improve the success rate of screening test in vitro. The high exclusion rate also could help to reduce the number of candidate compounds in CNPD and make the experiment more goal-oriented.
In the present study, two of five test compounds which inhibited CYP1A2 activities were flavonoid compounds. In the early experiment, five of 13 herb compounds which inhibited CYP1A2 activities were flavonoid compounds. The flavonoid compounds accounted for almost 40% (7/18) of the total of all inhibitors. This show that the potential for flavonoid inhibited CYP1A2 has tremendous possibilities. The ability of flavonoids to inhibit CYP1A2 has been extensively confirmed [34,35]. Flavones are present in a wide variety of fruits and vegetables whereas flavones are mainly found in cereals and herbs [35–37]. In some countries flavonoids are commonly used as therapeutic agents and some flavonoids are administered orally or intravenously as drugs [38,39]. However, there was little awareness of the potential for flavonoid interactions with conventional drugs. Some clinical studies have demonstrated that flavonoids can affect the metabolism of other drugs [39–41]. Accordingly, flavonoids’ effect on the activity of CYP1A2 should be given more attention. Other herb compounds were also selected using the pharmacophore model and it was validated that they inhibited CYP1A2 activities in vitro. Compound 300 was a coumarins compound and compound 284 and 616 were lignanoids compounds.
3. Materials and Methods
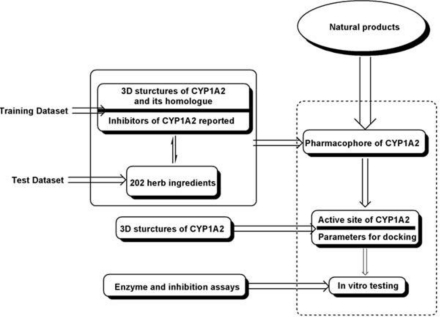
A tandem workflow is designed to screen the inhibitors of CYP1A2 from natural compounds. This workflow is outlined in Figure 5. Details are listed below: Our analysis was started by constructing the pharmaphore model of CYP1A2. The 3D structures of CYP1A2, its homologues and several reported inhibitors of CYP1A2 were used to test the basic templates. Then up to 202 herb ingredients tested in vitro were used to validate and modify the basic templates to derive the most suitable pharmaphore model of CYP1A2. In the following, based on the 3D structures of CYP1A2, an active site for docking was chosen as a complementary process for further screening. Finally, compounds having passed the former filters were tested in vitro.
Figure 5.
General workflow used in our study.
3.1. Pharmacophore Generation
The crystal structure of CYP1A2 and its homologue CYP2B4 were retrieved from the Protein Data Bank (PDB code 2HI4 [42] and 2BDM [20], respectively). The endogenic ligands α-naphthoflavone (BHF) and bifonazole (TMI) of those two crystals and several reported inhibitors of CYP1A2 were collected as the template molecules to train the pharmacophore model. 202 herb ingredients tested in vitro were used as the test dataset.
The purpose of pharmacophore searching is to facilitate 3D searches of conformation databases using molecule annotations related to ligand-receptor binding (like, H-bond donor, acceptor, hydrophobe, etc.). A pharmacophore is a set of structural features in a ligand that are related to the ligand’s recognition at a receptor site and its biological activity. In Molecular Operation Environment (MOE) [43] pharmacophoric structural features are represented by labeled points in space with a conformation of the ligand. Each ligand conformation is assigned a set of annotation points, which is a set of structural features that may contribute to the ligand’s pharmacophore. A database of conformations can be searched with a query that represents a pharmacophore hypothesis. The result of such a search is a set of matches that align the pharmacophoric features of the query to the pharmacophoric features present in the ligands of the searched database.
3.2. Docking Procedure
The crystal structure of CYP1A2 (PDB code 2HI4) was used to derive the active site and tune the stable parameters in docking.
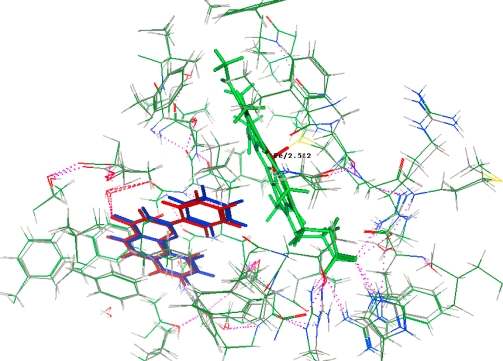
MOE-Dock 2008.10 [43] function was used for docking. Ligand placement was performed using alpha sphere triangle matching, with London dG scoring. The top 30 hits were retained and refined using MMFF94X force field energy minimization with Generalized Born solvation model, allowing the receptor residues within 9 Å to relax around the mobile ligand. The active site of receptor’s side chains were tethered with a force constant of 1.0 kcal/(mol Å2). Energy minimization was stopped when the root-mean-squared gradient cutoff of 0.0001 kcal/(mol Å) was reached. Final poses were ranked using the London dG method to calculate the free energy of binding. With this set of docking parameters, the crystallographic placement of α-naphthoflavone was accurately predicted in the crystal structure of Human Microsomal P450 1A2 receptor crystal structure, PDB: 2HI4 (Resolution (Å): 1.95) (Figure 6), with a root-mean-squared deviation (RMSD) of 0.3061 Å and a binding energy of −11.1076 kcal/mol.
Figure 6.
Crystallographic (Red) and docked (Blue) conformations of α-naphthoflavone in the Human Microsomal P450 1A2 receptor site S = −11.1076, rmsd = 0.3061. Note: Hydrogen bond is indicated by the pink dashed line.
3.3. Enzyme and Inhibition Assays
The basic incubation medium contained 100 mM potassium phosphate buffer (PH 7.4), 50 pmol recombinant human CYP1A2, 25 μM phenacetin and 1 μM active ingredients. The final incubation volume was 200 μL. After 5 min pre-incubation at 37 °C, the reaction was initiated by the addition of NADPH-generating system (5 mM MgCl2, 10 mM glucose 6-phosphates, 1 mM NADPH, and 2.5 U per mL glucose 6-phosphate dehydrogenase). Incubations were performed in a 37 °C shaking water bath for 1 h. Reactions were stopped by adding 0.4 mL of ice-cold acetonitrile containing 0.2 mg/L tinidazole (IS). Then 0.6 mL water was added to the mixture, vortex-mixing for 1.0 min and centrifuged at 10,000 g for 5 min, and separation for the supernatants were performed on a ZORBAX SB-C18 column (3.5 μm, 2.1 × 100 mm, Agilent Corporation, MA, USA) and a C18 guard column (5 μm, 4.0 × 2.0 mm, Phenomenex, CA, USA) with an isocratic elution of acetonitrile—0.1% formic acid (35:65, v/v) at a flow rate of 0.3 ml/min for the amount of metabolite (acetaminophen) formed. The column was maintained at 35 °C and the injection volume was 10 μL. The retention times of paracetamol and tinidazole were 1.872 and 3.912 min, respectively. The percent of metabolic activity remaining was calculated by comparing the enzyme activities in the control samples that did not contain active ingredients. The active ingredient demonstrating 10% or greater inhibition at 1 μM was considered to have inhibitory activity. Each set of incubation was carried out with four control samples that contained no active ingredients.
4. Conclusions
In this study, we have selected several template molecules to generate pharmacophores. 202 different herb compounds were used as test data to test their inhibitory activity against CYP1A2 in vitro and used to identify the best pharmacophore with the highest external predictive power. A highly predictive pharmacophore model was generated with three bifonazole (TMI) in different conformations with sophisticated optimization. A rigorously validated pharmacophore model was then used to screen our in-house database collection of a total of over one thousand herb compounds. 147 hits were filtered out by the selected pharmacophore model and were docked into the active site of CYP1A2. Finally, 18 hits were further filtered and experimentally validated. Five of them were confirmed to have inhibitory activities to CYP1A2, and the accuracy of the model was 27.8%. This study illustrates that the model developed here is efficient for virtual screening of large databases in the identification of CYP1A2 inhibitors or non-inhibitors. Accordingly, the models can play an important role to prevent the risk of e.g., herb–drug interactions through metabolism at an early stage of the drug development process.
Supplementary Materials
Acknowledgments
The work was supported by program NCET Foundation, NSFC (30725045), the Special Program for New Drug Innovation of the Ministry of Science and Technology, China (2009ZX09311-001, 2008ZX09308-005), Shanghai Leading Academic Discipline Project (B906) and in part by the Scientific Foundation of Shanghai China (09DZ1975700, 09DZ1971500), Ministry of Science and Technology China (2010CB833601, 2006AA02312, 2009zx10004-601,), National Natural Science Foundation of China (30976611), Research Fund for the Doctoral Program of Higher Education of China (20100072110008, 20100072120050).and Shanghai Municipal Education Commission (2000236018, 2000236016, 07ZR14085, 09dZ1972800).
References
- 1.Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: The Slone survey. JAMA. 2002;287:337–344. doi: 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- 2.Ernst E. Are herbal medicines effective? Int. J. Clin. Pharmacol. Ther. 2004;42:157–159. doi: 10.5414/cpp42157. [DOI] [PubMed] [Google Scholar]
- 3.Klepser TB, Doucette WR, Horton MR, Buys LM, Ernst ME, Ford JK, Hoehns JD, Kautzman HA, Logemann CD, Swegle JM, Ritho M, Klepser ME. Assessment of patients’ perceptions and beliefs regarding herbal therapies. Pharmacotherapy. 2000;20:83–87. doi: 10.1592/phco.20.1.83.34658. [DOI] [PubMed] [Google Scholar]
- 4.Delgoda R, Westlake AC. Herbal interactions involving cytochrome p450 enzymes: A mini review. Toxicol. Rev. 2004;23:239–249. doi: 10.2165/00139709-200423040-00004. [DOI] [PubMed] [Google Scholar]
- 5.Saxena A, Tripathi KP, Roy S, Khan F, Sharma A. Pharmacovigilance: Effects of herbal components on human drugs interactions involving cytochrome P450. Bioinformation. 2008;3:198–204. doi: 10.6026/97320630003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nebel A, Schneider BJ, Baker RK, Kroll DJ. Potential metabolic interaction between St. John’s wort and theophylline. Ann. Pharmacother. 1999;33:502. doi: 10.1345/aph.18252. [DOI] [PubMed] [Google Scholar]
- 7.Ameer B, Weintraub RA. Drug interactions with grapefruit juice. Clin. Pharmacokinet. 1997;33:103–121. doi: 10.2165/00003088-199733020-00003. [DOI] [PubMed] [Google Scholar]
- 8.Bailey DG, Malcolm J, Arnold O, Spence JD. Grapefruit juice-drug interactions. Br. J. Clin. Pharmacol. 1998;46:101–110. doi: 10.1046/j.1365-2125.1998.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kupferschmidt HH, Fattinger KE, Ha HR, Follath F, Krahenbuhl S. Grapefruit juice enhances the bioavailability of the HIV protease inhibitor saquinavir in man. Br. J. Clin. Pharmacol. 1998;45:355–359. doi: 10.1046/j.1365-2125.1998.t01-1-00687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faber MS, Jetter A, Fuhr U. Assessment of CYP1A2 activity in clinical practice: why, how, and when? Basic Clin. Pharmacol. Toxicol. 2005;97:125–134. doi: 10.1111/j.1742-7843.2005.pto_973160.x. [DOI] [PubMed] [Google Scholar]
- 11.Bapiro TE, Sayi J, Hasler JA, Jande M, Rimoy G, Masselle A, Masimirembwa CM. Artemisinin and thiabendazole are potent inhibitors of cytochrome P450 1A2 (CYP1A2) activity in humans. Eur. J. Clin. Pharmacol. 2005;61:755–761. doi: 10.1007/s00228-005-0037-3. [DOI] [PubMed] [Google Scholar]
- 12.Qiu F, Wang G, Zhao Y, Sun H, Mao G, A J, Sun J. Effect of danshen extract on pharmacokinetics of theophylline in healthy volunteers. Br. J. Clin. Pharmacol. 2008;65:270–274. doi: 10.1111/j.1365-2125.2007.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorski JC, Huang SM, Pinto A, Hamman MA, Hilligoss JK, Zaheer NA, Desai M, Miller M, Hall SD. The effect of echinacea (Echinacea purpurea root) on cytochrome P450 activity in vivo. Clin. Pharmacol. Ther. 2004;75:89–100. doi: 10.1016/j.clpt.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Appiah-Opong R, Commandeur JN, van Vugt-Lussenburg B, Vermeulen NP. Inhibition of human recombinant cytochrome P450s by curcumin and curcumin decomposition products. Toxicology. 2007;235:83–91. doi: 10.1016/j.tox.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Tang JC, Yang H, Song XY, Song XH, Yan SL, Shao JQ, Zhang TL, Zhang JN. Inhibition of cytochrome P450 enzymes by rhein in rat liver microsomes. Phytother. Res. 2009;23:159–164. doi: 10.1002/ptr.2572. [DOI] [PubMed] [Google Scholar]
- 16.Peterson S, Lampe JW, Bammler TK, Gross-Steinmeyer K, Eaton DL. Apiaceous vegetable constituents inhibit human cytochrome P-450 1A2 (hCYP1A2) activity and hCYP1A2-mediated mutagenicity of aflatoxin B1. Food Chem. Toxicol. 2006;44:1474–1484. doi: 10.1016/j.fct.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Fuhr U, Strobl G, Manaut F, Anders EM, Sorgel F, Lopez-de-Brinas E, Chu DT, Pernet AG, Mahr G, Sanz F, et al. Quinolone antibacterial agents: relationship between structure and in vitro inhibition of the human cytochrome P450 isoform CYP1A2. Mol. Pharmacol. 1993;43:191–199. [PubMed] [Google Scholar]
- 18.Sanz F, López-de-Briñas E, Rodríguez J, Manaut F. Theoretical Study on the Metabolism of Caffeine by Cytochrome P-450 1A2 and its Inhibition. Quant. Struct. Act. Relatsh. 1994;13:281–284. [Google Scholar]
- 19.Lee H, Yeom H, Kim YG, Yoon CN, Jin C, Choi JS, Kim BR, Kim DH. Structure-related inhibition of human hepatic caffeine N3-demethylation by naturally occurring flavonoids. Biochem. Pharmacol. 1998;55:1369–1375. doi: 10.1016/s0006-2952(97)00644-8. [DOI] [PubMed] [Google Scholar]
- 20.de Rienzo F, Fanelli F, Menziani MC, De Benedetti PG. Theoretical investigation of substrate specificity for cytochromes P450 IA2, P450 IID6 and P450 IIIA4. J. Comput. Aided Mol. Des. 2000;14:93–116. doi: 10.1023/a:1008187802746. [DOI] [PubMed] [Google Scholar]
- 21.Lozano JJ, Pastor M, Cruciani G, Gaedt K, Centeno NB, Gago F, Sanz F. 3D-QSAR methods on the basis of ligand-receptor complexes. Application of COMBINE and GRID/GOLPE methodologies to a series of CYP1A2 ligands. J. Comput. Aided Mol. Des. 2000;14:341–353. doi: 10.1023/a:1008164621650. [DOI] [PubMed] [Google Scholar]
- 22.Lewis DF. On the recognition of mammalian microsomal cytochrome P450 substrates and their characteristics: Towards the prediction of human p450 substrate specificity and metabolism. Biochem. Pharmacol. 2000;60:293–306. doi: 10.1016/s0006-2952(00)00335-x. [DOI] [PubMed] [Google Scholar]
- 23.Moon T, Chi MH, Kim D, Yoon CN, Choi Y. Quantitative Structure-Activity Relationships (QSAR) study of flavonoid derivatives for inhibition of cytochrome P450 1A2. Quant. Struct. Act. Relatsh. 2000;19:257–263. [Google Scholar]
- 24.Zhao Y, White MA, Muralidhara BK, Sun L, Halpert JR, Stout CD. Structure of microsomal cytochrome P450 2B4 complexed with the antifungal drug bifonazole: insight into P450 conformational plasticity and membrane interaction. J. Biol. Chem. 2006;281:5973–5981. doi: 10.1074/jbc.M511464200. [DOI] [PubMed] [Google Scholar]
- 25.Raychaudhuri S, Jain V, Dongre M. Identification of a constitutively active variant of LuxO that affects production of HA/protease and biofilm development in a non-O1, non-O139 Vibrio cholerae O110. Gene. 2006;369:126–133. doi: 10.1016/j.gene.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 26.Vieth M, Hirst JD, Brooks CR. Do active site conformations of small ligands correspond to low free-energy solution structures? J. Comput. Aided Mol. Des. 1998;12:563–572. doi: 10.1023/a:1008055202136. [DOI] [PubMed] [Google Scholar]
- 27.Chen IJ, Foloppe N. Conformational sampling of druglike molecules with MOE and catalyst: implications for pharmacophore modeling and virtual screening. J. Chem. Inf. Model. 2008;48:1773–1791. doi: 10.1021/ci800130k. [DOI] [PubMed] [Google Scholar]
- 28.Butler KT, Luque FJ, Barril X. Toward accurate relative energy predictions of the bioactive conformation of drugs. J. Comput. Chem. 2009;30:601–610. doi: 10.1002/jcc.21087. [DOI] [PubMed] [Google Scholar]
- 29.Zhu RX, Zhang XL, Dong XC, Chen MB. Searching Inhibitors of Adenosine Kinase by Simulation Methods. Chin. J. Chem. 2006;24:1493–1497. [Google Scholar]
- 30.Shen J, Xu X, Cheng F, Liu H, Luo X, Shen J, Chen K, Zhao W, Shen X, Jiang H. Virtual screening of natural products for discovering active compounds and target information. Curr. Med. Chem. 2003;10:2327–2342. doi: 10.2174/0929867033456729. [DOI] [PubMed] [Google Scholar]
- 31.Krovat EM, Steindl T, Langer T. Recent Advances in Docking and Scoring. Curr. Comput. Aided Drug Des. 2005;1:93–102. [Google Scholar]
- 32.Sousa SF, Fernandes PA, Ramos MJ. Protein-ligand docking: current status and future challenges. Proteins. 2006;65:15–26. doi: 10.1002/prot.21082. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, Shoichet BK. Molecular docking and ligand specificity in fragment-based inhibitor discovery. Nat. Chem. Biol. 2009;5:358–364. doi: 10.1038/nchembio.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cermak R, Wolffram S. The potential of flavonoids to influence drug metabolism and pharmacokinetics by local gastrointestinal mechanisms. Curr. Drug Metab. 2006;7:729–744. doi: 10.2174/138920006778520570. [DOI] [PubMed] [Google Scholar]
- 35.Hertog MGL, Hollman PCH, van de Putte B. Content of potentially anticarcinogenic flavonoids of tea infusions wines, and fruit juices. J. Agric. Food Chem. 1993;41:1242–1246. [Google Scholar]
- 36.Peterson J, Dwyer J. Taxonomic classification helps identify flavonoid-containing foods on a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1998;98:677–682. 685. doi: 10.1016/S0002-8223(98)00153-9. [DOI] [PubMed] [Google Scholar]
- 37.Bravo L. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998;56:317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- 38.State Food and Drug Administration . Catalog of the Drugs in China. State Food and Drug Administration; Beijing, China: 2008. [Google Scholar]
- 39.Choi JS, Choi BC, Choi KE. Effect of quercetin on the pharmacokinetics of oral cyclosporine. Am. J. Health Syst. Pharm. 2004;61:2406–2409. doi: 10.1093/ajhp/61.22.2406. [DOI] [PubMed] [Google Scholar]
- 40.Peng WX, Li HD, Zhou HH. Effect of daidzein on CYP1A2 activity and pharmacokinetics of theophylline in healthy volunteers. Eur. J. Clin. Pharmacol. 2003;59:237–241. doi: 10.1007/s00228-003-0596-0. [DOI] [PubMed] [Google Scholar]
- 41.Rajnarayana K, Reddy MS, Krishna DR. Diosmin pretreatment affects bioavailability of metronidazole. Eur. J. Clin. Pharmacol. 2003;58:803–807. doi: 10.1007/s00228-002-0543-5. [DOI] [PubMed] [Google Scholar]
- 42.Sansen S, Yano JK, Reynald RL, Schoch GA, Griffin KJ, Stout CD, Johnson EF. Adaptations for the oxidation of polycyclic aromatic hydrocarbons exhibited by the structure of human P4501A2. J. Biol. Chem. 2007;282:14348–14355. doi: 10.1074/jbc.M611692200. [DOI] [PubMed] [Google Scholar]
- 43.Molecular Operation Environment (MOE) Chemical Computing Group Inc; Montreal, Canada: 2008. version 200810; [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.