Abstract
Precision grip control is important for accurate object manipulation and requires coordination between horizontal (grip) and vertical (load) fingertip forces. Manifest Huntington’s disease (HD) subjects demonstrate excessive and highly variable grip force and delayed coordination between grip and load forces. Because the onset of these impairments is unknown, we examined precision grip control in premanifest HD (pre-HD) subjects. Fifteen pre-HD and 15 age- and sex-matched controls performed the precision grip task in a seated position. Subjects grasped and lifted an object instrumented with a force transducer that measured horizontal grip and vertical load forces. Outcomes were preload time, loading time, maximum grip force, mean static grip force, and variability for all measures. We compared outcomes across groups and correlated grip measures with the Unified Huntington’s Disease Rating Scale and predicted age of onset. Variability of maximum grip force (P < .0001) and variability of static grip force (P < .00001) were higher for pre-HD subjects. Preload time (P < .007) and variability of preload time (P < .006) were higher in pre-HD subjects. No differences were seen in loading time across groups. Variability of static grip force (r2 = 0.23) and variability of preload time (r2 = 0.59) increased with predicted onset and were correlated with tests of cognitive function. Our results indicate that pre-HD patients have poor regulation of the transition between reach and grasp and higher variability in force application and temporal coordination during the precision grip task. Force and temporal variability may be good markers of disease severity because they were correlated with predicted onset of disease.
Keywords: Huntington’s disease, premanifest, precision grip, motor control
Huntington’s disease (HD) is an autosomal dominant degenerative disorder caused by a CAG repeat expansion in the gene that encodes huntingtin.1 The length of the CAG repeat is related to disease onset and clinical progression.2,3 Clinically, HD is characterized by motor, cognitive, and behavioral impairments that vary in their presentation and rate of progression across subjects. Motor deficits are associated with functional limitations4,5 and include slow and variable eye movements,6–8 gait,9 and arm10 and hand movements.11,12
Grasping and manipulating objects is extremely important for everyday functional activities. Precision grip, the act of grasping objects between the thumb and index finger, is a complex sequential action that requires precise application of forces large enough to prevent objects from slipping but not too large to break fragile objects or cause fatigue.13,14 In healthy individuals, precision grip begins when the fingers contact the object of interest, leading to an increase in the grip force (preload phase). Following this, the grip force and load force increase in parallel (loading phase) until the load force overcomes the weight of the object, leading to the beginning of object transport. Finally, if the object is held in space (static phase), the grip and load forces exceed the minimum force required to prevent slipping until the object is returned to the surface.13 The magnitude and timing of fingertip forces are modulated to match variations in object size, weight, and surface texture for successful grasping.13,15 Precision grip control requires accurate prediction of object features (such as weight and texture) and timely reaction to sensory feedback from the hand and fingers.16,17
Precision grip impairments in manifest HD include temporal delays in the transition between the preload and loading phases, higher static grip force, and greater variability in grip force (particularly for lighter objects) compared with healthy control subjects.11,18–20 Variability in grip force is correlated with functional limitations, as defined by the Total Functional Capacity (TFC) scale11 and worsens with disease progression.21 These results suggest that precision grip may be impaired in HD because of a deficit in reactive control rather than predictive control either from degeneration of the basal ganglia–cortical loop or as compensation for the degeneration. Given that neuronal loss in the basal ganglia, specifically the putamen, begins 10 to 15 years before diagnosis,22 precision grip control may be impaired prior to the development of clinically diagnosed HD (premanifest HD [pre-HD]). Because regulation of sequential movements such as gait has been shown to be impaired in pre-HD,9 we hypothesized that pre-HD would also demonstrate impairments in regulation of precision grip control.
Subjects and Methods
Subjects
Fifteen premanifest Huntington’s disease (pre-HD) subjects (mean age, 38.86 years; range, 26–66 years) and 15 healthy controls (mean age, 37.55 years; range, 26–67 years) participated in the study. Pre-HD subjects were tested by a movement disorder specialist, and were included in the study if they had genetic confirmation of the HD mutation and did not meet the criteria for clinical diagnosis of HD based on a diagnostic confidence rating. Pre-HD subjects were recruited from the PREDICT-HD study, a multisite longitudinal observational study aimed at identifying the biological and clinical markers of HD.22 Healthy control subjects were recruited from among mutation negative family members of pre-HD or from the staff and student population at Columbia University. Control subjects were matched with pre-HD subjects for age (±1 year) and sex. The Institutional Review Board at Columbia University Medical Center approved study procedures, and all subjects provided written informed consent before participation.
Quantitative Assessment of Precision Grip
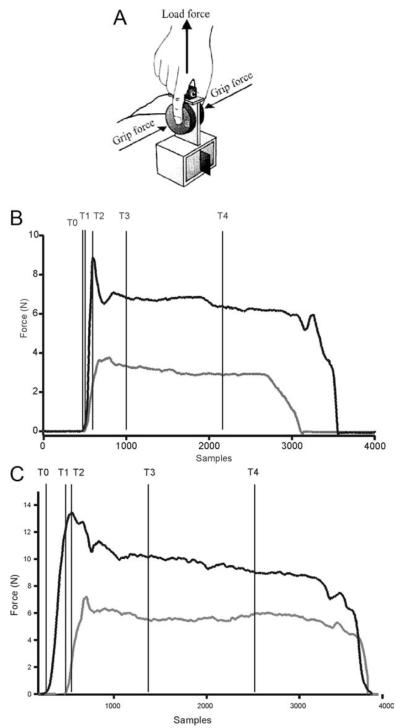
We used a custom instrumented object with a force transducer (25 mm diameter and 22 mm height; Nano F/T transducer, ATI Industrial Automation, Garner, NC) to measure the horizontal “grip” and vertical “load” forces exerted during precision grip.11,21 The contact surfaces of the object were covered with 200-grit sandpaper for the duration of this study. The object’s weight could be modified to 200, 500, or 800 g by inserting an exchangeable mass at the base of the object (Fig. 1A).
FIG. 1.
A: Schematic diagram of instrumented object with force transducer to measure grip force and load force. B: Sample grip force and load force traces from representative control subject. C: Sample grip force and load force traces from a premanifest HD (pre-HD) subject. Vertical lines in B and C indicate the temporal events marked: t0, grip force onset; t1, load force onset; t2, grip force maximum; t3, beginning of static phase; t4, end of static phase. Preload phase was measured as t0–t1, loading phase was measured from t1 to t2, and static phase was measured from t3 to t4.
All subjects washed their hands before participating in the experiment to remove excess dirt, sweat, or oils. Subjects were seated comfortably on a chair in front of a table. The height of the chair was adjusted such that the subjects’ forearms were parallel to the floor at the beginning of the task. Following a demonstration trial and 3 practice trials, each subject was asked to reach for and grasp the object between the index finger and thumb of the dominant hand, lift and hold the object at a height of 10 cm as indicated by a marker, before returning the object to the table. Each trial lasted 10 seconds. All subjects performed 5 trials at each of the 3 object weights (200, 500, and 800 g). The order of conditions was counterbalanced across subjects. We used a range of weights from light to heavy because magnitude and variability of static grip force were shown to be dependent on weight in manifest HD subjects.11,18–20
Quantitative data from the force transducer were sampled at a frequency of 400 samples per second, digitized with 12-bit resolution, and stored in a flexible laboratory computer system (SC/ZOOM, Department of Physiology, Umeå University, Sweden). An interactive graphics terminal was used to score force and temporal events. Figure 1B shows grip force and load force data from a typical trial from a control subject, and Figure 1C shows a representative trial from a pre-HD subject. We marked T0 as the onset of grip force, defined as the time at which the grip force first increased beyond 0.1N. The onset of load force (>0.1N) was marked as T1, and peak value of grip force was marked as T2. These temporal markers were used to define a preload phase (T1–T0) and loading phase (T2–T1). We marked T3 as the time at which load force stabilized once the object was held at the designated marker. Finally, we marked T4 3 seconds from T3. The period between T3 and T4 was defined the static phase.
Clinical Assessment
Premanifest HD subjects were administered the Unified Huntington’s Disease Rating Scale (UHDRS), a valid and reliable clinical rating scale that evaluates behavioral abnormalities and motor, cognitive, and functional skills.23
Motor Skills
A movement disorder specialist rated the motor items of the UHDRS. On the basis of the total motor score, the specialist made a judgment of confidence rating ranging from 0 to 4, defined as follows: 0, no motor abnormalities; 1, nonspecific motor abnormality (<50% confidence), 2, motor abnormalities that may be signs of HD (50%–89% confidence); 3, motor abnormalities that are likely signs of HD (90%–98% confidence); 4, motor abnormalities that are unequivocal signs of HD (≥99% confidence). Within the motor scale, we analyzed the total motor score and 3 items pertaining to hand function: finger tapping, pronation–supination, and the Luria test. The total motor score is computed from 31 items, with a maximum possible score of 124. Each item is rated from 0 (no impairment) to 4 (maximum impairment).
Cognitive Skills
We administered the following items from the UHDRS: Symbol Digit Modality test, a brief test of substitution; the Stroop test; Trail Making A and B, which measure executive function; and the Luria test, which is a measure of frontal lobe dysfunction.23
Functional Ability
Functional ability was assessed by the functional capacity scale (TFC), which evaluates capacity to perform activities of daily living and instrumental activities of daily living (scale of 0–13, with higher scores indicating greater ability).
Statistical Analysis
Differences in performance between the 2 groups (pre-HD and controls) were examined by conducting parametric and nonparametric analyses. Because the results were qualitatively similar, we only report results of the parametric statistical analysis. We compared the following dependent variables in a group (control, pre-HD) × weight (200, 500, 800 g) analysis of variance with repeated measures on weight: (1) maximum grip force, (2) coefficient of variation of maximum grip force, (3) static grip force (2 seconds), (4) coefficient of variation in static grip force within each trial, (5) preload time, (6) coefficient of variation of preload time, (7) loading time, and (8) coefficient of variation of loading time. Group comparisons were considered significant if P < .05.
Variables that were significantly different between pre-HD and controls were entered as dependent variables into a polynomial regression analysis with predicted years to onset for pre-HD. Estimated years to predicted clinical onset was computed from a parametric survival model based on the CAG repeat length and the person’s age at the time of motor testing.24 A blinded statistician for the PREDICT-HD study22 performed the computations of years to predicted onset. In addition, we conducted correlation analysis between selected grip variables and the clinical neurological exam (UHDRS), tests of cognitive function, and gait (from data published previously).9 Correlation with gait variables was conducted to evaluate whether motor variability, common to discrete (precision grip) and rhythmical (gait) movements in pre-HD, is related. Statistical analyses were carried out in SPSS (version 16.0). Group results are presented as mean ± standard deviation.
Results
Table 1 presents demographic and clinical characteristics for the 2 subject groups. There were no differences between groups in age and sex. Premanifest Huntington’s disease (pre-HD) subjects were, on average, 14.6 ± 1.84 years from predicted disease onset as computed from the probabilistic model.24 Pre-HD subjects either demonstrated no motor impairments (score of 0 for 5 subjects) or nonspecific motor impairments (score of 1 for 10 subjects) on diagnostic confidence rating. Total motor score for pre-HD subjects was 2.78 ± 1.62 from a total possible score of 124, indicating that clinical neurological exam revealed minimal nonspecific motor impairments. None of the pre-HD subjects demonstrated functional limitations (13 of 13 on the TFC).
TABLE 1.
Demographic and clinical characteristics of premanifest Huntington’s disease (pre-HD) and control subjects
| Demographic and clinical measure | Control | Pre-HD |
|---|---|---|
| Age (y) | 37.55 (11.78) | 38.86 (10.53) |
| Men/women | 6/9 | 6/9 |
| Race (% white) | 80% | 100% |
| UHDRS total motor score, range (SD) | NT | 2.78, 0–6 (1.62) |
| UHDRS upper limb score, range (SD) | NT | 2.18, 0–5 (1.72) |
| UHDRS, TFC score, range (SD) | NT | 13, 13–13 (0) |
| Mean CAG repeat length (SD) | NT | 42.84 (0.57) |
| Mean predicted yrs from onset (SD/range) |
NA | −14.6 (1.84/19.65) |
NT, not tested; NA, not applicable.
Force Control in Precision Grip
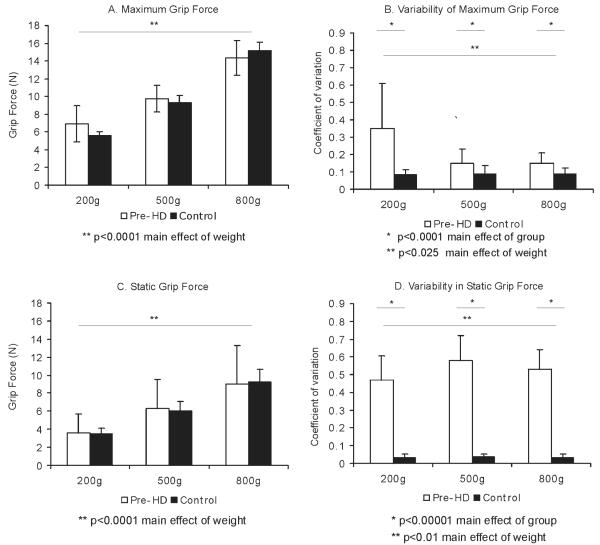
Control and pre-HD subjects were able to scale their grip force in a predictive manner to match object weight, as seen in Figure 2. Maximum grip force increased with object weight for both groups of subjects (Fig. 2A). Analysis of variance (ANOVA) for maximum grip force demonstrated a significant effect of weight (F = 29.25, P < .0001) but did not demonstrate differences between groups (F = 0.08, P = .78). We observed a similar pattern of results for static grip force, which increased with object weight (Fig. 2C). ANOVA demonstrated a significant effect for weight (F = 42.41, P < .0001) but did not demonstrate a significant effect for group (F = 0.17, P = .67).
FIG. 2.
Mean and standard deviation for grip force for premanifest HD (empty bars) and control subjects (black bars) for 3 object weights (200, 500, and 800 g). A: Maximum grip force. B: Variability of maximum grip force. C: Static grip force. D: Variability of static grip force. *Significant effect of group; **significant effect of condition (weight). Significance levels are listed below each graph.
Force variability was significantly higher for pre-HD subjects compared with controls (Fig. 2B,D). Coefficient of variation for maximum grip force was higher in pre-HD, as seen by a significant effect of group (F = 17.9, P = .0001). Variability in maximum grip force was highest for the lighter weight (200 g) compared with the heavier weights (F = 5.74, P = .025). Within-trial coefficient of variation of static grip force was also higher for pre-HD compared with controls (main effect of group, F = 231.38, P < .00001), particularly for the heavier weights (500 and 800 g), as seen by a significant effect of weight (F = 4.43, P < .018) and a significant interaction effect (F = 3.99, P < .025).
Temporal Control in Precision Grip
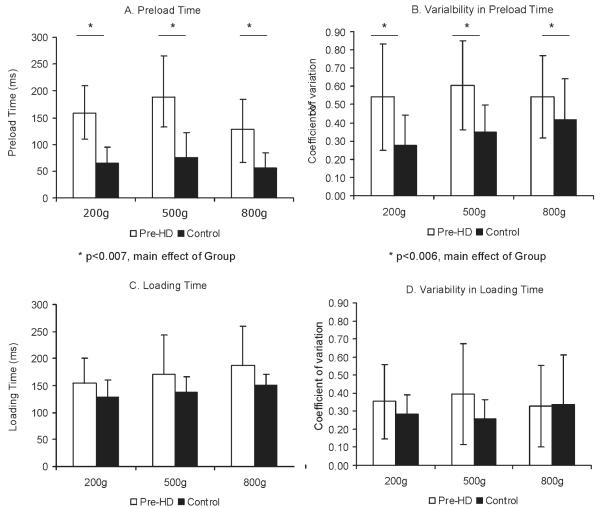
We compared duration of preload phase and loading phase during precision grip. Preload time was higher for pre-HD subjects compared with controls (Figure 3A), evidenced by a significant effect of group (F = 9.13, P < .007), but the effect was not modulated by weight (F = 1.97, P < .15). Variability in preload time was higher for pre-HD for all object weights (Figure 3B), as seen by a significant effect of group for coefficient of variation of preload time (F = 9.48, P < .006). There were no differences in loading time (F = 3.09, P < .09) or variability in loading time across groups (F = 2.36, P < .14), but as expected, this measure was longer for heavier weights (Figure 3B,D).
FIG. 3.
Mean and standard deviation for temporal control for premanifest HD (empty bars) and control subjects (black bars) for 3 object weights (200, 500, and 800 g). A: Preload time. B: Variability of preload time. C: Loading time. D: Variability of loading time. *Significantly different effect of group; **significantly different effect of condition (weight). Significance levels are listed below each graph.
Predictive Validity of Precision Grip
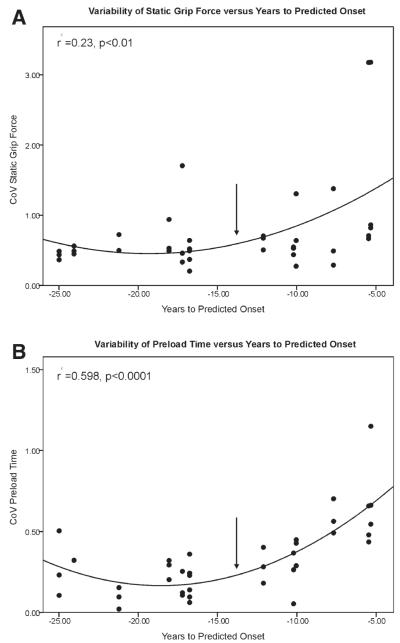
We conducted polynomial regression analysis (using a quadratic fit) of predicted years to onset for 4 variables that were significantly different across groups: preload time, coefficient of variation (CoV) preload time, CoV maximum grip force, and CoV static grip force. Of the 4 variables, CoV of static grip force and CoV preload time increased with predicted years to onset. CoV of static grip force increased as subjects approached disease onset (r2 = 0.23, P < .01), as seen in the top panel of Figure 4. CoV of preload time also increased as subjects approached predicted disease onset (r2 = 0.598, P < .0001), as seen in the bottom panel of Figure 4. It is interesting to note that the slope changed significantly between 10 and 15 years from predicted onset for both variables. These results indicate that variability of preload time, in particular, is a good predictor of estimated disease onset in pre-HD subjects.
FIG. 4.
Relationship between estimated years to onset of Huntington’s disease and variability of static grip force (A) and variability of preload time (B). Inflection point in each graph marks the change in slope between 15 and 10 years from onset.
Association of Precision Grip with Cognitive Function and Gait
Given the high predictive validity of coefficient of variation of preload time and static grip force, we examined their association (using correlation coefficients) with clinical measures of cognitive function and gait (Table 2). Coefficient of variation of preload time was significantly correlated with several measures of cognitive function, such as the Stroop test, Luria, and Trail Making B. In contrast, coefficient of variation of static grip force was significantly correlated only with the Luria test. Variability of preload time was correlated with stride length variability (r = 0.59), swing time variability (r = 0.59), and double support variability (r = 0.57). Variability of static grip force was correlated with variability of double support percentage (r = 0.6). Thus, precision grip variability appears to be a robust measure to differentiate between pre-HD and healthy controls and is also associated with measures of cognitive and gait impairments.
TABLE 2.
Correlation of selected measures of precision grip with cognitive tests and gait (significance level listed in parentheses)
| CoV preload time | CoV static grip force | |
|---|---|---|
| Cognitive test | ||
| Stroop Color Naming | 0.643 (P < .02) | 0.53 (P < .06) |
| Stroop Word Reading | 0.649 (P < .02) | 0.495 (P < .08) |
| Stroop Interference | −0.513 (P < .06) | −0.31 (P < .33) |
| Luria | 0.61 (P < .03) | 0.697 (P < .002) |
| Trail Making A | 0.14 (P < .84) | 0.245 (P < .84) |
| Trail Making B | 0.99 (P < .005) | 0.613 (P < .27) |
| Symbol Digit Modality Test | −0.55 (P < .06) | 0.29 (P < .35) |
| Gait | ||
| Stride length CoV | 0.59 (P < .03) | 0.5 (P < .09) |
| Swing time CoV | 0.59 (P < .03) | 0.23 (P < .47) |
| Double support % CoV | 0.57 (P < .04) | 0.6 (P < .03) |
Discussion
The precision grip task allows for the study of mechanisms underlying the control of complex, sequential voluntary movements in a functional context. Results of our study demonstrate that premanifest HD (pre-HD) subjects scaled their grip force appropriately with object weight similar to that of healthy control subjects. There were no differences in maximum grip force or static grip force between the 2 groups. However, variability of maximum grip force and static grip force was higher in pre-HD subjects. Pre-HD subjects also demonstrated specific impairments in temporal control, which included longer and highly variable preload time. Variability of preload time and static grip force was correlated with predicted disease onset and clinical measures of cognitive function, indicating that force and temporal variability may be good markers of disease onset and severity.
Manifest HD subjects are known to demonstrate increased grip force at load force onset and higher maximum grip force, particularly for lighter objects.11,12,20 This result suggests that HD subjects may apply higher grip force because of a deficit in processing sensory information. Given that higher grip force was not seen in pre-HD in our study, higher forces may be seen as a general compensation for disturbed sensory processing in manifest HD rather than a primary impairment specific to early HD.25 In contrast, impairments common to premanifest and manifest HD subjects are temporal delays during the preload phase, implicating poor regulation of the transition between reaching toward objects and grasping objects. In addition, pre-HD subjects demonstrated higher variability in temporal and force parameters, as has been demonstrated for manifest HD subjects.11,20,21 Because variability was correlated with predicted disease onset, it appears that increased motor variability is an additional significant feature of early basal ganglia pathology.9,26,27
What might cause delays in phase transitions and increased variability in premanifest subjects with no clinically observable impairments? For successful grasping of objects, accurate predictive control (to estimate object weight and produce appropriate grip forces) and reactive control (to modulate grip force in response to changing surface texture) is necessary.17 Given that pre-HD subjects in our study and manifest HD subjects (from prior work) are able to scale grip force with object weight, predictive control may not be impaired in HD. When an object is unexpectedly loaded, manifest HD subjects demonstrate appropriate magnitude but delayed onset in reactive increase in grip force.20 Because standard clinical examination of the hand and fingers does not detect sensory impairments,28 any sensory processing deficit may be central in origin.29,30 This is supported by the observation that long latency stretch reflexes and somatosensory evoked potentials are delayed in manifest HD.18,29,30 Increased activation of the globus pallidus has been shown in response to passive sensory stimulation in healthy subjects. However, patients with Parkinson’s disease and Huntington’s disease demonstrated decreased activation of the globus pallidus and putamen, highlighting the important of the basal ganglia as sensory analyzers.30
Because timely somatosensory information is necessary for the successful transition from reaching to grasping,31 it is possible that intact basal ganglia may be important for processing somatosensory information at phase transitions. Basal ganglia pathology begins well before onset of clinical symptoms in premanifest HD, as seen by decreased striatal volume computed from MRI scans.27,32,33 The presence of pathological changes may help to explain the increased variability seen during precision grip and during gait in the premanifest HD subjects.9
Another possibility is that higher variability in HD is related to a deficit in timing control, for which the basal ganglia are proposed to play a role.26,34 A recent study measured basal ganglia volume while pre-HD subjects completed a time reproduction task and reported that increased motor timing variability was associated with decreased striatal volume.33 These and additional data on increased variability on repetitive tapping tasks indicate that timing control may indeed be a putative deficit.26 However, none of the results discussed thus far allow us to resolve between the two proposed mechanistic deficits (sensory processing versus timing control). It may be useful in future work to better clarify mechanisms underlying impairments.
The similarity of impairments in precision grip and gait indicates that motor variability is a robust early neurobiological marker, rather than simply a task specific impairment. Future clinical trials may benefit from the use of motor variability as an outcome measure. In our study temporal and force variability demonstrated minimal impairment from 15 to 25 years from predicted onset. However, between 15 and 10 years from predicted onset, the slope of force and temporal variability increased, as was reported by the PREDICT-HD study with a larger sample size for tapping tasks, striatal volume, odor identification, and UDHRS motor exam score.35 Future work using the precision grip paradigm with larger samples will be important to validate the results of this study.
Footnotes
Relevant conflict of interest: Nothing to report.
References
- 1.A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 2.Mahant N, McCusker EA, Byth K, Graham S. Huntington’s disease: clinical correlates of disability and progression. Neurology. 2003;61:1085–1092. doi: 10.1212/01.wnl.0000086373.32347.16. [DOI] [PubMed] [Google Scholar]
- 3.Ravina B, Romer M, Constantinescu R, et al. The relationship between CAG repeat length and clinical progression in Huntington’s disease. Mov Disord. 2008;23:1223–1227. doi: 10.1002/mds.21988. [DOI] [PubMed] [Google Scholar]
- 4.Bylsma FW, Rothlind J, Hall MR, Folstein SE, Brandt J. Assessment of adaptive functioning in Huntington’s disease. Mov Disord. 1993;8:183–190. doi: 10.1002/mds.870080212. [DOI] [PubMed] [Google Scholar]
- 5.Marder K, Zhao H, Myers RH, et al. Rate of functional decline in Huntington’s disease. Huntington Study Group. Neurology. 2000;54:452–458. doi: 10.1212/wnl.54.2.452. [DOI] [PubMed] [Google Scholar]
- 6.Blekher T, Johnson SA, Marshall J, et al. Saccades in presymptomatic and early stages of Huntington disease. Neurology. 2006;67:394–399. doi: 10.1212/01.wnl.0000227890.87398.c1. [DOI] [PubMed] [Google Scholar]
- 7.Beenen N, Buttner U, Lange HW. The diagnostic value of eye movement recordings in patients with Huntington’s disease and their offspring. Electroencephalogr Clin Neurophysiol. 1986;63:119–127. doi: 10.1016/0013-4694(86)90005-2. [DOI] [PubMed] [Google Scholar]
- 8.Carella F, Bressanelli M, Piacentini S, et al. A study of arm movements in Huntington’s disease under visually controlled and blind-folded conditions. Neurol Sci. 2003;23:287–293. doi: 10.1007/s100720300003. [DOI] [PubMed] [Google Scholar]
- 9.Rao AK, Muratori L, Louis ED, Moskowitz CB, Marder KS. Spectrum of gait impairments in presymptomatic and symptomatic Huntington’s disease. Mov Disord. 2008;23:1100–1107. doi: 10.1002/mds.21987. [DOI] [PubMed] [Google Scholar]
- 10.Smith MA, Brandt J, Shadmehr R. Motor disorder in Huntington’s disease begins as a dysfunction in error feedback control. Nature. 2000;403:544–549. doi: 10.1038/35000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon AM, Quinn L, Reilmann R, Marder K. Coordination of prehensile forces during precision grip in Huntington’s disease. Exp Neurol. 2000;163:136–148. doi: 10.1006/exnr.2000.7348. [DOI] [PubMed] [Google Scholar]
- 12.Quinn L, Reilmann R, Marder K, Gordon AM. Altered movement trajectories and force control during object transport in Huntington’s disease. Mov Disord. 2001;16:469–480. doi: 10.1002/mds.1108. [DOI] [PubMed] [Google Scholar]
- 13.Johansson RS. Sensory input and control of grip. Novartis Found Symp. 1998;218:45–59. doi: 10.1002/9780470515563.ch4. discussion 59–63. [DOI] [PubMed] [Google Scholar]
- 14.Johansson RS, Edin BB. Neural control of manipulation and grasping. In: Forssberg H, Hirschfeld H, editors. Movement Disorders in Children (Medicine and Sport Science) Karger; Basel Switzerland: 1992. pp. 107–112. [Google Scholar]
- 15.Gordon AM, Forssberg H, Johansson RS, Westling G. Visual size cues in the programming of manipulative forces during precision grip. Exp Brain Res. 1991;83:477–482. doi: 10.1007/BF00229824. [DOI] [PubMed] [Google Scholar]
- 16.Nowak DA, Hermsdorfer J. Predictive and reactive control of grasping forces: on the role of the basal ganglia and sensory feedback. Exp Brain Res. 2006;173:650–660. doi: 10.1007/s00221-006-0409-7. [DOI] [PubMed] [Google Scholar]
- 17.Prodoehl J, Corcos DM, Vaillancourt DE. Basal ganglia mechanisms underlying precision grip force control. Neurosci Biobehav Rev. 2009;33:900–908. doi: 10.1016/j.neubiorev.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fellows S, Schwarz M, Schaffrath C, Domges F, Noth J. Disturbances of precision grip in Huntington’s disease. Neurosci Lett. 1997;226:103–106. doi: 10.1016/s0304-3940(97)00264-4. [DOI] [PubMed] [Google Scholar]
- 19.Serrien DJ, Burgunder JM, Wiesendanger M. Grip force scaling and sequencing of events during a manipulative task in Huntington’s disease. Neuropsychologia. 2001;39:734–741. doi: 10.1016/s0028-3932(00)00153-6. [DOI] [PubMed] [Google Scholar]
- 20.Schwarz M, Fellows SJ, Schaffrath C, Noth J. Deficits in sensorimotor control during precise hand movements in Huntington’s disease. Clin Neurophysiol. 2001;112:95–106. doi: 10.1016/s1388-2457(00)00497-1. [DOI] [PubMed] [Google Scholar]
- 21.Reilmann R, Kirsten F, Quinn L, Henningsen H, Marder K, Gordon AM. Objective assessment of progression in Huntington’s disease: a 3-year follow-up study. Neurology. 2001;57:920–924. doi: 10.1212/wnl.57.5.920. [DOI] [PubMed] [Google Scholar]
- 22.Paulsen JS, Langbehn DR, Stout JC, et al. Detection of Huntington’s disease decades before diagnosis: the Predict-HD study. J Neurology Neurosurg Psychiatry. 2008;79:874–880. doi: 10.1136/jnnp.2007.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Unified Huntington’s Disease Rating Scale: reliability and consistency. Huntington Study Group. Mov Disord. 1996;11:136–142. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- 24.Langbehn DR, Brinkman RR, Falush D, Paulsen JS, Hayden MR. A new model for prediction of the age of onset and penetrance for Huntington’s disease based on CAG length. Clin Genet. 2004;65(4):267–277. doi: 10.1111/j.1399-0004.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- 25.Lidsky TI, Manetto C, Schneider JS. A consideration of sensory factors involved in motor functions of the basal ganglia. Brain Res. 1985;356:133–146. doi: 10.1016/0165-0173(85)90010-4. [DOI] [PubMed] [Google Scholar]
- 26.Hinton SC, Paulsen JS, Hoffmann RG, Reynolds NC, Zimbelman JL, Rao SM. Motor timing variability increases in preclinical Huntington’s disease patients as estimated onset of motor symptoms approaches. J Int Neuropsychol Soc. 2007;13:539–543. doi: 10.1017/S1355617707070671. [DOI] [PubMed] [Google Scholar]
- 27.Tabrizi SJ, Langbehn DR, Leavitt BR, et al. Biological and clinical manifestations of Huntington’s disease in the longitudinal TRACK-HD study: cross-sectional analysis of baseline data. Lancet Neurol. 2009;8:791–801. doi: 10.1016/S1474-4422(09)70170-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abbruzzese G, Berardelli A. Sensorimotor integration in movement disorders. Mov Disord. 2003;18:231–240. doi: 10.1002/mds.10327. [DOI] [PubMed] [Google Scholar]
- 29.Thompson PD, Berardelli A, Rothwell JC, et al. The coexistence of bradykinesia and chorea in Huntington’s disease and its implications for theories of basal ganglia control of movement. Brain. 1988;111:223–244. doi: 10.1093/brain/111.2.223. [DOI] [PubMed] [Google Scholar]
- 30.Boecker H, Ceballos-Baumann A, Bartenstein P, et al. Sensory processing in Parkinson’s and Huntington’s disease: investigations with 3D H(2)(15)O-PET. Brain. 1999;122:1651–1665. doi: 10.1093/brain/122.9.1651. [DOI] [PubMed] [Google Scholar]
- 31.Johansson RS, Westling G. Roles of glabrous skin receptors and sensorimotor memory in automatic control of precision grip when lifting rougher or more slippery objects. Exp Brain Res. 1984;56:550–564. doi: 10.1007/BF00237997. [DOI] [PubMed] [Google Scholar]
- 32.Paulsen JS, Magnotta VA, Mikos AE, et al. Brain structure in preclinical Huntington’s disease. Biol Psychiatry. 2006;59:57–63. doi: 10.1016/j.biopsych.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 33.Zimbelman JL, Paulsen JS, Mikos A, Reynolds NC, Hoffmann RG, Rao SM. fMRI detection of early neural dysfunction in preclinical Huntington’s disease. J Int Neuropsychol Soc. 2007;13:758–769. doi: 10.1017/S1355617707071214. [DOI] [PubMed] [Google Scholar]
- 34.Freeman JSCF, O’Boyle DJ, Crauford D, Neary D, Snowden JS. Abnormalities of motor timing in Huntington’s disease. Parkinsonism Relat Disord. 1996;2:81–93. doi: 10.1016/1353-8020(96)00009-0. [DOI] [PubMed] [Google Scholar]
- 35.Paulsen JS, Langbehn DR, Stout JC, et al. Predict-HD Investigators and Coordinators of the Huntington Study Group Detection of Huntington’s disease decades before diagnosis: the Predict HD study. J Neurol Neurosurg Psychiatry. 2008;79:874–880. doi: 10.1136/jnnp.2007.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]