Abstract
Objective. Rheumatologic disorders are associated with sleep disturbances. This study examines sleep disturbance correlates in patients with SSc.
Methods. Participants are 180 SSc patients in an observational study. At baseline, patients completed the Medical Outcomes Study Sleep measure (MOS-Sleep scale). In addition, patients were administered other patient-reported outcome (PRO) measures including the 36-item short form (SF-36), HAQ disability index (HAQ-DI), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue), Center for Epidemiologic Studies Depression (CESD) scale and a University of California at Los Angeles Scleroderma Clinical Trial Consortium Gastrointestinal Tract Questionnaire (UCLA SCTC GIT 2.0). Descriptive statistics were assessed for six scales of MOS-Sleep and the 9-item sleep problem index (SLP-9; a composite index). We computed Spearman’s rank-order correlations between the MOS-Sleep scales and the HAQ-DI, FACIT-Fatigue, CESD, SSc-SCTC GIT 2.0 and SF-36 scales. In addition, we developed a regression model to assess predictors of SLP-9 scores. Covariates included demographics, physician variables of disease severity and patient-reported variables of worsening symptoms and the PRO measures.
Results. SSc patients reported a mean (s.d.) of 7.1 (1.73) h of sleep a night. Patients reported worse scores on four of six scales (except for snoring and sleep quantity) compared with the US general population (P < 0.001). SLP-9 was correlated with worsening pain and dyspnoea over the past 1 month, reflux scale of the UCLA SCTC GIT 2.0, CESD and FACIT-Fatigue (ρ 0.26–0.56). In the stepwise multivariate regression model, the CESD, worsening dyspnoea and reflux scale were significantly associated with SLP-9 index.
Conclusion. Sleep disturbances are common in SSc and are associated with worsening dyspnoea, depressed mood and severity of reflux symptoms.
Keywords: Systemic sclerosis, Scleroderma, Sleep, Depression, Gastroesophageal reflux, Quality of life, SF-36, HAQ disability index (HAQ-DI), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue), Center for Epidemiologic Studies Depression (CESD) scale, University of California at Los Angeles Scleroderma Clinical Trial Consortium Gastrointestinal Tract Questionnaire (UCLA SCTC GIT 2.0)
Introduction
SSc (scleroderma) is a chronic multisystem disease characterized by immune deregulation, vasculopathy and fibrosis. This immune dysfunction may cause chronic sleep disturbance that has detrimental effects on health and life expectancy [1]. Brain–immune interactions are an essential component in psychiatric and medical comorbidities that significantly impact health and sleep [2, 3]. SSc patients are believed to be at increased risk for sleep disturbance by polysomnographic findings [4, 5]. In one study, 27 consecutive SSc patients underwent all-night polysomnogram and investigators found that oesophageal dysmotility, dyspnoea and restless leg syndrome were associated with sleep disturbances. In other arthritides, sleep disturbances are associated with self-reported pain, depressed mood and fatigue [6–8].
Evaluation of a sleep disturbance requires assessment of multiple dimensions of sleep [9]. While not a diagnostic tool, the Medical Outcomes Study Sleep (MOS-Sleep) scale has been studied in other chronic diseases and these studies provide support for the feasibility, reliability and validity of the MOS-Sleep scale [10–13]. This study examines the correlates of sleep disturbance in patients with SSc in an effort to better understand the interventions that may improve disease outcomes. Based on previous studies, we hypothesized that: (i) patients with SSc will have greater sleep disturbance compared with the US general population; and (ii) self-reported pain, depressed mood and upper gastrointestinal tract involvement will be associated with sleep disturbances.
Patients and methods
Patients
University of California at Los Angeles (UCLA) Scleroderma Quality of Life Study is a single-centre ongoing longitudinal observational study where patients with SSc are invited to participate during their clinic visits. The current analysis reports the baseline data. Potential participants were approached at the time of a scheduled clinic visit and completed written consent and Health Insurance Portability and Accountability Act (HIPAA) forms. The study was approved by UCLA Institutional Review Board (IRB), study number 7-07-061-01.
Inclusion criteria include adult patients (≥18 years) with diagnosis of SSc. Patients with SSc were further divided into limited SSc, diffuse SSc and overlap syndrome according to ACR criteria [14, 15]. Limited SSc is defined as skin thickening distal, but not proximal, to the knees and elbows, with or without facial involvement; diffuse SSc is defined as skin thickening distal and proximal to the knees and elbows, with or without facial involvement; and overlap syndrome is defined as patients with SSc and another rheumatic disease such as inflammatory myositis or RA.
Patient-reported outcome measures
MOS-Sleep measure, the 36-item Medical Outcome Survey Short Form (SF-36), the HAQ-disability index (HAQ-DI), the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue), the MOS-Social Support scale, the Center for Epidemiologic Studies Depression (CESD) scale and the UCLA Scleroderma Clinical Trial Consortium Gastrointestinal Tract Questionnaire (UCLA SCTC GIT 2.0) were administered to each patient.
The MOS-Sleep is a 12-item instrument with six scales. These include: (i) sleep disturbance, which assesses trouble falling asleep, how long to fall asleep, sleep was not quiet, awaken during your sleep time and have trouble falling asleep again; (ii) sleep adequacy, which asks if a person gets enough sleep to feel rested upon waking in the morning, gets amount of sleep needed; (iii) daytime somnolence, which assesses drowsy during day, have trouble staying awake during the day, take naps; (iv) snoring; (v) awaken short of breath or with headache; and (vi) quantity of sleep. Items are administered using a past 4-week recall interval. Quantity of sleep is scored as the average hours slept per night. Five of the above six scales (except sleep quantity) is scored into a 9-item sleep problem index (SLP-9) are scored on a 0–100 possible range. For all scales and SLP-9, higher score relates to poor health-related quality of life (HRQOL) (except for sleep adequacy where higher score relates to better HRQOL). The MOS-Sleep scale is available online (http://www.rand.org/health/surveys/sleepscale/), and a scoring guide can be found at http://www.gim.med.ucla.edu/FacultyPages/Hays/sleep.htm.
The SF-36 version 2 is a generic health status measure consisting of 36 items assessing eight domains [16]: physical functioning (10 items); bodily pain (2 items); role limitations due to physical health problems (4 items); general health perceptions (5 items); mental health (5 items); role limitations due to emotional problems (3 items); vitality (4 items); and social functioning (2 items). The eight scales are summarized into physical component summary (PCS) and mental component summary (MCS) scores. The summary scores are normalized to the US general population, with mean scores of 50 and s.d. of 10. We used the standard 4-week recall version of the SF-36 v. 2 [17].
The HAQ-DI is an arthritis-targeted measure intended for assessing functional ability in arthritis [18]. It is a self-administered 20-question instrument that assesses a patient’s level of functional ability and includes questions that involve both upper and lower extremities. The HAQ-DI score is determined by summing the highest item score in each of eight domains (dressing, arising, eating, walking, hygiene, reach, grip and common daily activities) and dividing the sum by eight, which results in a score from 0 (no disability) to 3 (severe disability).
Depressive symptoms were measured with the 10-item CESD (CESD-10) [19]. The CESD-10 is a subset of the 20-item CESD scale and has been used extensively in general populations and patients with chronic illness. The items include depressed mood, feelings of guilt, worthlessness and helplessness, psychomotor retardation, loss of appetite and sleep difficulties. Both versions have an emphasis on affective symptoms. The CESD-10 uses a 4-point categorical response scale (range 0–30) with higher scores representing greater depressive symptoms. The cut-off of ≥10 is indicative of depressed mood.
The FACIT-Fatigue is a 13-item questionnaire that assesses self-reported fatigue and its impact upon daily activities and function. It uses a 5-point categorical response scale (0 = not at all; 4 = very much). The range of possible scores is 0–52, with lower scores reflecting more fatigue.
UCLA SCTC GIT 2.0 assesses GIT symptom severity and its impact on social and emotional well-being in SSc [20, 21]. This 34-item instrument has seven scales: reflux, distention/bloating, diarrhoea, faecal soilage, constipation, emotional well-being and social functioning and a total GIT score. The total GIT score is the average of six of seven scales (excludes constipation) and total gastrointestinal (GI) score is scored from 0 (better HRQOL) to 3 (worse HRQOL) except for diarrhoea scale with a maximum score of 2 (worse HRQOL) and constipation scale with a score of 2.5 (worse HRQOL). The UCLA SCTC GIT 2.0 captures SSC-related GIT symptom severity and its impact on HRQOL and can be used both in clinical trials and day-to-day care [20]. It is available free online at http://uclascleroderma.researchcore.org/.
The patient global assessment was assessed using a one-item question: In general, would you say your health is? The choices ranged from 1 = excellent health and 5 = poor health. Pain was assessed using a single-item scale: How much pain did you have in the past 7 days? Very mild, mild, moderate, severe and very severe? Worsening of SSc-associated symptoms was captured using single-item questions from the scleroderma disease activity index. These questions asked about any new symptoms or worsening of symptoms over the past 1 month of the skin, RP or digital ulcers, GI symptoms (upper and lower) and shortness of breath or chest pain in patients. Physicians were asked to assess the overall health of their patient in the last week. On a scale of 0–10, how was your patient’s overall health in the last week? Responses are rated on a scale of 0–10, where 0 = excellent health and 10 = extremely poor health.
MOS-Sleep scores in the US general population and participants of the MOS
For the US general population, Hays et al. administered the MOS-Sleep measure by telephone to a nationally representative sample of 1011 US adults aged ≥18 years in January 2001 [22, 23]. The observations were weighted (using current population survey data) by age, gender, race, education, number of adults and number of voice/telephone lines in the household to reflect the adult (≥18 years) population. More detail on the instrument and population is available in Hays et al. [22].
MOS participants
MOS was an observational study to assess variations in physician practice styles and patient outcomes recruited in three large cities: Los Angeles, Boston and Chicago [24, 25]. Non-institutionalized English-speaking adult patients were screened and a subset of these patients (n = 2471) with one or more of four conditions (hypertension, diabetes, advanced coronary artery disease and depression) were enrolled in a longitudinal study. This group formed the baseline cohort for development and validation of the MOS-Sleep instrument.
Statistics and analysis
Mean scores, s.d.s, ranges and percentages of respondents scoring the minimum (floor) and maximum (ceiling) possible scores were calculated to evaluate distributions for all instruments. For easy interpretability, floor effect is presented as worst score and ceiling as best irrespective of the direction of the scale. Internal consistency reliability for HRQOL instruments was estimated using Cronbach’s α [13]. An α of >0.70 is considered satisfactory. We compared the mean (s.d.) scores of our SSc patients with the US general population and MOS population. We used standard t-test to compare the two groups.
Each dimension of the MOS-Sleep questionnaire: sleep disturbance (SLPD); sleep adequacy (SLPA); daytime somnolence (SLPS); snoring (SLPSNR); awakening short of breath or with headache (SLPSOB); and quantity of sleep (SLPQ) as well as the SLP-9 were assessed by Spearman’s rank correlation with the HAQ-DI, FACIT-Fatigue, MOS-Social Support, CESD, UCLA SCTC GIT 2.0 total score, SF-36 PCS and MCS and single-item questions. Correlations ≤ 0.29 were considered to be small, between 0.30 and 0.49 were moderate and ≥0.50 were large [26].
We developed multivariate regression models to assess the factors associated with sleep disturbance in SSc patients. SLP-9 was modelled as a continuous variable using a series of nested models to document the unique variance accounted for by different groups of variables, including demographics, physician- and patient-reported variables. Demographic variables (age, sex, ethnicity and BMI), physician-reported measures [patient global assessment on 0–100 visual analogue scale (VAS) and modified Rodnan skin score (MRSS)] and patient-reported measures—the HAQ-DI, FACIT-Fatigue, CESD, UCLA SCTC GIT 2.0 total score and single items (pain VAS, patient global assessment VAS, new/worsening of skin, RP or digital ulcers, and shortness of breath or chest pain) were used based on previous literature in SSc and other arthritides [27, 28]. Since Prado et al. [4] found that reflux was associated with poor sleep in SSc, we also modelled five separate scales of the UCLA SCTC GIT 2.0 (reflux, distention/bloating, constipation, diarrhoea and faecal soilage). We did not include the GIT 2.0 social functioning and emotional well-being subscales or the SF-36 scales due to overlap with HAQ-DI, FACIT-Fatigue and CESD. We used a multivariable linear regression model for demographics (Model 1) and physician-reported measures (Model 2). For Model 3, we used stepwise regression with forward selection (variables were included if P < 0.15) and backward elimination (variables were excluded if P > 0.15) and forcing the variables from Models 1 and 2. This was done as there were large numbers of variables for the patient-reported measures. The coefficient of determination, R2, was used in the context of this model to account for the proportion of variability in the data set that is accounted for by the statistical model. It provides a measure of how well future outcomes are likely to be predicted by the model. All analyses were performed using Stata 10.2 (Statacorp, College Station, TX, USA) and P < 0.05 was indicative of statistical significance.
Results
A total of 180 patients completed the MOS-Sleep scale and form the cohort in this study. Of these participants, 82% were female. The mean (s.d.) age of the participants was 51.1 (15.2) years, disease duration was 7.5 (8.1) years, 69% were Caucasian and 50.9% had limited SSc (Table 1). Patient global assessments were excellent/very good in 24 (13.3%), good in 69 (38.3%) and fair/poor in 87 (48.3%). Patients had mild functional disability (mean HAQ-DI 0.94) and mean PCS and MCS were 1.2 and 0.2 s.d. less than that in the US general population, respectively [29]. There were 65 (36.1%) patients who had depressed mood (defined as CESD ≥10).
Table 1.
Baseline characteristics of study participants
| Variables | Total sample (n = 180) | |
|---|---|---|
| Age, mean (s.d.), years | 51.1 (15.2) | |
| Gender: female, n (%) | 148 (82.2) | |
| Race, n (%) | ||
| White | 122 (67.8) | |
| African-American | 13 (7.2) | |
| Asian | 21 (11.7) | |
| Others | 16 (8.9) | |
| Education, n (%)a | ||
| Less than or equal to high-school graduate | 30 (16.8) | |
| Some college | 62 (34.8) | |
| College graduate | 38 (21.4) | |
| Graduate degree | 86 (48.4) | |
| Type of SSc, n (%)b | ||
| Limited | 90 (50.9) | |
| Diffuse | 71 (40.1) | |
| Overlap | 11 (6.2) | |
| Swollen joint count (0–12) | 0.2 (0.8) | |
| Tender joint count (0–12) | 1.2 (2.4) | |
| Patient global (categorical) | ||
| Excellent/very good | 24 (13.3) | |
| Good | 69 (38.3) | |
| Fair/poor | 87 (48.3) | |
| Pain over past 7 days (0–10) | ||
| Clinical status worsening over past 1 month | ||
| Gastrointestinal symptoms | 72 (40.5) | |
| Skin thickening | 49 (27.2) | |
| Digital ulcers | 62 (34.6) | |
| Dyspnoea | 65 (36.1) | |
| MRSS, mean (s.d.) | 8.8 (8.5) | |
| Physician global assessment (0–10 scale) | 3.6 (2.1) | |
| HRQOL, mean (s.d) | ||
| SF-36 PCS | 38.1 (10.1) | |
| SF-36 MCS | 48.5 (12.3) | |
| HAQ-DI (0–3) | 0.94 (0.67) | |
| CESD (0–30) | 8.4 (5.9) | |
| FACIT-Fatigue (0–52) | 31.9 (12.55) | |
| UCLA SCTC GIT total score (0–3) | 0.55 (0.46) |
aData not available for two patients. bData not available for three patients. A higher score on the SF-36 and FACIT represents better HRQOL; a higher score on the HAQ-DI, CESD, and UCLA SCTC GIT 2.0 represents worse HRQOL.
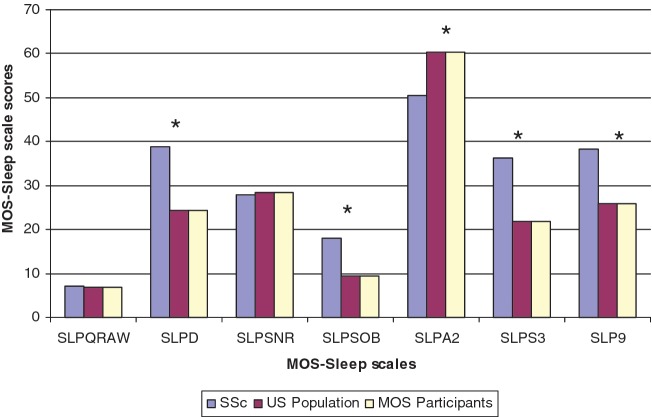
SSc patients reported a mean (s.d.) of 7.1 (1.73) h sleep a night (range 0–11 h) and reported worse scores on four of six sleep scales (except for snoring and sleep quantity) compared with the US general population and MOS population (Fig. 1). The general population had an average age of 46 years (range 18–94 years); 51% were females and 81% were white [20]. The MOS participants (n = 2471) had a mean (s.d.) age of 55.5 (16.3) years, 60.5% were women and 79.6 were white. Minimal floor effects (suggesting most severe symptoms) were noted on scales and ranged from 1% (snoring) to 8% (sleep adequacy and snoring). There were high-ceiling effects for snoring and shortness of breath scales (41 and 59%), similar to the MOS population (33 and 64%, respectively) [30].
Fig. 1.
Comparison of MOS-Sleep scales between SSc patients, previous MOS participants and US general population. *P < 0.05. SLPQRAW: sleep quantity (h); SLPD: sleep disturbance; SLPSNR: snoring; SLPSOB: awakening short of breath or with headache; SLPA2: sleep adequacy; SLPS3: daytime somnolence. All scales (except sleep quantity) are scored from 0 to 100. In addition, all scales (except sleep adequacy) are scored from better (0) to worse (100). Sleep adequacy is scored from worse (0) to better (100).
SLP-9 index had moderate correlations with HAQ-DI and total GIT score (ρ 0.32) to large correlations with the CESD and FACIT-Fatigue (ρ 0.56 and 0.50; see Supplementary data available at Rheumatology Online). Within the GIT 2.0 scales: reflux had the largest correlation (0.38), followed by distention (0.29), emotional (0.26), diarrhoea (0.22), faecal soilage (0.17) and constipation (0.12). Other correlation coefficients are shown in Supplementary data (available at Rheumatology Online) and are notable for low correlations to tender joint count (0.04), swollen joint count (0.09) and digital ulceration (0.08).
We then modelled SLP-9 index in a hierarchical multivariate regression model. With the exception of BMI, demographic variables had no impact on the model (adjusted R2 = 0.02) with an additional variance of 14% explained by addition of MRSS and physician global assessment of disease severity. Addition of patient-reported measures accounted uniquely for an additional 42% variance, with the CESD, total GIT 2.0 score and increasing gastrointestinal symptoms and shortness of breath over past 1 month significantly associated with the SLP-9 index (Table 2). When we modelled the individual scales of UCLA SCTC GIT 2.0, we found that the reflux scale of UCLA SCTC GIT 2.0, CESD and FACIT-Fatigue scores were significantly associated with SLP-9 index with a variance of 42%.
Table 2.
Hierarchical regression models to assess predictors of sleep problems index
| Variables | β Coeff. | P-value | Adj. R2 |
|---|---|---|---|
| Model 1 | |||
| Age, years | 0.04 | 0.74 | 0.0186 |
| Sex | 7.2 | 0.087 | |
| Non-white | 0.81 | 0.819 | |
| BMI | 0.57 | 0.031 | |
| Model 2 | |||
| Age, years | 0.05 | 0.62 | 0.1432 |
| Sex | 9.43 | 0.03 | |
| Non-white | −1.74 | 0.63 | |
| BMI | 0.49 | 0.07 | |
| MRSS | −0.13 | 0.50 | |
| Physician global severity | 3.48 | <0.001 | |
| Model 3A—Total GIT score modela | |||
| Age, years | −0.01 | 0.87 | 0.4221 |
| Sex | 7.60 | 0.04 | |
| Non-white | −2.67 | 0.39 | |
| BMI | 0.21 | 0.38 | |
| MRSS | 0.05 | 0.76 | |
| Physician global severity | 0.96 | 0.18 | |
| Worsening of GI symptoms during last month | −6.59 | 0.04 | |
| Worsening of shortness of breath during past 1 month | 8.10 | 0.01 | |
| FACIT-Fatigue | −0.28 | 0.072 | |
| CESD | 1.31 | <0.001 | |
| Swollen joint count | −3.12 | 0.09 | |
| Total GIT score | 6.29 | 0.074 | |
| Model 3B—GI scales modela | |||
| Age, years | −0.04 | 0.708 | 0.4236 |
| Sex | 7.83 | 0.03 | |
| Non-white | −2.29 | 0.48 | |
| BMI | 0.21 | 0.40 | |
| MRSS | −0.03 | 0.84 | |
| Physician global severity | 0.78 | 0.30 | |
| Worsening of GI symptoms during last month | −5.77 | 0.07 | |
| Worsening of shortness of breath during past 1 month | 5.33 | 0.12 | |
| FACIT-Fatigue | −0.29 | 0.05 | |
| CESD | 1.07 | 0.001 | |
| Swollen joint count | −3.40 | 0.07 | |
| Reflux scale of UCLA SCTC GIT 2.0 | 8.02 | 0.009 |
aStepwise regression model.
Discussion
This study examines the quality and quantity of sleep in patients with SSc. We found that patients with SSc have detrimental effects on their sleep compared with the US general population. We also found that presence of reflux, new or recent worsening of shortness of breath and gastrointestinal symptoms, and depressed mood were independent predictors of poor sleep. Patient-reported pain, physician assessment of disease and demographics did not predict sleep disturbances.
When compared with the US general population and MOS participants, our study showed that although sleep duration is comparable to that of the general population, on average, individuals with SSc have significantly more sleep difficulties on all scales, with the exception of snoring. In particular, fatigue, depressive symptoms and upper gastrointestinal involvement are important correlates to poor sleep quality in this population.
The MOS-Sleep instrument captures important psychometric aspects to assess sleep quality and quantity, and is endorsed by the OMERACT [12]. It is feasible, reliable and has acceptable construct validity in the US general population and in patients with other chronic diseases, such as overactive bladder, refractory partial-onset epilepsy and painful peripheral neuropathy [22, 31–34]. MOS-Sleep was found to be responsive to change in a clinical trial on pre-gabalin use for neuropathic pain [22]. Our study shows that the MOS-Sleep scale is feasible, shows satisfactory internal consistency (≥0.70) and construct validity in SSc.
Prado et al. [4] assessed sleep in SSc and found that oesophageal dysmotility, dyspnoea and restless leg syndrome were associated with sleep disturbances. In his study, sleep complaints were intertwined with pain, depression and inflammation in many rheumatologic disorders. Prado et al. [4] suggest an algorithm for identifying associated sleep disorders, which highlights the importance of mood and pharmaceutical interventions. In our multivariate analysis, depressed mood, presence of reflux and new or recent onset of shortness of breath or gastrointestinal symptoms were associated with sleep disturbances. This is an especially important finding since acid reflux during sleep may be associated with delayed oesophageal clearance and aspiration [35]. Reflux has also been linked to SSc-associated interstitial lung disease and we recently showed that reflux symptoms are independently associated with depressed mood [21]. It remains to be seen if treatment of reflux and depression will improve quality of sleep in SSc. Until then, patients should be advised to sleep at an angle (wedge or raise bed) with aggressive pharmacological management of their reflux disease [36].
A detailed review of the sleep in different arthritides showed that pain is an independent predictor of sleep disturbance [5]. Although our univariate analysis showed that pain during the past 7 days was associated with SLP-9 (ρ = 0.30), multivariate analysis did not show this association. Also, tender and swollen joint counts were not associated with SLP-9. We have previously shown that patients with SSc and RA perceive pain differently [37] and we did not assess pain using a validated instrument. Also, low prevalence of the tender and swollen joint counts may have also limited the ability to show an association.
Effects of sleep and sleep deprivation on cytokines and immune dysfunction are recognized [38]. The pain, fatigue, distress and sleep disturbance observed in cancer patients during concurrent chemoradiation therapy is thought to be due to over-expression of pro-inflammatory cytokines [39]. TNF-α from peripheral blood mononuclear cells has been described as an important factor in excessive daytime sleepiness [40, 41]. This over-expression of this cytokine and other immunological abnormalities observed in patients with SSc including chronic mononuclear cell infiltration of affected tissues, dysregulation of lymphokine and growth factor production, and autoantibody production [42] are reported, but the effect of sleep and sleep deprivation on these disturbances in SSc patients has not been studied. Further studies on the correlation of cytokines with sleep disturbance, depression and gastrointestinal disease in SSc are warranted.
Our study has many strengths. First, this is the first comprehensive description of sleep disturbances using the MOS-Sleep in patients with SSc. Secondly, the comprehensive HRQOL data allowed us to assess correlates of sleep disturbance. It supports previous work that suggests that upper gastrointestinal disease and depression correlate with sleep disorders in SSc patients [4]. Our study is not without limitations. First, this is a cross-sectional study so causal inferences cannot be made. Although we developed a regression model to assess predictors of poor sleep, association between sleep, depressed mood, reflux symptoms and shortness of breath may be bi-directional. Longitudinal analysis will assess causal associations. Also, we did not explore the reasons for worsening shortness of breath in our patient group. This may be related to gastric aspiration, asthma or new/worsening of cardio-pulmonary disease. Secondly, the use of immunosuppressive medications, anti-reflux medications, hypnotics and anti-depressants was not captured and could affect the MOS-Sleep, thus the effect of intervention strategies is not known. Thirdly, cytokines were not analysed as part of this study to assess relationships between the immune system and sleep disturbances. This, in particular, is an important aspect for future studies and brain–immune interactions are an essential component in psychiatric and medical comorbidities [3]. Lastly, complications and severity of end-organ complications were not captured using a severity index. We relied on overall global assessment of disease severity by physician and the patient.
The importance of understanding sleep in SSc patients is recognized as an important area of research [43]. Although the multi-casual pathway of sleep disturbance is complex, in this study we found that reflux and depressed mood are important correlates that may be amenable to intervention. Future studies of sleep disturbance in SSc and development of successful interventions for reflux and depressed mood in this population are of great need given the high prevalence and potential effect of sleep on overall disease burden.

Supplementary data
Supplementary data are available at Rheumatology Online.
Acknowledgements
Funding: UCLA Scleroderma Study is supported by grants from the National Institutes of Health/National Institute of Arthritis, Musculoskeletal and Skin Diseases (NIH/NIAMS) and the Scleroderma Foundation.
Disclosure statement: D.K. is supported by NIAMS K23 AR053858-04 and the Scleroderma Foundation (New Investigator Award). UCLA Scleroderma Quality of Life Study is supported by NIAMS. D.E.F. is a consultant for and has received honoraria from Abbott, Actelion, Amgen, BMS, Biogen Idec, Centocor, Genentech, Gilead, Corrona, GSK, NIH, Nitec, Novartis, Pfizer, Roche and UCB. P.J.C. has a consultancy with Gilead. All other authors have declared no conflicts of interest.
References
- 1.Bollinger T, Bollinger A, Oster H, Solbach W. Sleep, immunity, and circadian clocks: a mechanistic model. Gerontology. 2010;56:574–80. doi: 10.1159/000281827. [DOI] [PubMed] [Google Scholar]
- 2.Eisenberger NI, Inagaki TK, Mashal NM, Irwin MR. Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain Behav Immun. 2010;24:558–63. doi: 10.1016/j.bbi.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun. 2007;21:374–83. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Prado GF, Allen RP, Trevisani VM, Toscano VG, Earley CJ. Sleep disruption in systemic sclerosis (scleroderma) patients: clinical and polysomnographic findings. Sleep Med. 2002;3:341–5. doi: 10.1016/s1389-9457(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 5.Abad VC, Sarinas PS, Guilleminault C. Sleep and rheumatologic disorders. Sleep Med Rev. 2008;12:211–28. doi: 10.1016/j.smrv.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Mermigkis C, Stagaki E, Tryfon S, et al. How common is sleep-disordered breathing in patients with idiopathic pulmonary fibrosis? Sleep Breath. 2010;14:387–90. doi: 10.1007/s11325-010-0336-5. [DOI] [PubMed] [Google Scholar]
- 7.Flume PA, Ciolino J, Gray S, Lester MK. Patient-reported pain and impaired sleep quality in adult patients with cystic fibrosis. J Cyst Fibros. 2009;8:321–5. doi: 10.1016/j.jcf.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Reading SR, Crowson CS, Rodeheffer RJ, Fitz-Gibbon PD, Maradit-Kremers H, Gabriel SE. Do rheumatoid arthritis patients have a higher risk for sleep apnea? J Rheumatol. 2009;36:1869–72. doi: 10.3899/jrheum.081335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim H, Young T. Subjective daytime sleepiness: dimensions and correlates in the general population. Sleep. 2005;28:625–34. doi: 10.1093/sleep/28.5.625. [DOI] [PubMed] [Google Scholar]
- 10.Redeker NS, Muench U, Zucker MJ, et al. Sleep disordered breathing, daytime symptoms, and functional performance in stable heart failure. Sleep. 2010;33:551–60. doi: 10.1093/sleep/33.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saldana MT, Navarro A, Perez C, Masramon X, Rejas J. Patient-reported-outcomes in subjects with painful lumbar or cervical radiculopathy treated with pregabalin: evidence from medical practice in primary care settings. Rheumatol Int. 2010;30:1005–15. doi: 10.1007/s00296-009-1086-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wells GA, Li T, Kirwan JR, et al. Assessing quality of sleep in patients with rheumatoid arthritis. J Rheumatol. 2009;36:2077–86. doi: 10.3899/jrheum.090362. [DOI] [PubMed] [Google Scholar]
- 13.Hays RD, Stewart AL. Measuring functioning and well-being: the medical outcomes study approach. In: Ware ALSJE, editor. Sleep measures. Durham, NC: Duke University Press; 1992. pp. 235–59. [Google Scholar]
- 14.Preliminary criteria for the classification of systemic sclerosis (scleroderma) Subcommittee for scleroderma criteria of the American rheumatism association diagnostic and therapeutic criteria committee. Arthritis Rheum. 1980;23:581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 15.Salliot C, Gottenberg JE, Bengoufa D, Desmoulins F, Miceli-Richard C, Mariette X. Anticentromere antibodies identify patients with Sjogren's syndrome and autoimmune overlap syndrome. J Rheumatol. 2007;34:2253–8. [PubMed] [Google Scholar]
- 16.Ware JE, Snow KK, Kosinski M, et al. SF-36 health survey: manual and interpretation guide. Boston: New England Medical Center, The Health Institute; 1993. [Google Scholar]
- 17.Khanna D, Furst DE, Clements PJ, et al. Responsiveness of the SF-36 and the Health Assessment Questionnaire Disability Index in a systemic sclerosis clinical trial. J Rheumatol. 2005;32:832–40. [PubMed] [Google Scholar]
- 18.Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980;23:137–45. doi: 10.1002/art.1780230202. [DOI] [PubMed] [Google Scholar]
- 19.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (center for epidemiologic studies depression scale) Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 20.Khanna D, Hays RD, Maranian P, et al. Reliability and validity of the University of California, Los Angeles Scleroderma Clinical Trial Consortium gastrointestinal tract instrument. Arthritis Rheum. 2009;61:1257–63. doi: 10.1002/art.24730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bodukam V, Hays RD, Maranian P, et al. Association of gastrointestinal involvement and depressive symptoms in patients with systemic sclerosis. Rheumatology. 2011;50:330–4. doi: 10.1093/rheumatology/keq296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hays RD, Martin SA, Sesti AM, Spritzer KL. Psychometric properties of the medical outcomes study sleep measure. Sleep Med. 2005;6:41–4. doi: 10.1016/j.sleep.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Hayes D., Jr Obstructive sleep apnea syndrome: a potential cause of lower airway obstruction in cystic fibrosis. Sleep Med. 2006;7:73–5. doi: 10.1016/j.sleep.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Hays RD, Wells KB, Sherbourne CD, Rogers W, Spritzer K. Functioning and well-being outcomes of patients with depression compared with chronic general medical illnesses. Arch Gen Psychiatry. 1995;52:11–9. doi: 10.1001/archpsyc.1995.03950130011002. [DOI] [PubMed] [Google Scholar]
- 25.Tarlov AR, Ware JE, Jr, Greenfield S, Nelson EC, Perrin E, Zubkoff M. The medical outcomes study. An application of methods for monitoring the results of medical care. JAMA. 1989;262:925–30. doi: 10.1001/jama.262.7.925. [DOI] [PubMed] [Google Scholar]
- 26.Cohen JCP. Applied multiple regression/correlation analysis for the behavioral sciences. 2nd edition. Hillsdale, NJ: Lawrence Erlbaum; 2003. [Google Scholar]
- 27.Walker UA, Tyndall A, Czirjak L, et al. Clinical risk assessment of organ manifestations in systemic sclerosis: a report from the EULAR scleroderma trials and research group database. Ann Rheum Dis. 2007;66:754–63. doi: 10.1136/ard.2006.062901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gazi H, Pope JE, Clements P, et al. Outcome measurements in scleroderma: results from a delphi exercise. J Rheumatol. 2007;34:501–9. [PubMed] [Google Scholar]
- 29.Khanna D, Clements PJ, Postlethwaite AE, Furst DE. Does incorporation of aids and devices make a difference in the score of the health assessment questionnaire-disability index? Analysis from a scleroderma clinical trial. J Rheumatol. 2008;35:466–8. [PubMed] [Google Scholar]
- 30.Spritzer KL, Hays RD. MOS sleep scale: a manual for use and scoring, version 1.0. Los Angeles, CA: RAND; 2003. [Google Scholar]
- 31.Lau DT, Morlock RJ, Hill CD. Psychometric evaluation of the medical outcomes study-sleep scale in persons with overactive bladder. Clin Ther. 2006;28:2119–32. doi: 10.1016/j.clinthera.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Wells GA, Boers M, Li T, Tugwell PS. Investigating the validity of the minimal disease activity state for patients with rheumatoid arthritis treated with abatacept. J Rheumatol. 2009;36:260–5. doi: 10.3899/jrheum.080059. [DOI] [PubMed] [Google Scholar]
- 33.Xu X, Brandenburg NA, McDermott AM, Bazil CW. Sleep disturbances reported by refractory partial-onset epilepsy patients receiving polytherapy. Epilepsia. 2006;47:1176–83. doi: 10.1111/j.1528-1167.2006.00591.x. [DOI] [PubMed] [Google Scholar]
- 34.Viala-Danten M, Martin S, Guillemin I, Hays RD. Evaluation of the reliability and validity of the medical outcomes study sleep scale in patients with painful diabetic peripheral neuropathy during an international clinical trial. Health Qual Life Outcomes. 2008;6:113. doi: 10.1186/1477-7525-6-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stein E, Katz PO. Gerd: Gerd and insomnia—first degree relatives or distant cousins? Nat Rev Gastroenterol Hepatol. 2010;7:8–10. doi: 10.1038/nrgastro.2009.217. [DOI] [PubMed] [Google Scholar]
- 36.Khanna D. Gastrointestinal involvement in systemic sclerosis. In: Font J, Ramos-Casals M, Rodés J, editors. Digestive involvement in systemic autoimmune disease. Handbook of systemic autoimmune diseases. Vol. 8. New York: Elsevier; 2008. pp. 51–61. [Google Scholar]
- 37.Hay MCFD, Strathmann C, Khanna D. Systemic sclerosis (SSc) patients experience pain differently than rheumatoid arthritis (RA) patients ACR. Abstract. 2005 [Google Scholar]
- 38.Marshall L, Born J. Brain-immune interactions in sleep. Int Rev Neurobiol. 2002;52:93–131. doi: 10.1016/s0074-7742(02)52007-9. [DOI] [PubMed] [Google Scholar]
- 39.Wang XS, Shi Q, Williams LA, et al. Inflammatory cytokines are associated with the development of symptom burden in patients with NSCLC undergoing concurrent chemoradiation therapy. Brain Behav Immun. 2010;24:968–74. doi: 10.1016/j.bbi.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guasti L, Marino F, Cosentino M, et al. Cytokine production from peripheral blood mononuclear cells and polymorphonuclear leukocytes in patients studied for suspected obstructive sleep apnea. Sleep Breath. 2009 doi: 10.1007/s11325-009-0315-x. November 19 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 41.Gozal D, Serpero LD, Kheirandish-Gozal L, Capdevila OS, Khalyfa A, Tauman R. Sleep measures and morning plasma TNF-alpha levels in children with sleep-disordered breathing. Sleep. 2010;33:319–25. doi: 10.1093/sleep/33.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Besliu AN, Banica LM, Lonescu R, et al. Role of cellular immunity in systemic sclerosis pathogenesis: update on CD4+ t cells population studies. Roum Arch Microbiol Immunol. 2009;68:5–13. [PubMed] [Google Scholar]
- 43.Thombs BD, van Lankveld W, Bassel M, et al. Psychological health and well-being in systemic sclerosis: state of the science and consensus research agenda. Arthritis Care Res. 2010;62:1181–9. doi: 10.1002/acr.20187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.