Abstract
The authors describe a new variant of guanosine triphosphate (GTP)- cyclohydrolase deficiency in a young man with severe and disabling major depressive disorder with multiple near-lethal suicide attempts. His cerebrospinal fluid levels showed that the concentration of tetrahydrobiopterin (BH4), neopterin, 5-hydroxyindoleacetic acid and homovanillic acid were below the reference range, suggesting a defect in the pterin biosynthetic pathway and in synthesis of dopamine and serotonin indicative of GTP-cyclohydrolase deficiency. Patient was started on sapropterin, a BH4 replacement protein, for the defect in the above pathway. In addition, the authors started 5-hydroxytryptophan titrated to 400 mg orally twice daily with concomittant carbidopa 37.5 mg orally four times a day, and he responded with remission of suicidal ideation and significant improvement in depression and function.
Background
Our hope is that other patients presenting with treatment-refractory, life-threatening depression will be evaluated for defects in this pathway. A brief summary of the scientific bases for selecting the replacement therapies is included. For future research, we propose that potential pharmacogenetic characterisation might also be evaluated so that these ‘rare’ syndromes are treated, if possible, at an earlier age.
Case presentation
Guanosine triphosphate-cyclohydrolase (GTPCH) deficiency is an autosomal-recessive genetic disorder associated with neurologic abnormalities. Type I includes hyperphenylalaninemia due to deficiency of tetrahydrobiopterin (BH4).1 In milder variants, defective monoamine production is prominent because of tetrahydrobiopterin-dependent tyrosine and tryptophan hydroxylases.2 BH4 is a cofactor for the conversion of phenylalanine-4-hydroxylase (to phenylalanine), tyrosine-3-hydroxylase(to catecholamines) and tryptophan-5-hydroxylase (to serotonin). Neopterin and biopterin are by-products of these reactions. Nearly 200 different mutant alleles are identified.3 Repletion with sapropterin (Kuvan)4 and/or the deficient monoamine may be successful.5
We report clinical applications of multiple enzymatic product corrections in the treatment of 19-year-old patient, with GTPCH deficiency, treatment-refractory suicidal ideation, severe major depressive disorder, gifted IQ and the absence of neurologic abnormalities. He presented at 14 years of age with suicide attempt by overdose, by 15, he overdosed again requiring treatment in the pediatric intensive care unit. Despite treatment with serotonin-norepinephrine reuptake inhibitors (SNRIs), riluzole, antipsychotics, mood stabilisers and electroconvulsive therapy (ECT) (figure 1), the patient did not improve. Serotonin reuptake inhibitors (SSRIs) resulted in worsening. At age 17, after 32 treatments of ECT, he remitted for only 1 week followed by immediate onset of suicidal intent and an aborted suicide attempt. The patient refused further ECT due to non-response. He demonstrated significant suicidality during a 49-day hospitalisation. With maximal dose polypharmacy, the patient experienced neither improvement nor side effects. Neurologic examination remained normal throughout.
Figure 1.
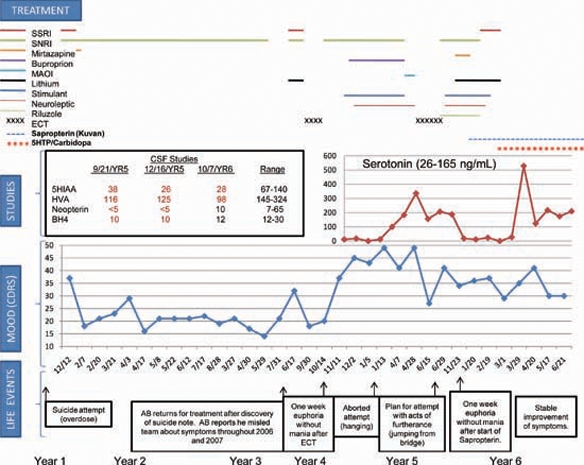
Summary of assessment and treatment. BH4, tetrahydrobiopterin; CDRS, Children’s Depression Rating Scale; ECT, electroconvulsive therapy; 5-HIAA, 5-hydroxyindoleacetic acid; 5-HTP, 5-hydroxytryptophan; HVA, homovanillic acid; MAOI, monoamine oxidase inhibitor; SNRI, serotonin-norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor.
Investigations
Testing revealed normal cytochrome P450 (3A4, 2D6 and C19) metabolism and homozygous long allele of the serotonin transporter promoter gene (SLC64A) locus. Brain MRI was normal. Cerebrospinal fluid (CSF) showed undetectable neopterin, biopterin 10 nM/l (12–30), 5-hydroxyindoleacetic acid (5-HIAA) 38 nM/l (67–140) and homovanillic acid (HVA) 116 nM/l (145–324), indicative of GTPCH deficiency and thus a defect in the pterin biosynthetic pathway. Blood phenylalanine level was 6 μmol/dl (nl<12) and sequencing of the GTPCH gene showed no pathogenic mutation.
Treatment
Due to BH4 deficiency, the patient was given sapropterin 800 mg (10 mg/kg) supplementation. Beginning treatment day 6, he reported euphoric mood, then euthymia; on day 17, hyperactivity, euphoria and normal sleep. By day 24, mood fluctuated markedly with gradually decreasing frequency. The patient endorsed mood incongruent impulses and strong feelings of love and emotion. By day 31, the effect plateaued. Sapropterin was increased to 800 mg orally twice daily (20 mg/kg). On day 40, the CSF neurotransmitter pattern revealed little change (figure 1). He began to have episodes of shaking, insomnia and word-finding difficulty. Sapropterin was reduced to 800 mg orally, once a day in the morning and 400 mg orally, once a day at bed time. After 2.5 months, the patient reported stable improvement, but mood remained low. He received 5-hydroxytryptophan (5-HTP) supplementation with carbidopa to block peripheral effects of serotonin and increase conversion to serotonin in the central nervous system (CNS). Sapropterin was stabilised at 700 mg orally twice daily and carbidopa 25 mg daily was added. 5-HTP was started at 50 mg and titrated to 200 mg, the patient had normal sleep duration and continuity, and at 250 mg, he reported improved mood and continued relief of suicidal ideation. Mood remained euthymic for 4 months with titration of 5-HTP to 400 mg orally twice daily. Nausea responded to promethazine. Eight months status-post sapropterin treatment, CSF neopterin and biopterin were in the normal range (10 and 12 nM/l respectively). The patient continues to report only minimal residual symptoms and improved function.
Outcome and follow-up
In summary, we describe a patient with severe, treatment-refractory depression and suicidal behaviour. CSF revealed deficient neopterin, biopterin, 5-HIAA and HVA. Suicide symptoms improved with sapropterin replacement, but depression symptoms remained disabling. Following 5-HTP and carbidopa, the patient reported relief of depression.
Discussion
To our knowledge, this is the first report of GTPCH deficiency with isolated severe psychiatric symptoms in the absence of neurologic abnormality. The aetiology of the patient’s severe CNS biopterin deficiency remains unclear, as GTPCH molecular studies were normal. However, this enzyme requires an additional protein subunit for activation, a defect which would reduce GTPCH activity. Previously unrecognised negative regulatory mechanisms on the enzyme may also be at play.
The metabolic profile reported here is unique to GTPCH deficiency or a defect in the GTP activator protein. While there is no known demonstrated defect in this protein, it would cause similar functional deficiency. Other enzymatic defects in the pterin synthetic pathway present with very different metabolic profiles. It should be noted that little is known about alterations or responses in this pathway with neuroactive metabolic agents. For example, there could be non-specific secondary changes mimicking deficiencies that are not clinically relevant. Alterations in the pathway could be related to a pathophysiologic process or even pharmacotherapy.
While it is possible that the discovery of a defect in the pterin biosynthetic pathway is coincidental to symptoms of severe, treatment-resistant depression and suicidality, the patient’s clinical response to replacement of BH4 with 5-HTP supplementation makes this unlikely. We do not recommend this treatment in the absence of a documented pterin synthesis defect. Successful reversal of life-threatening depression refractory to known interventions does suggest that metabolomic abnormalities should be considered in similar cases.
Learning points.
-
▶
Our patient with life threatening major depressive disorder and suicidal behaviour responded to no known treatment for his illness.
-
▶
Further evaluation elucidated a disorder of the pterin synthetic pathway that was amenable to treatment with sapropterin and 5-HTP supplementation.
-
▶
Successful reversal of life-threatening depression refractory to known interventions suggests that metabolomic abnormalities should be considered in similar cases.
Acknowledgments
The authors would like to thank the patient for his involvement in the preparation of this manuscript and his willingness to share this information to help others.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Nichol CA, Smith GK, Duch DS. Biosynthesis and metabolism of tetrahydrobiopterin and molybdopterin. Annu Rev Biochem 1985;54:729–64 [DOI] [PubMed] [Google Scholar]
- 2.Blau N, Barnes I, Dhondt JL. International database of tetrahydrobiopterin deficiencies. J Inherit Metab Dis 1996;19:8–14 [DOI] [PubMed] [Google Scholar]
- 3.Thöny B, Blau N. Mutations in the BH4-metabolizing genes GTP cyclohydrolase I, 6-pyruvoyl-tetrahydropterin synthase, sepiapterin reductase, carbinolamine-4a-dehydratase, and dihydropteridine reductase. Hum Mutat 2006;27:870–8 [DOI] [PubMed] [Google Scholar]
- 4.Burton BK, Grange DK, Milanowski A, et al. The response of patients with phenylketonuria and elevated serum phenylalanine to treatment with oral sapropterin dihydrochloride (6R-tetrahydrobiopterin): a phase II, multicentre, open-label, screening study. J Inherit Metab Dis 2007;30:700–7 [DOI] [PubMed] [Google Scholar]
- 5.Swodoba KJ. Inherited disorders of amine biosynthesis. Future Neurol 2006;1: 605–14 [Google Scholar]


