Abstract
Patients' functional recovery at home following surgery may be evaluated by monitoring their activities of daily living. Existing tools for assessing these activities are labor-intensive to administer and rely heavily on recall. This study describes the use of a wireless ear-worn activity recognition sensor to monitor postoperative activity levels continuously using a Bayesian activity classification framework. The device was used to monitor the postoperative recovery of five patients following abdominal surgery. Activity was classified into four groups ranging from very low (level 0) to high (level 3). Overall, patients were found to be undertaking a higher proportion of level 0 activities on postoperative day 1 which was gradually replaced by higher-level activities over the next 3 days. This study demonstrates how a pervasive healthcare technology can objectively monitor functional recovery in the unsupervised home setting. This may be a useful adjunct to existing postoperative monitoring systems.
Introduction
Postoperative recovery is a multifaceted and dynamic process involving biological, physiological, functional, and psychological components. Increasing physical independence, a gradual return to activities of daily living (ADLs), and a return to normal mobility are all aspects of functional recovery that act as global indicators of a patient's well-being following surgery. For most patients, the evaluation of their ability to perform ADLs occurs only in the hospital setting where, prior to discharge, a patient has to demonstrate the ability to transfer independently, feed themselves, toilet themselves, dress themselves, and finally bathe.1 There is, however, a case for continuing to monitor functional recovery at home using ADLs in patients such as older people, those living alone who still require significant rehabilitation, the disabled, and those with neurological deficit (such as after a stroke). While a number of ADL assessment tools have been developed for such patients (examples include the ‘Barthel Index’2 and the ‘Extended Activities of Daily Living scale’3), these are labor-intensive to administer, often take the patient out of their normal daily routine, and rely too heavily on patient recall, which is variably reliable. What is required is a system that monitors a patient's ADLs continuously and objectively in their home environment, yet in a manner that is non-disruptive to their daily lives.
As a sensor modality, accelerometers have been shown to be of use in monitoring physical activity. Inouez et al used an ankle-worn system to measure cumulative acceleration of physical activity over a 24 h period in patients for 7 days following gastric surgery.4 Cumulative acceleration measure in this way acts as a means of activity quantification, but is unable to differentiate between a patient's activity types. Kang et al used a waist-mounted triaxial accelerometer to classify activities such as sitting, standing, lying down, and walking in healthy volunteers.5 Long et al used a triaxial waist-worn accelerometer to compare a Bayesian activity classifier to a Decision Tree activity classification system in the differentiation of activities such as walking, running, driving, and sports.6 Finally, Manohar et al used an ear-worn accelerometer wired to a data-collection unit to determine activities such as lying, standing, and walking.7 While these studies have all demonstrated the potential of accelerometer-based devices in activity classification, factors that would limit their use in real patients include a lack of wireless data capability transfer, thereby limiting patients' activities in their homes, and a lack of ‘on-node’ processing which limits the ability to monitor a patient's progress in real-time.
This brief communication describes an innovative means of pervasive functional postoperative recovery monitoring using a wireless ear-worn activity recognition (e-AR) body sensor network (BSN) device to collect biomechanical data on patients' ADLs in their homes. A Bayesian activity classifier framework with multivariate Gaussians was used to model activity classes.
Methods
The e-AR device was used to monitor postoperative recovery of five patients who had undergone abdominal surgery. Patients were asked to wear the device preoperatively, and on 4 days following home discharge. In order to be included, patients had to be scheduled for elective surgical procedures, have an anticipated postoperative length of hospital stay of less than 7 days, and be older than 60 years in age. Patients who were selected and agreed to take part were trained to wear the e-AR and had a base-station laptop installed in their home. They were asked to wear the device for 4 h on a day in the week preceding their operation, and 4 h a day while at home following discharge from hospital. Patients wore the sensor at the same time of day on each occasion, and not while sleeping at night or bathing. Approval for this study was obtained from our local research ethics committee with informed consent obtained from all participants. Regulatory approval for the experimental use of the e-AR device in this study was obtained from the UK Medicines and Healthcare Products Regulatory Agency. Prior to use with real patients, the device and its frameworks had to be validated in healthy volunteers. These validation studies and their outcomes are presented in the online appendix associated with this report (http://www.jamia.org/).
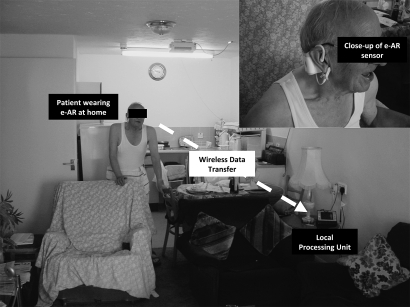
The e-AR device comprises a BSN node, a battery power supply (Unionfortune 041528 lithium-polymer battery, Guangdong, China), a three-dimensional accelerometer (Analog Devices ADXL330 3-axis MEMS accelerometer, Norwood, MA, USA), and a pulse oximeter, all of which are enclosed within a casing that is designed to be worn on the outer ear.8 The ADXL330 accelerometer measures acceleration with a range of ±3 g. Analog-to-digital conversion (ADC) of the BSN node will digitalize the sensor signals into x, y, and z axis accelerometer channel outputs ranging from 0 to 4095, representing 0–3 V. Higher output corresponds to higher acceleration. This arrangement allows the determination of head displacement in three dimensions, namely x (anterior/posterior), y (medial/lateral), and z (superior/inferior) axes. The output of the accelerometer reflects its orientation, which is controlled by the subject's posture, type of activity being performed, and its intensity.9 At rest, the output of the accelerometer is determined by its orientation relative to the gravitational vector. As a result, provided that the orientation of the accelerometer relative to the subject is known, the e-AR sensor can be used to determine the orientation and movements of the subject. The BSN node provides a wireless link to a local processing unit that can send information at the required intervals to a centralized patient database via a wireless local area network or mobile phone network. The BSN node contains a Texas Instruments (Dallas, TX, USA) MSP430 16-bit ultra-low-power reduced instruction set computer processor that acts as the ‘brains’ of the node–sensor complex, containing 60 KB+256 B flash memory, 2 KB of random access memory. Low-power wireless communication is provided using a Chipcon CC2420 (IEEE 802.15.4 compliant) 2.4 GHz wireless radio transceiver, which has a throughput of 250 kbps and a range over 50 m. The TinyOS operating system is used to manage the network and its resources. The local processing unit is a tablet PC equipped with a BSN node receiver which serves as the router that bridges the signals from the e-AR sensor to the centralized database. Figure 1 outlines the architecture of this system and the flow of data, and shows a close-up of a sensor being worn by a patient.
Figure 1.
Outline of the architecture of this system, showing the flow of data and a close-up of a sensor being worn by a patient. e-AR, ear-worn activity recognition.
Activity classification analysis was undertaken using a Matlab environment-based software using a framework described by Lo et al.10 This uses a Bayesian classifier with multivariate Gaussians to model activity classes. The inputs to the classifier are the three-dimensional variance across the three accelerometer axes, as well as the amplitude of the raw data. A 4 s window of streaming data is assigned an activity class if the probability of belonging to this class was higher than the probability of belonging to all other classes. The activity classes are assumed to have equal prior probabilities. To enhance the accuracy of the classification, the signal amplitude is used to identify static activities (such as lying down or sleeping), and the classifier is used to determine other dynamic activities (such as running and walking). The software allows activities to be grouped into ‘very low’ (Class 0), ‘low’ (Class 1), ‘medium’ (Class 2), and ‘high’ (Class 3) levels. A binary logistic regression model was used to determine if the activity classifier could correctly predict the intensity of activity being undertaken by a subject (mean accelerometer variance). Class 0 and 1 activities were grouped as ‘low level’ (including activities such as lying down, sleeping, sitting, and eating), and Class 2 and 3 activities were classified as ‘high level’ (including standing, walking, cleaning, and cooking). The mean accelerometer variance (+/–SD) for periods during which patients were undertaking these activities was calculated, and a logistic regression analysis performed to assess the association between the mean variance and activity classification level. The effect of this association was expressed as OR and its 95% CIs. Data analysis was performed using SPSS version 16.0 for Windows.
Observations
The demographics and operative procedures of five patients who took part in this study are shown in table 1:
Table 1.
Demographics of patients selected for the postoperative home recovery study
| Patient | Age (sex) | Comorbidity | Exercise tolerance | Lives with | Operation | Postop complication | Data collected |
| 1 | 79 (M) |
|
Limited (366 m (400 yards) due to shortness of breath) | Son | Open right inguinal hernia repair | None |
|
| 2 | 84 (M) |
|
Unlimited | Alone | Open left inguinal hernia repair | Seroma |
|
| 3 | 80 (F) |
|
Unlimited | Husband | Laparoscopic sigmoid colectomy | None |
|
| 4 | 65 (M) |
|
Unlimited | Alone | Open right inguinal hernia repair | Wound infection |
|
| 5 | 64 (F) |
|
Unlimited | Husband | Open cholecystectomy | Intra-abdominal collection (readmitted to hospital) |
|
COPD, chronic obstructive pulmonary disease; HT, hypertension; IDDM, insulin-dependent diabetes mellitus; IHD, ischemic heart disease; NIDDM, non-insulin dependent diabetes mellitus.
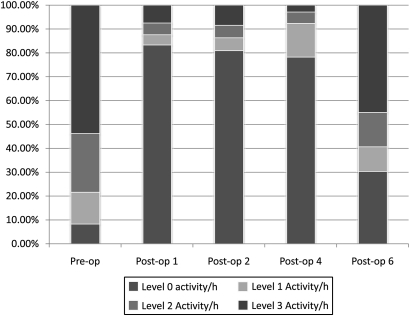
The activity classification per hour profile for patient 1 is shown in figure 2. He was a 79-year old male with a large right inguinal hernia, who left his house only twice a week, largely due to his shortness of breath which limited his walking to about 366 m (400 yards) at a time. From his activity profile, it can be seen that while, preoperatively, the patient was seen to spend 53.8% of his time undertaking level three activities and 8.3% of his time on level 0 activities per hour, on postoperative home recovery day 1 this changed significantly, with the subject spending over 70% of his time undertaking level 0 activities. This suggests that the patient was much less active soon after surgery. The amount of activity the patient undertook was seen to increase daily following surgery, and by postoperative home recovery day 6 the activity profile of the subject was seen to return back almost to its preoperative levels.
Figure 2.
Activity classification per hour profile for patient 1 during pre- and postoperative periods.
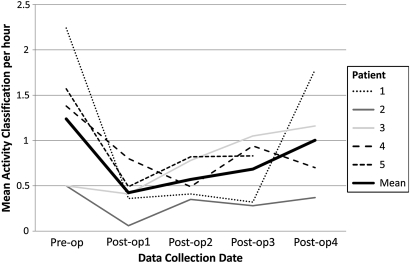
The average activity classification per hour for each of the five subjects is shown below in figure 3, along with the overall mean activity classification per hour when all five subjects' data were combined. This shows that the mean activity level for all subjects was higher on the preoperative assessment compared to postoperative day 1, and rose daily during the postoperative period. In the case of patient 3, the activity level was higher on day 4 compared to before the operation.
Figure 3.
Average activity classification per hour profile for each of the five patients during preoperative and postoperative periods. The mean for all five subjects is also shown (dotted line).
Logistic regression analysis of the data showed that the mean accelerometer variance was significantly greater during high level activities (p=0.003) and was strongly predictive of the level of activity the subjects were undertaking in their home, with a high OR of 55.2 (95% CI 4.044 to 754.082).
Discussion
This study demonstrates the ability of the e-AR device and its activity classifier to detect changes in patients' postoperative activity levels in their home environment. While this measure is unlikely to completely replace the conventional monitoring of patients' ADLs, the ‘activity level’ as measured by the e-AR device may be a useful adjunct in following a patient's functional recovery. Thus, for example, while a patient may report that they are able to perform an ADL, the e-AR activity classifier provides an objective and unbiased view of how often they are being active. Such pervasive wireless technologies may also be of particular interest to those who care for patients who are rehabilitating from a major event such as a stroke, myocardial infarction, or spinal injury, in whom the quantification of daily meaningful activity is of importance. Importantly, patients were enthusiastic about wearing the device, and did not report any interference with their eye glasses. While none of the patients wore hearing aids, the sensor may be worn on either ear to avoid interference.
The activity classifier used in this study was able to standardize and quantify activity levels over time. In all five patients, an increase in level 0 activity, compared to the level before surgery, was seen to occur on postoperative home recovery day 1 due to longer sedentary periods. This is clinically significant because it shows how a patient is most vulnerable to complications secondary to immobility (such as deep vein thrombosis) at time of discharge following surgery. Pervasive functional postoperative recovery monitoring in this way may lead to safer discharge from hospital at a time when a number of healthcare initiatives are driving the reduction in postoperative length of hospital stay. In the existing healthcare systems, BSN technologies may support focused community nursing, prioritizing patients with poor postoperative recovery. This in turn may have an impact on reducing patient morbidity and ultimately hospital readmission. Preoperative assessment of a patient's activity profile may help identify those who are less active and therefore potentially at higher risk of complications secondary to their stasis. Finally, the ability to demonstrate that an operation has raised the patient's baseline functional activity (such as in patient 3), is a powerful method of evaluating the outcome from the intervention.
It is important at this stage to mention several limitations of this study. First, the energy consumption of the BSN earpiece required a battery change every 8 h. This was partly as a result of the energy consumption of the sensor, but also because of the processor and wireless data transfer. Second, the size of the e-AR device meant that although the sensor was easy to wear and stayed on the ear in most cases, some patients required the additional support of a headband. A reduction in weight of the device would help increase its acceptability and long-term wear. Advances in key areas such as power supply miniaturization, increased battery duration, reduced energy consumption, and power scavenging will be essential to achieving this goal. Third, the use of the device by patients for the same 4 h of the day attempted to standardize the impact of time of day on activity performed by patients. Addressing the limitations mentioned will result in a device that can be worn for a 24 h period and collect a full day's worth of data. Finally, as this system involves unsupervised monitoring of activity level, it cannot differentiate pathological movements (such as during a seizure) from normal activity.
Pervasive BSN technologies such as the e-AR sensor have the potential to revolutionize healthcare through their ability to collect data continuously, and in any environment. This ubiquitous diagnostic ability will not only allow us to monitor our patients better, but also help us learn about postoperative recovery and the impact that our interventions have on patients' lives. Clinical use will need to include an assimilation of these technologies into global healthcare systems, representing the next great challenge.
Supplementary Material
Footnotes
Funding: Imperial College London.
Competing interests: None.
Patient consent: Obtained.
Ethics approval: Ethics approval was provided by St Mary's Hospital.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Amemiya T, Oda K, Ando M, et al. Activities of daily living and quality of life of elderly patients after elective surgery for gastric and colorectal cancers. Ann Surg 2007;246:222–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sainsbury A, Seebass G, Bansal A, et al. Reliability of the Barthel Index when used with older people. Age Ageing 2005;34:228–32 [DOI] [PubMed] [Google Scholar]
- 3.Harwood RH, Ebrahim S. A comparison of the responsiveness of the Nottingham extended activities of daily living scale, London handicap scale and SF-36. Disabil Rehabil 2000;22:786–93 [DOI] [PubMed] [Google Scholar]
- 4.Inouez Y, Kimura T, Fujita S, et al. A new parameter for assessing postoperative recovery of physical activity using an accelerometer. Surg Today 2003;33:645–50 [DOI] [PubMed] [Google Scholar]
- 5.Kang DW, Choi JS, Lee JW, et al. Real-time elderly activity monitoring system based on a tri-axial accelerometer. Disabil Rehabil Assist Technol 2010;5:247–53 [DOI] [PubMed] [Google Scholar]
- 6.Long X, Yin B, Aarts RM. Single-accelerometer-based daily physical activity classification. Conf Proc IEEE Eng Med Biol Soc 2009;2009:6107–10 [DOI] [PubMed] [Google Scholar]
- 7.Manohar C, McCrady S, Pavlidis IT, et al. An accelerometer-based earpiece to monitor and quantify physical activity. J Phys Act Health 2009;6:781–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aziz O, Atallah L, Lo B, et al. A pervasive body sensor network for measuring postoperative recovery at home. Surg Innov 2007;14:83–90 [DOI] [PubMed] [Google Scholar]
- 9.Najafi B, Aminian K, Paraschiv-Ionescu A, et al. Ambulatory system for human motion analysis using a kinematic sensor: monitoring of daily physical activity in the elderly. IEEE Trans Biomed Eng 2003;50:711–23 [DOI] [PubMed] [Google Scholar]
- 10.Lo B, Atallah L, Aziz O, et al. Real-time pervasive monitoring for postoperative care. In: Leonhardt S, Falck T, Mähönen P, eds. 4th International Workshop on Wearable and Implantable Body Sensor Networks (BSN 2007). Berlin: Springer, 2007:122–7 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.