Abstract
G protein-coupled receptors (GPCRs) have key roles in cell–cell communication. Recent data suggest that these receptors can form large complexes, a possibility expected to expand the complexity of this regulatory system. Among the brain GPCRs, the heterodimeric GABAB receptor is one of the most abundant, being distributed in most brain regions, on either pre- or post-synaptic elements. Here, using specific antibodies labelled with time-resolved FRET compatible fluorophores, we provide evidence that the heterodimeric GABAB receptor can form higher-ordered oligomers in the brain, as suggested by the close proximity of the GABAB1 subunits. Destabilizing the oligomers using a competitor or a GABAB1 mutant revealed different G protein coupling efficiencies depending on the oligomeric state of the receptor. By examining, in heterologous system, the G protein coupling properties of such GABAB receptor oligomers composed of a wild-type and a non-functional mutant heterodimer, we provide evidence for a negative functional cooperativity between the GABAB heterodimers.
Keywords: cooperativity, GABA, GABAB , GPCR, receptor oligomer
Introduction
G protein-coupled receptors (GPCRs) have key roles in many physiological processes, including cell–cell communication, cell differentiation, metabolism or synaptic transmission. Representing about 3% of encoding genes in mammals, these receptors constitute a major target in drug development (Overington et al, 2006; Lim, 2007). Over the last 10 years, numerous studies have suggested that these signalling proteins display a high degree of complexity due to their ability (i) to adopt several conformations each linked to specific signalling cascades (Gay et al, 2004; Moniri et al, 2004; Galandrin et al, 2007) and (ii) to form dimers or even larger oligomeric complexes, offering new possible synergistic receptor responses (Terrillon and Bouvier, 2004; Carriba et al, 2008; Gonzalez-Maeso et al, 2008; Gurevich and Gurevich, 2008; Panetta and Greenwood, 2008). However, the existence and the functional consequences of such receptor oligomers in a physiological context remain elusive.
The main inhibitory neurotransmitter, γ-aminobutyric acid (GABA), acts on both ionotropic (GABAA) and G protein-coupled (GABAB) receptors. The GABAB receptor modulates both pre- and post-synaptic elements by regulating calcium or potassium channels through the activation of Gi/o types of G proteins (Bettler et al, 2004; Kornau, 2006; Ulrich and Bettler, 2007). Not surprisingly, this receptor represents an interesting target for the treatment of various CNS diseases, including spasticity, anxiety, depression, drug addiction or pain (Cryan et al, 2004; Marshall, 2005; Bowery, 2006). The GABAB receptor was the first GPCR found to be an obligatory heterodimer constituted of two subunits, GABAB1 (GB1) that binds agonists and GABAB2 (GB2) responsible for G protein activation (Jones et al, 1998; Kaupmann et al, 1998; White et al, 1998; Galvez et al, 2001; Robbins et al, 2001; Duthey et al, 2002). It was therefore considered as an excellent model to study the functional significance of GPCR dimerization.
Recently, we have shown that the GABAB heterodimeric receptor can spontaneously form dimers of heterodimers, or possibly larger complexes in heterologous system via the interaction of GB1 subunits (Maurel et al, 2008). In the rest of the manuscript, we will refer to tetramers for simplicity, and as this is the most likely possibility, as discussed later. This newly described quaternary structure raised a number of possibilities that may help to elucidate the various functional and pharmacological properties reported for the GABAB receptor in vivo (Bowery et al, 2002), that is a change in oligomeric state may differently regulate the receptor properties. In addition, this concept could be generalized to other receptors and thus would provide important information to understanding the relevance of GPCR oligomerization.
Here, we bring additional data supporting our initial model of GABAB oligomer and its organization in heterologous system. Most importantly, we bring evidence for the existence of higher-ordered GABAB oligomers in the brain using fluorescent antibodies directed against the native receptors and compatible for time-resolved FRET. This led us to decipher the functional properties of the various oligomeric states of this receptor—that is dimer versus tetramer. Our data revealed a lower G protein coupling efficacy per GABAB heterodimer when assembled into tetramers. We conclude on a negative allosteric coupling between the heterodimeric functional units in the oligomer, thus reporting a new example of allosteric GPCR oligomer.
Results
New evidences for the assembly of GABAB receptors into larger entities
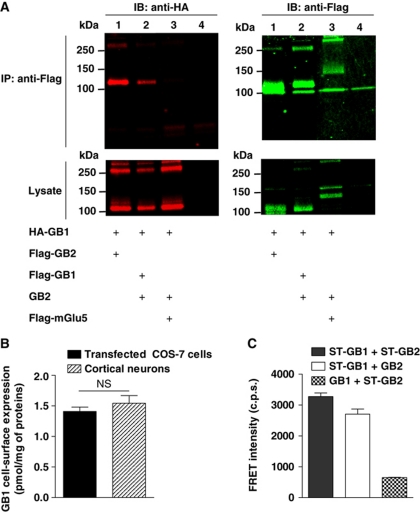
To strengthen our FRET-based observation that GABAB receptor heterodimers spontaneously form larger complexes at the cell surface (Maurel et al, 2008), we performed co-immunoprecipitation of cell-surface proteins. Flag-GB1 was found to efficiently co-immunoprecipitate HA-GB1 while Flag-mGlu5 failed (GB2 being co-expressed in all conditions), demonstrating the specificity and the stability of the GB1–GB1 interaction in transiently transfected COS-7 cells (Figure 1A). Of note, unless stated otherwise, we used the GB1a isoform in the whole study.
Figure 1.
GB1–GB1 interaction in heterologous system. (A) GB1–GB1 interaction detected by cell-surface co-immunoprecipitation. Anti-HA (left) and anti-Flag (right) immunoblots performed on anti-Flag immunoprecipitate (top) and the corresponding lysate (bottom) obtained from COS-7 cells transfected with the indicated constructs. Lane 4 corresponds to mock-transfected cells. The gels are representative of three independent experiments. The bands at high molecular weight (over 250 kDa) represent the multimeric forms of the receptors. (B) GB1 cell-surface expression assessed by binding of the GB1 selective non-permeant antagonist [3H]-CGP54626A either on COS-7 cells expressing GB1 and GB2 or on mouse cortical neurons (E15.5)—15 DIV expressed as pmol of bound ligand per mg of total amount of proteins. No [3H]-CGP54626A binding could be detected on non-transfected COS-7 cells. Data are mean±s.e.m. of three individual experiments each performed in triplicate. P>0.05 in a paired t-test. (C) FRET intensity measured on COS-7 cells between the indicated Snap-tag-labelled subunits. Data are mean±s.e.m. of four individual experiments each performed in triplicate.
To rule out overexpression-induced clustering artifact in heterologous expression system, we optimized the transfection condition to ensure a similar receptor density to that measured in cortical neurons using the non-permeant tritiated GABAB antagonist [3H]-CGP54626A (Figure 1B). In this condition, a large and significant TR-FRET signal was still measured between Snap-tagged (ST) GB1 subunits expressed in HEK-293 cells, confirming the detection of tetramers at physiological density (Figure 1C). Of note, this signal was comparable to that measured between GB1 and GB2 within the heterodimer, consistent with a direct interaction between the GB1 subunits from two GABAB heterodimers (Figure 1C). In contrast, the signal recorded between two ST-GB2 was very low, consistent with a tetrameric organization where the GB2 subunits are further apart at a distance not compatible with efficient TR-FRET (Figure 1C) (Supplementary Figure S1).
Detection of GB1–GB1 proximity in native brain membranes
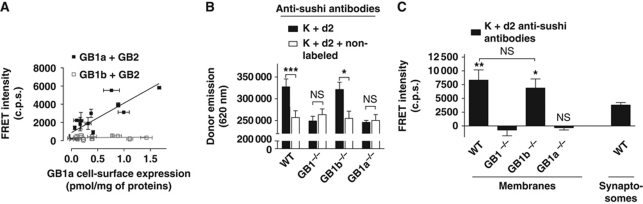
The question whether GABAB tetramers exist in native tissue remains unanswered mostly due to the lack of technical solution. To address this issue, we took advantage of monoclonal antibodies (Tiao et al, 2008) directed against the sushi domains present at the N-terminus of the native GB1a isoform but not of GB1b (Kaupmann et al, 1997; Hawrot et al, 1998). The anti-sushi antibody was conjugated with TR-FRET compatible fluorophores, either Lumi4-Tb (K, donor) or d2 (acceptor) as previously described (Brinkley, 1992). Optimal antibody concentrations for FRET measurements (Supplementary Figure S2) and the signal specificity were determined on HEK-293 cells, expressing GB1a and GB2 compared with cells expressing GB1b and GB2 (negative control) (Figure 2A). Of note, the TR-FRET signal from cells expressing GB1a and GB2 is linear over a large range of receptor density, indicative for a constant FRET emission per cell-surface protein thus compatible with detection of proximity between GB1a subunits at physiological density.
Figure 2.
Detection of GABAB tetramers on brain membrane. (A) FRET intensity measured using Lumi4-Tb (K) and d2-conjugated monoclonal anti-sushi antibodies on COS-7 cells expressing increasing amounts of GB1a and GB2 (black squares) or GB1b and GB2 (open squares). The background signal was determined in the absence of d2-conjugated antibodies. Cell-surface expression was measured by binding assay. (B) Specific K anti-sushi antibody labelling on brain membrane prepared from wild-type, and GB1−/−, GB1a−/− or GB1b−/− mice determined in the presence of d2 anti-sushi antibodies with (white bars) or without (black bars) an excess of non-conjugated anti-sushi antibodies. The specific antibody labelling for each condition tested is given by the difference between the black and the white bars. (C) FRET intensity between K and d2-conjugated anti-sushi antibodies measured on brain membrane prepared from wild-type, GB1b−/−, GB1a−/− or GB1b−/− mice or on synaptosomes prepared from wild-type mice. The background signal was determined on samples labelled with K and an optimized amount of non-conjugated antibodies to obtain the same donor emission as in the assay (data not shown). Data in (A–C) are mean±s.e.m. of three individual experiments each performed in triplicates. *, ** and *** represent P<0.05, P<0.01 and P<0.001, respectively, in a paired t-test; in (C) samples were compared with GB1−/−.
We next used these antibodies to detect the possible oligomerization of GABAB heterodimers on brain membrane prepared from wild-type (WT), GB1−/−, GB1a−/− or GB1b−/− mice (expressing no GB1 isoforms, GB1b only or GB1a only, respectively) (Schuler et al, 2001; Vigot et al, 2006). After labelling with mixtures of donor- and acceptor-conjugated antibodies, we first measured the donor emission as a read-out for antibody binding. For the WT and GB1b−/−, the donor emission was significantly decreased by addition of an excess of non-conjugated antibodies, indicative for a specific binding of the conjugated antibodies although the non-specific signal was high (Figure 2B). By contrast, no significant specific binding was detected for GB1−/− or GB1a−/−, in agreement with the absence of the epitope recognized by the antibody (Figure 2B).
On the same samples, we also measured the acceptor signal following excitation of the donor. A similar FRET signal was measured on membrane from WT and GB1b−/− mice, while no significant FRET signal could be measured on membranes from mice lacking GB1a (GB1−/− and GB1a−/−) (Figure 2C). Since using total brain membranes, we could not discriminate between the GB1–GB1 FRET originating from intracellular receptor pools, from those at the cell surface, we repeated FRET experiments on preparations enriched in synaptosomes (purified neuron terminals). The FRET signal measured was in a similar range to that measured on brain membranes (Figure 2C). A significant FRET signal was also obtained between the native GB1 on rat brain but not on rat mammary gland membrane (assumed not to express GABAB receptors) proving that GB1–GB1 interaction is not restricted to one species (Supplementary Figure S3B–D). Of note, we also checked that the FRET recorded did not result from a non-specific collisional FRET or from artifactual aggregation during membrane preparation or from antibody-induced aggregates (Supplementary Figure S3). Altogether, these results revealed a close proximity of GB1a subunits in native brain membranes, consistent with the existence of GABAB tetramers in the brain.
In order to analyse whether GABAB receptor tetramerization led to a functional crosstalk from one heterodimer to the other, we used HEK-293 cells as a model system, as it allows an easy characterization of various combinations of subunits, either chimeric constructs, or subunits carrying specific mutations.
GB1-VFTs are important for the GB1–GB1 interaction
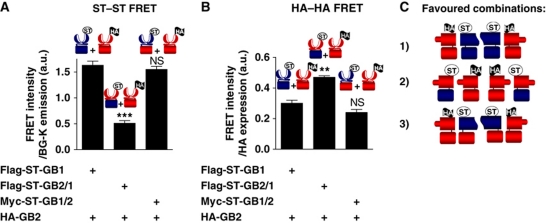
To analyse whether specific functional interactions exist between the GABAB dimers, one would need to disrupt the GB1–GB1 interaction. We previously reported that a truncated GB1 subunit corresponding to the isolated 7TM region (7TM-GB1-ΔCT) could prevent to some extent the formation of GABAB tetramers (Maurel et al, 2008). However, to be efficient, it required a large amount of the competitor, and very low expression of the WT GABAB, suggesting that regions other than the 7TM domain are involved in the GB1–GB1 association. To test our hypothesis, we took advantage of the following chimeric constructs: GB2/1 bearing the GB2-VFT and the GB1-TM; GB1/2 where GB1-VFT is linked to the TM of GB2 (Galvez et al, 2001). Each subunit carried either a Snap-tag or an HA epitope at their N-terminus allowing their specific labelling at the cell surface with TR-FRET compatible fluorophores using either antibodies or the Snap-tag substrates. Of interest, the same set of transfected cells were used to monitor by TR-FRET the proximity between either Snap-tagged or HA-tagged subunits. All experiments were conducted under conditions where all HA-tagged constructs were expressed at the same density at the cell surface, as determined by ELISA (Supplementary Figure S4). When comparing TR-FRET data obtained with the ST-GB1+HA-GB2 and ST-GB2/1+HA-GB2 combinations, the absence of the GB1-VFT in the later resulted in a large decrease in the ST–ST FRET signal, suggesting that the GB1-VFT has an important role in the GABAB tetramer assembly (Figure 3A). In contrast, HA–HA FRET was increased, supporting a possible reorganization of the tetramers that would bring the GB2-VFTs closer to each other (Figure 3B). In the combination ST-GB1/2+HA-GB2, where the GB1-TM was replaced by that of GB2, both ST–ST and HA–HA FRET signals were equivalent to that measured with the combination of WT subunits. Altogether, these data revealed that the VFTs of the GABAB subunits have a critical role in determining the assembly mode between the heterodimers, either because of a strong and favoured interaction between the GB1-VFTs or because the GB2-VFTs cannot interact with each other when associated with GB1 possibly due to steric or ionic hindrance. In contrast, the GB1-TM does not appear to have a critical role, giving an explanation on the toughness to prevent the formation of the tetramer with 7TM-GB1-ΔCT (Maurel et al, 2008).
Figure 3.
Determination of the GB1 domains involved in the interaction between GABAB heterodimers. (A) FRET intensity using the fluorescent BG substrates measured on COS-7 cells expressing wild-type or chimeric GABAB subunits bearing an ST or an HA tag. FRET signals for the same amount of HA construct at the cell surface (see Supplementary Figure S4) are represented over the BG-K emission. GB1 domains are illustrated in blue and GB2 in red. Wild-type GABAB receptor was used as a positive control. (B) FRET intensity over the HA cell-surface expression measured after fluorescent anti-HA antibodies labelling on the same transfected COS-7 cells than that used for the experiment depicted in (A). For (A, B), data are representative of three independent experiments, each performed in triplicates. ** and *** represent P<0.01 and P<0.001, respectively, in a t-test compared with Flag-ST-GB1+HA-GB2. (C) Schematic representation of the different favoured associations for each subunit combination used as suggested by the results of FRET experiments.
Dissociation of GABAB tetramers leads to an enhanced G protein coupling efficacy
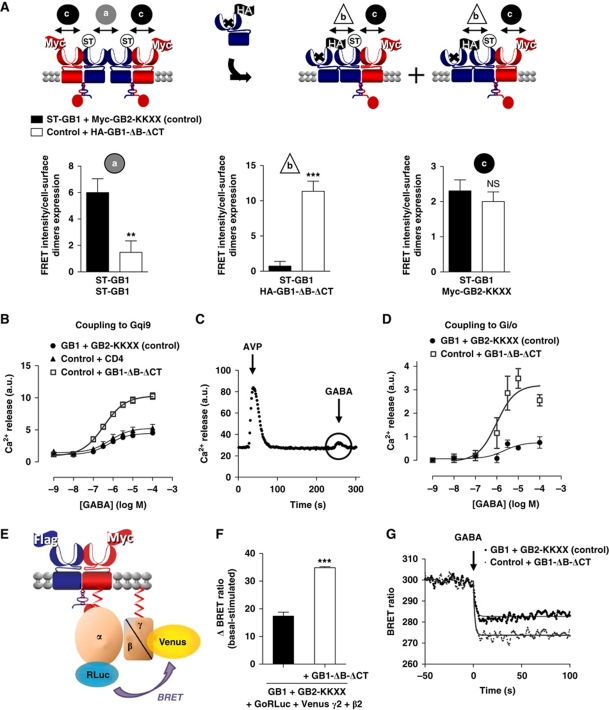
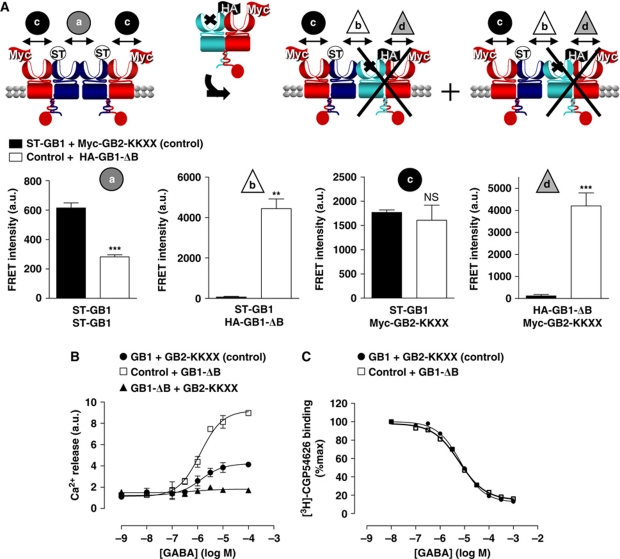
The above data indicate that the best competitor to prevent GABAB receptor oligomerization should compete GB1–GB1 interaction at the VFT level. In agreement, we developed a new competitor (GB1-ΔB-ΔCT) based on GB1 in which (i) GABA binding was prevented by inserting two mutations in the binding site: S246A and E465A (Galvez et al, 1999, 2000; Kniazeff et al, 2002) and (ii) the C-terminal tail was deleted (ΔCT) to allow cell surface targeting. Indeed, the C-terminal tail of GB1 contains an ER retention signal that is masked upon coiled-coil interaction with GB2 C-terminus (Kuner et al, 1999; Margeta-Mitrovic et al, 2000; Pagano et al, 2001). In addition, an ER retention signal was inserted at the C-terminal end of GB2 (GB2-KKXX) such that only GB1 with its intact C-terminus and not GB1-ΔB-ΔCT masks the retention signal promoting the targeting of GB1–GB2-KKXX dimers to the cell surface (Maurel et al, 2008). Hence, the competitor should not disturb the GB1–GB2 heterodimers (Figure 4A). Of note, neither ST on GB1, nor the KKXX motif in GB2 modified the function of the receptor (Maurel et al, 2008; data not shown).
Figure 4.
Functional implication of the GABAB higher-ordered oligomers formation assessed using a competitor of the GB1–GB1 interaction. (A) Dissociation of the GABAB tetramers using as a competitor HA-GB1-ΔB-ΔCT: a GB1 unable to bind GABA and deleted of its C-terminal tail as illustrated in the top scheme. Only the combinations reaching the cell surface are represented. ST-GB1 is represented in blue and Flag-GB2-KKXX in red. FRET intensity over the wild-type GABAB cell-surface expression (ELISA) measured on cells expressing either ST-GB1 and Myc-GB2-KKXX (control) (black bars) or control with GB1-ΔB-ΔCT (white bars), (a) between ST-GB1 subunits labelled with BG-K and BG-d2, (b) between ST-GB1 and HA-GB1-ΔB-ΔCT labelled with BG-d2 and K anti-HA antibody and (c) between ST-GB1 and Myc-GB2-KKXX labelled with BG-d2 and K anti-Flag antibody. ** and *** represent P<0.01 and P<0.001, respectively, in a t-test. (B) Calcium signal measured upon stimulation of the chimeric G protein Gqi9 by increasing concentration of GABA in HEK-293 cells transfected with GB1 and GB2 (control) (black-filled circles), or co-expressed with CD4 (negative control) (black triangles) or with GB1-ΔB-ΔCT (grey open squares). (C) Calcium release kinetics on cells expressing the GABAB receptor recorded for a period of 300 s, including the addition of 10−7 M AVP and 10−4 M GABA at 20 and 240 s, respectively (see also Supplementary Figure S6). (D) Measurement of GABAB signalling through native Gi/o coupling upon application of increasing concentration of GABA in a calcium assay after pre-stimulation of an endogenous Gq-coupled receptor. Experiments were conducted as in (B). (E) Schematic representation of the BRET assay with Go fused to Rluc and Gγ2 to Venus leading to a BRET signal that is decreased upon activation of the G protein. (F) Variation of BRET signal between Go–Rluc and Venus-Gγ2 measured on HEK-293 cells also expressing GB1 and GB2-KKXX (black bar) or GB1, GB2-KKXX and the competitor GB1-ΔB-ΔCT (white bar). The results are shown as the difference between the ΔBRET ratio recorded with PBS (basal) minus the ΔBRET ratio recorded with 1 mM GABA (stimulated). **P<0.01 in a paired t-test. (G) BRET variation kinetics determined on HEK-293 cells co-transfected with the same constructs as described in (F). GABA (1 mM) was added at 150 s of reading. The kinetics recorded for the control is in black and the kinetics measured on cells overexpressing the competitor GB1-ΔB-ΔCT is in grey. For (A, D, G), data are representative of three to six independent experiments. For (B, F), data are mean±s.e.m. of three to four independent experiments each performed in triplicates.
To validate this approach, the competition efficiency was first assessed using TR-FRET. When expressing the competitor, a decrease in FRET signal between ST-GB1 subunits was observed, while a FRET signal between the ST-GB1 and the HA-tagged competitor appeared (Figure 4A). We also verified that the heterodimer formation was not much affected by the co-expression of the competitor as measured with FRET between ST-GB1 and Flag-GB2 (Figure 4A).
To compare the G protein signalling properties of two ‘disrupted’ GABAB heterodimers to that of one tetramer, we expressed the WT subunits in the presence or absence of the competitor together with the chimeric G protein Gqi9 that allows the receptor to activate phospholipase C in HEK-293 cells. The amount of ST-GB1–GB2-KKXX heterodimers at the cell surface was equivalent in both conditions (Supplementary Figure S5A), such that one tetramer corresponds to two heterodimers (each associated with the competing subunit). The maximal response measured in the presence of the competitor was twice higher than that obtained in the absence of it (Figure 4B). In comparison, when transfected at a similar cell-surface expression, CD4 did not affect the GABAB maximal response (Figure 4B). CD4 was chosen as control because it does not perturb the GABAB tetramer (Maurel et al, 2008) and has the advantage of not affecting G protein signalling.
The effect of the competitor on the native pertusis toxin-sensitive Gi coupling was then directly assessed using a ‘priming’ approach, that is, recording Ca2+ responses induced by stimulation of a Gi/o-coupled receptor following a pre-activation of a Gq-coupled receptor (Figure 4C; Supplementary Figure S6) (Park et al, 1993; Quitterer and Lohse, 1999; Rives et al, 2009). In agreement with the data obtained using the chimeric Gqi9 protein (Figure 4B), competing with the formation of WT tetramers using GB1-ΔB-ΔCT, resulted in an increased Ca2+ response for a similar amount of WT GABAB heterodimers at the cell surface (Figure 4D). To measure more directly G protein activation, we monitored G protein rearrangement upon activation by BRET between Gαo and Gγ2 fused to RLuc and Venus-YFP, respectively (Gales et al, 2006; Ayoub et al, 2007) (Figure 4E). BRET signal after GABA stimulation was measured on cells expressing these G protein subunits with either GABAB only or GABAB+GB1-ΔB-ΔCT. The change in BRET signal was significantly higher (around two-fold) in the presence of the competitor, consistent with the activation of twice as much G proteins in the presence of the competitor for an equivalent amount of GB1−GB2-KKXX heterodimer (Figure 4F; Supplementary Figure S5B). Of note, the use of the competitor did not affect G protein activation kinetics (τ1/2=0.59±0.18 s and 0.61±0.19 for GB1+GB2-KKXX and GB1+GB2-KKXX+GB1-ΔB-ΔCT, respectively) (Figure 4G). Altogether, these data indicate that G protein coupling efficacy is regulated by the oligomeric state of the GABAB receptor.
Mutation destabilizing the tetramer enhances G protein coupling efficacy
In an attempt to destabilize the tetramer formation by mutagenesis, we used the glycan wedge approach that has been used previously to destabilize the GB1–GB2 interaction (Rondard et al, 2008). It consists of inserting N-linked glycosylation consensus sites (NxS/T) that will link large carbohydrates during maturation, hence generating steric hindrance to disturb protein–protein interaction. Here, we inserted such consensus sites at different area of GB1-VFT based on a 3D molecular model. In a first set of mutants, consensus sites were introduced opposite to the GB1–GB2 heterodimerization interface (Supplementary Figure S7), but none of the expressed mutants appeared to affect the GB1–GB1 interaction as measured by FRET using the ST (data not shown). We also combined these mutants in order to further increase the steric hindrance, but again, none of the double mutants destabilize the GB1–GB1 interaction as indicated by similar FRET signals between the WT and mutants (Supplementary Figure S7).
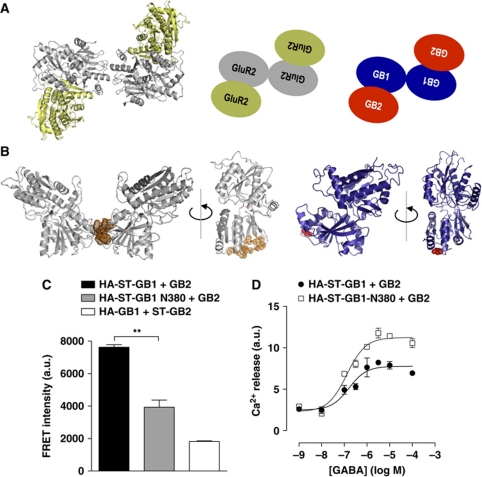
A crystal structure of a VFT tetramer has been recently published with the determination of the full-length tetrameric GluR2 AMPA receptor structure at 3.6 Å (Sobolevsky et al, 2009). It is organized as a loose dimer of tight dimers (Figure 5A). One could easily imagine that this organization is well transposable to loose dimer of tight GB1–GB2 heterodimers (Figure 5A). Of note, the structural elements at the interface of the tight AMPA-VFT dimer are similar to those identified at the GB1–GB2 interface (Rondard et al, 2008; Jin et al, 2009; Sobolevsky et al, 2009). Based on these observations, we hypothesized the GB1–GB1 contact area may correspond, at least in part, to the small area defined in the crystal structure (Figure 5B).
Figure 5.
Mutation at the GB1–GB1 contact area destabilizes the tetramer and increase G protein coupling efficacy. (A) Crystal structure (left, pdb code 3KG2) and schematic representation (centre) of GluR2 N-terminal domain tetramer, illustrating the assembly of the four VFTs into a loose dimer of tight dimers. Putative organization of GABAB tetramer (right). (B) Illustration of contact area of GluR2 N-terminal domain (left), 3D model of the corresponding GB1-VFT (right). The identified contact area in GluR2 is highlighted in orange and the position 380 in GB1 in red. (C) FRET intensity measured on HEK-293 cells between the indicated ST-labelled subunits. ** represents P<0.01 in a t-test. (D) Calcium signal measured upon stimulation of the chimeric G protein Gqi9 by increasing concentration of GABA in HEK-293 cells transfected with wild-type (black-filled circles) or N380 (open squares) GB1 together with GB2. For (C, D) data are representative of four independent experiments.
In order to disturb this potential GB1–GB1 interface, we generated HA-ST-GB1-N380 in which Glu380 and Leu382 were mutated into Asn and Thr, respectively. When transfecting HEK-293 cells with HA-ST-GB1 WT or N380 together with GB2, the specific FRET signal measured between ST-GB1 was significantly lower (about 35% of WT) for an equivalent cell-surface expression (Figure 5C; Supplementary Figure S7E). This indicates that this mutant affects the GABAB receptor oligomerization.
Measurements of Ca2+ release was measured upon GABA addition on HEK cells co-transfected with Gqi9 showed that N380 containing GABAB receptors were more efficient in G protein stimulation. Indeed, for a smaller cell-surface expression (60% of WT; Supplementary Figure S7F), HA-ST-GB1-N380 led to a larger response (170% of WT), as illustrated in Figure 5D. This is in agreement with the previous experiments using the competitor.
Binding of a single agonist in the tetramer leads to the maximal response
The data reported above indicate that a heterodimer dissociated from a tetramer leads to a maximal G protein coupling efficacy. This gives rise to questioning the stoichiometry of active units in a tetramer. A first issue is to assess whether one agonist binding site per tetramer is sufficient to induce the maximal response.
Our aim was to substitute in the tetramer, one WT GB1 by a mutant unable to bind GABA (with the two mutations S246A and E465A: GB1-ΔB) with its intact C-terminal tail such that it interacts with GB2-KKXX. In that way, we expect to have only one functional heterodimer per tetramer. We co-expressed ST-GB1+Myc-GB2-KKXX in the presence or absence of HA-GB1-ΔB, in HEK-293 cells (Figure 6). We ensured that, in our conditions, the amount of ST-GB1 at the cell surface was equivalent in both cases (Supplementary Figure S8A). Upon expression of HA-GB1-ΔB, the FRET signal between ST-GB1 was decreased whereas a FRET signal between HA-GB1-ΔB and either ST-GB1 or Myc-GB2-KKXX appeared. Moreover, the FRET signal between WT heterodimers was two-fold decreased (Figure 6A). These data confirmed that when HA-GB1-ΔB was co-transfected: (i) the mutant could dimerize with Myc-GB2-KKXX and oligomerize with ST-GB1; (ii) the interaction between two ST-GB1 was decreased and (iii) the amount of functional heterodimers at the cell surface remained constant. That allowed us to compare GABA responses from cells expressing WT tetramers with those obtained in cells expressing twice as many tetramers most having a single site able to bind the agonist. Although other combinations may exist at the cell surface (Supplementary Figure S9), we used conditions where the predominant functional tetramer is composed of one WT heterodimer and a mutant heterodimer (Figure 6A).
Figure 6.
One binding site per tetramer is sufficient to induce the maximal agonist response. (A) Schematic representation of the oligomeric GABAB receptor in the absence (left) or in the presence of a GB1 unable to bind the GABA (GB1-ΔB), which can substitute one wild-type GB1 to form tetramers with a single functional heterodimer per tetramer (right). FRET intensity measured on cells expressing either ST-GB1 and Myc-GB2-KKXX (control) (black bars) or control with an optimized amount of GB1-ΔB (white bars), (a) between ST-GB1 subunits labelled with BG-K and BG-d2, (b) between ST-GB1 and HA-GB1-ΔB labelled with BG-d2 and K anti-HA antibody, (c) between ST-GB1 and Myc-GB2-KKXX labelled with BG-d2 and K anti-Myc antibody and (d) between HA-GB1-ΔB and Myc-GB2-KKXX labelled with K anti-HA and d2 anti-Myc antibodies. Data are mean±s.e.m. of three independent experiments each performed in triplicate. ** and *** represent P<0.01 and P<0.001, respectively, in a paired t-test. (B) Intracellular calcium response curve mediated by GABA on cells expressing GB1 and GB2-KKXX (control) (black-filled circles), GB1-ΔB and GB2-KKXX (negative control) (black triangles) and control and GB1-ΔB (grey open squares) in the presence of a chimeric Gqi9. Data are representative of four independent experiments each performed in triplicate. (C) Displacement of [3H]-CGP54626A binding by GABA on wild-type GB1 measured on cells expressing GB1 and GB2-KKXX (control) (black-filled circles), and control with GB1-ΔB (grey open squares). Data are representative of three independent experiments, each performed in triplicates.
The GABA-mediated Ca2+ response was analysed in both conditions. In cells expressing HA-GB1-ΔB, the functional response efficacy was higher than that obtained with WT receptor only (200±20% of the WT) (Figure 6B). As expected, no Ca2+ release could be detected when the mutant heterodimer was expressed alone (GB1-ΔB+GB2-KKXX) (Figure 6B). These data indicate that the Ca2+ response generated per functional GABAB receptor heterodimer is higher when they are associated with a non-functional heterodimer, consistent with one GABA-binding site per tetramer being sufficient to induce maximal G protein activation.
In addition, GABA affinity on GB1 was measured by displacement of 1.4 nM of [3H]-CGP54626A (corresponding to the Kd of the antagonist) on cells expressing the WT tetramer or the tetramer with a single GABA-binding site. We could not see any difference in GABA (Ki=2.02 μM (1.02–3.96 μM) and 2.78 μM (1.51–5.10 μM) for WT and WT+GB1-ΔB, respectively) (Figure 6C) or in cold CGP54626A affinity (Ki=1.80 nM (1.43–2.26 nM) and 2.33 nM (1.93–2.80 nM)) for WT and WT+GB1-ΔB, respectively).
Dimerization of GABAB heterodimers regulates G protein coupling efficacy
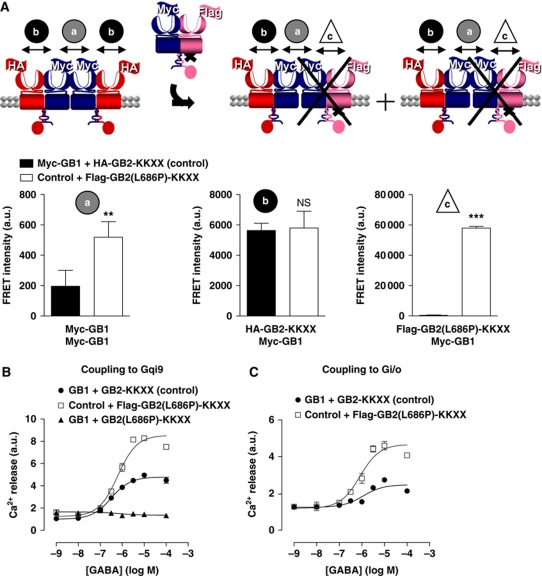
Next, we tried to assess the stoichiometry for G protein coupling in the tetramer. To that aim, we chose to substitute one WT GB2 subunit by a mutant that cannot activate G proteins (GB2(L686P)-KKXX) (Havlickova et al, 2002). The G protein coupling being dependant on WT GB2, the conditions of transfection were optimized to have the same HA-GB2-KKXX expression level whether the mutant is present or not (Supplementary Figure S8B). Accordingly, when Flag-GB2(L686P)-KKXX is co-expressed, the amount of Myc-GB1 at the cell surface increased and the FRET signal between Myc-GB1 subunits is higher (Figure 7A). Moreover, the formation of heterodimers between Myc-GB1 and Flag-GB2(L686P)-KKXX was confirmed (Figure 7A). Note that the FRET between Myc-GB1 and HA-GB2-KKXX remained constant. This is in agreement with the expected reorganization (Figure 7A). As indicated above, although additional combinations exist at the cell surface, the conditions are such that the complexes composed of a WT and a mutated heterodimer are predominant (Supplementary Figure S9).
Figure 7.
Regulation of G protein coupling depends on the dimerization of the GABAB heterodimers. (A) Schematic representation of the oligomeric GABAB receptor in the absence (left) or in the presence (right) of a GB2 subunit unable to activate the G protein and carrying a KKXX retention signal motif (GB2(L686P)-KKXX), which can substitute one wild-type GB2 to form tetramers with a single functional heterodimer per tetramer (right). FRET intensity measured on COS-7 cells expressing the indicated constructs: control (black bars) and control with GB2(L686P)-KKXX (white bars) (a) between the Myc-GB1 subunits after labelling with a mixture of K and d2 anti-Myc antibodies, (b) between Myc-GB1 and HA-GB2-KKXX after labelling with K anti-HA and d2 anti-Myc antibodies and finally (c) between Flag-GB2(L686P)-KKXX and Myc-GB1 labelled with K anti-Flag and d2 anti-Myc antibodies. Data are mean±s.e.m. of three independent experiments each performed in triplicates. ** and *** represent P<0.01 and P<0.001, respectively, in a t-test. (B) Calcium dose-response recorded after stimulation by the GABA on cells expressing GB1 and GB2-KKXX (control) (black-filled circles), GB1 and GB2(L686P)-KKXX (negative control) (black triangles) and control with GB2(L686P)-KKXX (grey open squares) in the presence of a Gqi9 chimeric protein. (C) GABA-induced response of the native Gi/o protein by measurement of the calcium formation after pre-stimulation of an endogenous Gq-coupled receptor. For (B, C), representative of three to four independent experiments, each performed in triplicates.
In those conditions and using Gqi9, we observed that the Ca2+ release induced by GABA was enhanced compared with the WT in the presence of Flag-GB2(L686P)-KKXX (152±14% of the WT%) (Figure 7B). To exclude any contamination of the signal by putative Myc-GB1/Flag-GB2(L686P)-KKXX heterodimers, we checked that these heterodimers did not give rise to Ca2+ release (Figure 7B). To eliminate any artifact coming from the use of a chimeric G protein, we successfully reproduced these results by activation of native Gi proteins in the ‘priming’ assay (213±42% of the WT) (Figure 7C). The extent of G protein activation when a single GB2 in the tetramer can couple suggests that only a single G protein can be activated by the GABAB tetramer.
Discussion
Oligomerization of GPCRs has been proposed to offer additional possibilities to regulate their function, especially in the brain (Carriba et al, 2008; Gonzalez-Maeso et al, 2008; van Rijn et al, 2010). However, the existence of such oligomers is still a matter of intense debate (Park et al, 2004; Chabre et al, 2009). Among the large GPCR family, the GABAB receptor is well recognized as being an obligatory heterodimer, and recent data suggest that it may even form larger complexes composed of at least two heterodimers (Maurel et al, 2008). In the present study, we first bring further evidence supporting this phenomenon: (i) we showed that the tetramers are stable enough to resist to solubilization, as demonstrated by co-immunoprecipitation experiments; (ii) the interaction between heterodimers does not result from overexpression since these are detected at an expression level similar to that measured in cultured neurons and (iii) most importantly, our data support the existence of such tetramers in the brain. Second, by comparing the functional properties of the GABAB heterodimers and tetramers, we show here that there is a negative cooperativity in terms of G protein coupling between heterodimers in the tetramers.
The detection of GABAB tetramers was achieved using the TR-FRET technology that has several advantages compared with conventional FRET: (i) a 100-fold better signal-to-noise ratio and (ii) an absence of dipole orientation constraint of the fluorophores, such that the signal depends only on the distance between the fluorophores (Bazin et al, 2002; Selvin, 2002). Thanks to the use of antibodies directed against the native GB1a protein (Tiao et al, 2008), we could use TR-FRET to detect oligomers in native tissues. Our data revealed a close proximity between GB1a subunits both in membranes from COS-7 cells expressing the GB1a and GB2 subunits, as well as in brain membrane of WT mice and rats and in mice synaptosomes. This does not result from random clustering of the GABAB receptors in specific microdomains, since no close proximity could be detected between GB2 subunits (see also Maurel et al, 2008). Although this could not be directly demonstrated with the native receptors due to the lack of specific antibodies recognizing the GB2 extracellular domain, no TR-FRET between GB2 subunits was detected in membranes prepared from COS-7 cells transfected with HA-GB2 and GB1. This is consistent with an interaction of two heterodimers via the GB1 subunits, while the GB2-VFTs are further apart. Even though we used membranes to measure the TR-FRET signal between native GB1a, we are convinced that it does not simply result from intracellular homomeric GB1 not assembled with GB2. Actually, GB1–GB1 interaction was also detected in synaptosomes preparations and a dramatic decrease in GB1 expression observed in the GB2−/− mice (Gassmann et al, 2004) suggests that most GB1 is associated with GB2 in WT brain. Thus, this represents a first direct evidence for large GPCR complexes in the brain.
The exact stoichiometry of GABAB receptor oligomers remains unknown; however, there are several pieces of argument in favour of a tetrameric assembly: (i) the absence of measurable FRET between GB2-VFTs implies that the distance between two GB2 is over 77 Å (according to the Ro (58 Å) of the FRET pair used in this study), which is not compatible with most of the possible arrangements (Supplementary Figure S1); (ii) our data indicate that the GB1–GB1 contact area is likely to be similar with the tetramerization interface in the GluR2 VFT tetramer, which supports a symmetrical interaction of two VFT limiting at four the total number of subunits and (iii) size analysis of native GABAB receptor complexes from mouse brain on native gels indicates that the complex is likely limited to two GABAB heterodimers (i.e. a tetramer) each associated with accessory proteins KCTD (Schwenk et al, 2010).
The study of the GABAB tetramer signalling properties provides new insights on the functional consequences of GPCR oligomerization and mainly the allosteric phenomena involved. Actually, we showed that, for a similar amount of WT receptors at the cell surface, the tetramers exhibit a lower G protein coupling efficacy than two disrupted heterodimers as revealed by using competitors of the GB1–GB1 interaction or a destabilized one through mutation at the contact area (Figures 4 and 5; see also Maurel et al, 2008). This could be the result either of a less efficient activation of G proteins by each heterodimers within the tetramer, or of the inability of the tetramer to activate two G proteins at a time. Such hypotheses are reminiscent to what has been proposed for class A GPCR dimers, where a single G protein is likely interacting with a dimer (Baneres and Parello, 2003; Bayburt et al, 2007; Whorton et al, 2007; Kuszak et al, 2009). Nevertheless, it appears that a class A GPCR dimer activates a G protein equally well as a monomer, such that a pair of (non-interacting) monomers can activate twice as much G proteins than a dimer (White et al, 2007; Arcemisbehere et al, 2010). In the GABAB receptor context, the heterodimer GB1–GB2 could be compared with a monomeric class A receptor. However, in the case of class A GPCRs, steric hindrance is assumed to prevent two G proteins to interact simultaneously with a dimer (one per protomer). This appears unlikely to be the case for the GABAB receptor since the GB2 subunits that are responsible for G protein activation, are at a large distance within the tetramer (over 77 Å according to our FRET data), thus giving theoretically enough space for each GB2 subunit to contact and activate a G protein in the tetramer. We also confirmed that in a tetramer where a single heterodimer was able to signal (the second one being invalidated for either ligand binding or G protein activation) the response was roughly equivalent to that of the WT tetramer. This could be interpreted as a single heterodimer per tetramer being able to adopt an active conformation at a time. Altogether, our data imply that a negative cooperativity exists between the two assembled GABAB receptors, such that only one functions at a time. Even if the receptors formed higher-ordered oligomers, the functional analysis of the GABAB complex would still be valid. Indeed, in the competition experiment, the size of the oligomers would decrease, such that the larger functional response observed still supports a negative cooperativity between GABAB heterodimers. This reasoning is also true for the replacement experiments, where the number of functional subunits per oligomer is decreased, then limiting negative cooperativity between functional heterodimers.
What would be the physiological significance of the tetrameric organization of the GABAB receptor? A key issue to answer this question will be to know whether all GABAB receptors are tetramers, or whether an equilibrium can exist between dimeric and tetrameric entities. If such equilibrium exists, it is tempting to speculate that this offers new possible ways to regulate the GABAB receptor function, such as limiting GABAB-mediated G protein responses through oligomerization, depending on the activity of the receptor, or its specific location in particular subcellular compartments. One would easily imagine tetramers to constitute receptor stores, as proposed for rhodopsin oligomers (Govardovskii et al, 2009), that associate or dissociate depending on various signals like the basal GABA concentration, interacting proteins, ligands etc. Of interest, some class A GPCRs have been shown to undergo such association–dissociation events (Dorsch et al, 2009; Ilien et al, 2009; Hern et al, 2010). In contrast, if all GABAB receptors are tetrameric in vivo, it is quite surprising that such organization appears to limit G protein-mediated signalling. However, several points must be considered: (i) the GABAB receptor assembly into tetramers may be beneficial for other GABAB receptor properties that need to be identified, such as receptor stability, trafficking, turnover, desensitization or internalization; (ii) the assembly into tetramers indeed offers larger possibilities for interacting proteins to regulate GABAB receptor activity and (iii) it is possible that non-G protein-mediated responses may be activated preferentially by the tetrameric state of the receptor. Furthermore, if GB1a is part of GABAB tetramers, we have yet no direct evidence that GB1b forms tetramers in vivo due to the lack of GB1b-specific antibody. Although GB1b containing tetramers have been observed in heterologous system (Maurel et al, 2008), it is still possible that the oligomeric state of GB1a and GB1b containing GABAB receptors might be different in the brain. This would offer alternatives in the strength of signalling of pre- versus post-synaptic receptors as GB1a and GB1b are segregated in these synaptic compartments, respectively (Vigot et al, 2006; Biermann et al, 2010).
Materials and methods
Chemicals and reagents
Unless stated otherwise, the compounds were purchased from Sigma.
Plasmids and site-directed mutagenesis
Plasmids encoding the WT GABAB1a or GABAB1b and GABAB2 subunits bearing an HA, Flag or ST epitope at their N-terminus were described previously (Kniazeff et al, 2004; Maurel et al, 2008).
The HA-GB1(S246AE465A)-ΔCT was generated by subclonings of the S246A or the E465A mutations previously described (Galvez et al, 1999, 2000; Kniazeff et al, 2002) and by adding a stop codon at position 875 using a Quick-Change® strategy (Stratagene). The N-glycosylation mutants were generated using a Quick-Change strategy. The final constructs were verified by sequencing (Genome express, Meylan, France).
The ST chimeric GABAB constructs were generated by inserting the ST sequence in the chimeras previously described (Galvez et al, 2001).
The Go–Rluc fusion protein used for BRET assay was previously described (Ayoub et al, 2007, 2009). The Venus-tagged Gγ2 subunit was provided by Dr C Galès (INSERM U858, Toulouse, France) (Gales et al, 2006).
Cell culture and transfection
HEK-293 and COS-7 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% FBS. Culture medium, FCS and other products used for cell culture were purchased from GIBCO/BRL/Life Technologies (Cergy Pontoise, France). Cells were transiently transfected either by lipofection with the Lipofectamine 2000 following the manufacturer's protocol (Invitrogen Life Technologies, Gaithersburg, MD) or by electroporation (Maurel et al, 2004).
Cell-surface Co-IP
The method is thoroughly detailed in Supplementary data. Briefly, after incubation with mouse Flag-M2 Ab and N-ethyl-maleimide treatment, cells were lysed with RIPA lysis buffer. After centrifugation of the soluble fraction, the supernatant was applied on Protein A/G beads and incubated for 3 h at 4°C. The precipitate was then loaded on a 7.5% SDS–PAGE.
For the western blot, primary antibodies used were rabbit anti-HA Ab (71-5500; Zymed) and mouse anti-Flag Ab (F3165; Sigma). The secondary antibodies used were Alexa Fluor® 680 goat anti-rabbit IgG (A-21076; Molecular Probes) and DyLight® 800-conjugated anti-mouse IgG (610-145-121; Rockland). The fluorescence signals were recorded by the Odyssey® (LI-COR Biosciences).
ELISA for quantification of cell-surface expression
ELISA on intact cells was performed as previously described (Maurel et al, 2008) using the anti-HA monoclonal antibody (clone 3F10; Roche Bioscience, Basel, Switzerland) or the anti-Flag-M2 monoclonal antibody (Sigma-Aldrich, St Louis, MO, USA), both conjugated with horseradish peroxidase.
Intracellular calcium measurements
HEK-293 cells were transfected with plasmids encoding the indicated GABAB subunits and a chimeric protein Gqi9 and seeded out in a 96-well plate at 200 000 cells/well. Intracellular calcium measurements were performed as previously described (Maurel et al, 2008) after addition of various concentrations of GABA.
For ‘priming’ experiments, HEK-293 cells were transfected only with plasmids encoding the GABAB subunits without Gqi9. The cells were treated in the same way, besides 50 μl of 2X-AVP (vasopressin 2.10−7 μM) was added after 20 s reading and then 50 μl of 3X-GABA solution at various concentrations after 240 s.
Binding assay on intact cells
Cells were incubated with 10 nM of [3H]-CGP54626, for 4 h at 4°C. Non-specific binding was determined by addition of GABA (1 mM). After incubation, cells were washed with ice-cold Tris-KREBS buffer (20 mM Tris pH 7.4, 118 mM NaCl, 5.6 mM glucose, 1.2 mM KH2PO4, 1.2 mM MgSO4, 4.7 mM KCl, 1.8 mM CaCl2) in order to eliminate the excess of free radioactive ligand. Cells were then lysed using NaOH at 0.1 M for 10 min and transferred in flasks containing scintillant (OptiPhase Supermix, Perkin-Elmer). Radioactivity was counted on a Beta counter Cobra (Hewlett Packard). The bound radioactivity was determined considering the specific activity of the [3H]-CGP54626 and the volume per well.
IC50 determination
Cells were incubated with a concentration corresponding to the Kd of the [3H]-CGP54626 (1.4 nM) and increasing concentrations of the competitor cold ligand, either CGP54626 (10 pM to 1 μM) or GABA (0.1 μM to 1 mM) for 4 h at 4°C. Then, the cells were treated as in the binding assay.
TR-FRET between two Snap-tags or between Snap-tags and labelled antibodies
TR-FRET experiments were performed in 96-well plates (Greiner CellStar) as previously described (Maurel et al, 2008). Note that the donor-conjugated benzyl guanine (BG) used was the BG-Lumi4-Tb bearing a terbium cryptate known as a Tag-Lite substrate (Cisbio, Bagnols/Cèze, France). The specific FRET signal is calculated as indicated in Supplementary data.
Mice brain membrane preparation
Brains from WT, GB1−/−, GB1a−/− or GB1b−/− mice were homogenized in 20 vol of ice-cold sucrose (0.32 M) using a potter. The homogenate was centrifuged at 1000 g for 10 min at 4°C. The supernatant was centrifuged at 17 000 g for 20 min at 4°C. Then, the pellet (P2) was lysed in 40 vol. of ice-cold distilled water for 45 min. After a centrifugation at 20 000 g for 35 min at 4°C, the pellet was washed three times in 40 vol. of ice-cold distilled water. The final pellet was resuspended in 50 mM Tris-citrate buffer, pH 7.4 (Pin et al, 1984).
Mice brain synaptosomes preparation
We proceeded as previously described (Karten et al, 2006). Briefly, after brain homogeneization and cell debris removal, the total brain membrane fraction was loaded on a percoll gradient (3, 10 and 23%) and set for ultracentrifugation (100 000 g, 14 min). The synaptosomes fraction was collected and resuspended after centrifugation in 0.32 M sucrose, 10 mM hepes pH 7.4.
TR-FRET using anti-sushi antibodies on brain membrane
A measure of 200 μg of brain membrane diluted in 50 mM Tris-citrate ice-cold buffer were incubated overnight at 4°C under rotation with the monoclonal anti-sushi antibodies (Tiao et al, 2008). Three conditions were tested, each in triplicate: (i) Lumi4-Tb Ab (16.7 nM)+cold Ab (1.35 nM); (ii) Lumi4-Tb Ab (16.7 nM)+d2 Ab (8.3 nM) and (iii) Lumi4-Tb Ab (16.7 nM)+d2 Ab (8.3 nM)+cold Ab in large excess (1 μM). The indicated concentrations were optimized (Supplementary Figure S2) in order to ensure the highest FRET signal. Then, samples were centrifuged at 17 000 g for 15 min at 4°C and the resultant pellet was washed twice with 1 ml of 50 mM Tris-citrate buffer. The final pellet was resuspended in 100 μl of the same buffer and distributed in a 96-well plate Greiner CellStar. The FRET signal was recorded at 665 nm between 50 and 450 μs after laser excitation at 337 nm using a time-resolved fluorimeter (RubyStar, BMG Labtechnologies, Champigny-sur-Marne, France). FRET intensity was expressed as the Δ665=(total signal recorded at 665 nm)−(background at 665 nm given by the Lumi4-Tb Ab+cold Ab). The non-specific FRET signal given by the membranes labelled with Lumi4-Tb+d2+an excess of cold Ab was also subtracted.
BRET measurements
BRET measurements were recorded after 1 mM GABA stimulation on the Mithras LB 940 plate reader (Berthold Biotechnologies, Bad Wildbad, Germany) as previously described (Ayoub et al, 2007). For BRET kinetics, 1 mM GABA was added using the injection system at 150 s of reading and the BRET signal was recorded for a total time of 250 s at 0.1 s intervals, each channels being alternatively recorded every 0.05 s.
Animals
The generation of GB1−/−, GB1a−/−, GB1b−/− mice has been previously described (Schuler et al, 2001; Vigot et al, 2006). The mice were kept in the BALB/c inbred background. Animal experiments were subjected to institutional review, conducted in accordance with Swiss guidelines and approved by the veterinary office of Basel-Stadt.
Data analysis
Data were analysed using the GraphPad software, San Diego, CA, USA. For calcium measurements, binding experiments and BRET assays, the fitting equations used are indicated in Supplementary data.
Supplementary Material
Acknowledgments
We thank Dr Mohammed Akli Ayoub for initiating us to the BRET experiments and for helpful and constructive discussions. We are also thankful to Dr Cyril Goudet for his support, to Dr Gilles Labesse for generating the GB1 3D model and to Dr Céline Galès for providing us the Venus-tagged Gγ2 plasmid. The FRET, BRET and Flex experiments have been performed using the ARPEGE (Pharmacology Screening-Interactome) platform facility at the Institute of Functional Genomics (Montpellier, France). JPP's work was supported by CNRS, INSERM, Cisbio and by grants from the French Ministry of Research, Agence Nationale de la Recherche (ANR-06-BLAN-0087 and ANR-09-BLAN-0272) and by an unrestricted grant from Senomyx. BB was supported by the Swiss Science Foundation (3100A0-117816) and the European Community's 7th Framework Programme (FP7/2007-2013) under Grant Agreement 201714. LCA was supported by a CIFRE fellowship from CisBio and the French government.
Author contributions: LCA conceived, performed and analysed all the experiments, with contribution of JK, LNL and DM. MG and BB provided the anti-GB1a Ab and brains from the KO animals and NG labelled the anti-GB1a Ab. BB, TD and LP participated to constructive discussions and had important conceptual inputs to the project. LCA and JK wrote the paper. ET supervised the work using the labelled antibodies and TR-FRET data. JK conceived and supervised the analysis of tetramer destabilizing mutants. JPP conceived and supervised the project and finalized the paper. All the authors understand their responsibilities connected to authorship.
Footnotes
The authors declare that they have no conflict of interest.
References
- Arcemisbehere L, Sen T, Boudier L, Balestre MN, Gaibelet G, Detouillon E, Orcel H, Mendre C, Rahmeh R, Granier S, Vives C, Fieschi F, Damian M, Durroux T, Baneres JL, Mouillac B (2010) Leukotriene BLT2 receptor monomers activate the G(i2) GTP-binding protein more efficiently than dimers. J Biol Chem 285: 6337–6347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoub MA, Damian M, Gespach C, Ferrandis E, Lavergne O, De Wever O, Baneres JL, Pin JP, Prevost GP (2009) Inhibition of heterotrimeric G protein signaling by a small molecule acting on Galpha subunit. J Biol Chem 284: 29136–29145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoub MA, Maurel D, Binet V, Fink M, Prezeau L, Ansanay H, Pin JP (2007) Real-time analysis of agonist-induced activation of protease-activated receptor 1/Galphai1 protein complex measured by bioluminescence resonance energy transfer in living cells. Mol Pharmacol 71: 1329–1340 [DOI] [PubMed] [Google Scholar]
- Baneres JL, Parello J (2003) Structure-based analysis of GPCR function: evidence for a novel pentameric assembly between the dimeric leukotriene B4 receptor BLT1 and the G-protein. J Mol Biol 329: 815–829 [DOI] [PubMed] [Google Scholar]
- Bayburt TH, Leitz AJ, Xie G, Oprian DD, Sligar SG (2007) Transducin activation by nanoscale lipid bilayers containing one and two rhodopsins. J Biol Chem 282: 14875–14881 [DOI] [PubMed] [Google Scholar]
- Bazin H, Trinquet E, Mathis G (2002) Time resolved amplification of cryptate emission: a versatile technology to trace biomolecular interactions. J Biotechnol 82: 233–250 [DOI] [PubMed] [Google Scholar]
- Bettler B, Kaupmann K, Mosbacher J, Gassmann M (2004) Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev 84: 835–867 [DOI] [PubMed] [Google Scholar]
- Biermann B, Ivankova-Susankova K, Bradaia A, Abdel Aziz S, Besseyrias V, Kapfhammer JP, Missler M, Gassmann M, Bettler B (2010) The Sushi domains of GABAB receptors function as axonal targeting signals. J Neurosci 30: 1385–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery NG (2006) GABAB receptor: a site of therapeutic benefit. Curr Opin Pharmacol 6: 37–43 [DOI] [PubMed] [Google Scholar]
- Bowery NG, Bettler B, Froestl W, Gallagher JP, Marshall F, Raiteri M, Bonner TI, Enna SJ (2002) International Union of Pharmacology. XXXIII. Mammalian gamma-aminobutyric acid(B) receptors: structure and function. Pharmacol Rev 54: 247–264 [DOI] [PubMed] [Google Scholar]
- Brinkley M (1992) A brief survey of methods for preparing protein conjugates with dyes, haptens, and cross-linking reagents. Bioconjug Chem 3: 2–13 [DOI] [PubMed] [Google Scholar]
- Carriba P, Navarro G, Ciruela F, Ferre S, Casado V, Agnati L, Cortes A, Mallol J, Fuxe K, Canela EI, Lluis C, Franco R (2008) Detection of heteromerization of more than two proteins by sequential BRET-FRET. Nat Methods 5: 727–733 [DOI] [PubMed] [Google Scholar]
- Chabre M, Deterre P, Antonny B (2009) The apparent cooperativity of some GPCRs does not necessarily imply dimerization. Trends Pharmacol Sci 30: 182–187 [DOI] [PubMed] [Google Scholar]
- Cryan JF, Kelly PH, Chaperon F, Gentsch C, Mombereau C, Lingenhoehl K, Froestl W, Bettler B, Kaupmann K, Spooren WP (2004) Behavioral characterization of the novel GABAB receptor-positive modulator GS39783 (N,N′-dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine): anxiolytic-like activity without side effects associated with baclofen or benzodiazepines. J Pharmacol Exp Ther 310: 952–963 [DOI] [PubMed] [Google Scholar]
- Dorsch S, Klotz KN, Engelhardt S, Lohse MJ, Bunemann M (2009) Analysis of receptor oligomerization by FRAP microscopy. Nat Methods 6: 225–230 [DOI] [PubMed] [Google Scholar]
- Duthey B, Caudron S, Perroy J, Bettler B, Fagni L, Pin JP, Prezeau L (2002) A single subunit (GB2) is required for G-protein activation by the heterodimeric GABA(B) receptor. J Biol Chem 277: 3236–3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galandrin S, Oligny-Longpre G, Bouvier M (2007) The evasive nature of drug efficacy: implications for drug discovery. Trends Pharmacol Sci 28: 423–430 [DOI] [PubMed] [Google Scholar]
- Gales C, Van Durm JJ, Schaak S, Pontier S, Percherancier Y, Audet M, Paris H, Bouvier M (2006) Probing the activation-promoted structural rearrangements in preassembled receptor-G protein complexes. Nat Struct Mol Biol 13: 778–786 [DOI] [PubMed] [Google Scholar]
- Galvez T, Duthey B, Kniazeff J, Blahos J, Rovelli G, Bettler B, Prezeau L, Pin JP (2001) Allosteric interactions between GB1 and GB2 subunits are required for optimal GABA(B) receptor function. EMBO J 20: 2152–2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez T, Parmentier ML, Joly C, Malitschek B, Kaupmann K, Kuhn R, Bittiger H, Froestl W, Bettler B, Pin JP (1999) Mutagenesis and modeling of the GABAB receptor extracellular domain support a venus flytrap mechanism for ligand binding. J Biol Chem 274: 13362–13369 [DOI] [PubMed] [Google Scholar]
- Galvez T, Prezeau L, Milioti G, Franek M, Joly C, Froestl W, Bettler B, Bertrand HO, Blahos J, Pin JP (2000) Mapping the agonist-binding site of GABAB type 1 subunit sheds light on the activation process of GABAB receptors. J Biol Chem 275: 41166–41174 [DOI] [PubMed] [Google Scholar]
- Gassmann M, Shaban H, Vigot R, Sansig G, Haller C, Barbieri S, Humeau Y, Schuler V, Muller M, Kinzel B, Klebs K, Schmutz M, Froestl W, Heid J, Kelly PH, Gentry C, Jaton AL, Van der Putten H, Mombereau C, Lecourtier L et al. (2004) Redistribution of GABAB(1) protein and atypical GABAB responses in GABAB(2)-deficient mice. J Neurosci 24: 6086–6097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay EA, Urban JD, Nichols DE, Oxford GS, Mailman RB (2004) Functional selectivity of D2 receptor ligands in a Chinese hamster ovary hD2L cell line: evidence for induction of ligand-specific receptor states. Mol Pharmacol 66: 97–105 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, Lopez-Gimenez JF, Zhou M, Okawa Y, Callado LF, Milligan G, Gingrich JA, Filizola M, Meana JJ, Sealfon SC (2008) Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature 452: 93–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govardovskii VI, Korenyak DA, Shukolyukov SA, Zueva LV (2009) Lateral diffusion of rhodopsin in photoreceptor membrane: a reappraisal. Mol Vis 15: 1717–1729 [PMC free article] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV (2008) GPCR monomers and oligomers: it takes all kinds. Trends Neurosci 31: 74–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havlickova M, Prezeau L, Duthey B, Bettler B, Pin JP, Blahos J (2002) The intracellular loops of the GB2 subunit are crucial for G-protein coupling of the heteromeric gamma-aminobutyrate B receptor. Mol Pharmacol 62: 343–350 [DOI] [PubMed] [Google Scholar]
- Hawrot E, Xiao Y, Shi QL, Norman D, Kirkitadze M, Barlow PN (1998) Demonstration of a tandem pair of complement protein modules in GABA(B) receptor 1a. FEBS Lett 432: 103–108 [DOI] [PubMed] [Google Scholar]
- Hern JA, Baig AH, Mashanov GI, Birdsall B, Corrie JE, Lazareno S, Molloy JE, Birdsall NJ (2010) Formation and dissociation of M1 muscarinic receptor dimers seen by total internal reflection fluorescence imaging of single molecules. Proc Natl Acad Sci USA 107: 2693–2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilien B, Glasser N, Clamme JP, Didier P, Piemont E, Chinnappan R, Daval SB, Galzi JL, Mely Y (2009) Pirenzepine promotes the dimerization of muscarinic M1 receptors through a three-step binding process. J Biol Chem 284: 19533–19543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Singh SK, Gu S, Furukawa H, Sobolevsky AI, Zhou J, Jin Y, Gouaux E (2009) Crystal structure and association behaviour of the GluR2 amino-terminal domain. EMBO J 28: 1812–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KA, Borowsky B, Tamm JA, Craig DA, Durkin MM, Dai M, Yao WJ, Johnson M, Gunwaldsen C, Huang LY, Tang C, Shen Q, Salon JA, Morse K, Laz T, Smith KE, Nagarathnam D, Noble SA, Branchek TA, Gerald C (1998) GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature 396: 674–679 [DOI] [PubMed] [Google Scholar]
- Karten B, Campenot RB, Vance DE, Vance JE (2006) The Niemann-Pick C1 protein in recycling endosomes of presynaptic nerve terminals. J Lipid Res 47: 504–514 [DOI] [PubMed] [Google Scholar]
- Kaupmann K, Huggel K, Heid J, Flor PJ, Bischoff S, Mickel SJ, McMaster G, Angst C, Bittiger H, Froestl W, Bettler B (1997) Expression cloning of GABA(B) receptors uncovers similarity to metabotropic glutamate receptors. Nature 386: 239–246 [DOI] [PubMed] [Google Scholar]
- Kaupmann K, Malitschek B, Schuler V, Heid J, Froestl W, Beck P, Mosbacher J, Bischoff S, Kulik A, Shigemoto R, Karschin A, Bettler B (1998) GABA(B)-receptor subtypes assemble into functional heteromeric complexes. Nature 396: 683–687 [DOI] [PubMed] [Google Scholar]
- Kniazeff J, Galvez T, Labesse G, Pin JP (2002) No ligand binding in the GB2 subunit of the GABA(B) receptor is required for activation and allosteric interaction between the subunits. J Neurosci 22: 7352–7361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniazeff J, Saintot PP, Goudet C, Liu J, Charnet A, Guillon G, Pin JP (2004) Locking the dimeric GABA(B) G-protein-coupled receptor in its active state. J Neurosci 24: 370–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornau HC (2006) GABA(B) receptors and synaptic modulation. Cell Tissue Res 326: 517–533 [DOI] [PubMed] [Google Scholar]
- Kuner R, Kohr G, Grunewald S, Eisenhardt G, Bach A, Kornau HC (1999) Role of heteromer formation in GABAB receptor function. Science 283: 74–77 [DOI] [PubMed] [Google Scholar]
- Kuszak AJ, Pitchiaya S, Anand JP, Mosberg HI, Walter NG, Sunahara RK (2009) Purification and functional reconstitution of monomeric mu-opioid receptors: allosteric modulation of agonist binding by Gi2. J Biol Chem 284: 26732–26741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim WK (2007) GPCR drug discovery: novel ligands for CNS receptors. Recent Pat CNS Drug Discov 2: 107–112 [DOI] [PubMed] [Google Scholar]
- Margeta-Mitrovic M, Jan YN, Jan LY (2000) A trafficking checkpoint controls GABA(B) receptor heterodimerization. Neuron 27: 97–106 [DOI] [PubMed] [Google Scholar]
- Marshall FH (2005) Is the GABA B heterodimer a good drug target? J Mol Neurosci 26: 169–176 [DOI] [PubMed] [Google Scholar]
- Maurel D, Comps-Agrar L, Brock C, Rives ML, Bourrier E, Ayoub MA, Bazin H, Tinel N, Durroux T, Prezeau L, Trinquet E, Pin JP (2008) Cell-surface protein-protein interaction analysis with time-resolved FRET and snap-tag technologies: application to GPCR oligomerization. Nat Methods 5: 561–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel D, Kniazeff J, Mathis G, Trinquet E, Pin JP, Ansanay H (2004) Cell surface detection of membrane protein interaction with homogeneous time-resolved fluorescence resonance energy transfer technology. Anal Biochem 329: 253–262 [DOI] [PubMed] [Google Scholar]
- Moniri NH, Covington-Strachan D, Booth RG (2004) Ligand-directed functional heterogeneity of histamine H1 receptors: novel dual-function ligands selectively activate and block H1-mediated phospholipase C and adenylyl cyclase signaling. J Pharmacol Exp Ther 311: 274–281 [DOI] [PubMed] [Google Scholar]
- Overington JP, Al-Lazikani B, Hopkins AL (2006) How many drug targets are there? Nat Rev Drug Discov 5: 993–996 [DOI] [PubMed] [Google Scholar]
- Pagano A, Rovelli G, Mosbacher J, Lohmann T, Duthey B, Stauffer D, Ristig D, Schuler V, Meigel I, Lampert C, Stein T, Prezeau L, Blahos J, Pin J, Froestl W, Kuhn R, Heid J, Kaupmann K, Bettler B (2001) C-terminal interaction is essential for surface trafficking but not for heteromeric assembly of GABA(b) receptors. J Neurosci 21: 1189–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panetta R, Greenwood MT (2008) Physiological relevance of GPCR oligomerization and its impact on drug discovery. Drug Discov Today 13: 1059–1066 [DOI] [PubMed] [Google Scholar]
- Park D, Jhon DY, Lee CW, Lee KH, Rhee SG (1993) Activation of phospholipase C isozymes by G protein beta gamma subunits. J Biol Chem 268: 4573–4576 [PubMed] [Google Scholar]
- Park PS, Filipek S, Wells JW, Palczewski K (2004) Oligomerization of G protein-coupled receptors: past, present, and future. Biochemistry 43: 15643–15656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pin JP, Bockaert J, Recasesn M (1984) The Ca2+/C1- dependent L-[3H]glutamate binding: a new receptor or a particular transport process? FEBS Lett 175: 31–36 [DOI] [PubMed] [Google Scholar]
- Quitterer U, Lohse MJ (1999) Crosstalk between Galpha(i)- and Galpha(q)-coupled receptors is mediated by Gbetagamma exchange. Proc Natl Acad Sci USA 96: 10626–10631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rives ML, Vol C, Fukazawa Y, Tinel N, Trinquet E, Ayoub MA, Shigemoto R, Pin JP, Prezeau L (2009) Crosstalk between GABA(B) and mGlu1a receptors reveals new insight into GPCR signal integration. EMBO J 28: 2195–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins MJ, Calver AR, Filippov AK, Hirst WD, Russell RB, Wood MD, Nasir S, Couve A, Brown DA, Moss SJ, Pangalos MN (2001) GABA(B2) is essential for g-protein coupling of the GABA(B) receptor heterodimer. J Neurosci 21: 8043–8052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondard P, Huang S, Monnier C, Tu H, Blanchard B, Oueslati N, Malhaire F, Li Y, Trinquet E, Labesse G, Pin JP, Liu J (2008) Functioning of the dimeric GABA(B) receptor extracellular domain revealed by glycan wedge scanning. EMBO J 27: 1321–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler V, Luscher C, Blanchet C, Klix N, Sansig G, Klebs K, Schmutz M, Heid J, Gentry C, Urban L, Fox A, Spooren W, Jaton AL, Vigouret J, Pozza M, Kelly PH, Mosbacher J, Froestl W, Kaslin E, Korn R et al. (2001) Epilepsy, hyperalgesia, impaired memory, and loss of pre- and postsynaptic GABA(B) responses in mice lacking GABA(B(1)). Neuron 31: 47–58 [DOI] [PubMed] [Google Scholar]
- Schwenk J, Metz M, Zolles G, Turecek R, Fritzius T, Bildl W, Tarusawa E, Kulik A, Unger A, Ivankova K, Seddik R, Tiao JY, Rajalu M, Trojanova J, Rohde V, Gassmann M, Schulte U, Fakler B, Bettler B (2010) Native GABA(B) receptors are heteromultimers with a family of auxiliary subunits. Nature 465: 231–235 [DOI] [PubMed] [Google Scholar]
- Selvin PR (2002) Principles and biophysical applications of lanthanide-based probes. Annu Rev Biophys Biomol Struct 31: 275–302 [DOI] [PubMed] [Google Scholar]
- Sobolevsky AI, Rosconi MP, Gouaux E (2009) X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature 462: 745–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrillon S, Bouvier M (2004) Roles of G-protein-coupled receptor dimerization. EMBO Rep 5: 30–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiao JY, Bradaia A, Biermann B, Kaupmann K, Metz M, Haller C, Rolink AG, Pless E, Barlow PN, Gassmann M, Bettler B (2008) The sushi domains of secreted GABA(B1) isoforms selectively impair GABA(B) heteroreceptor function. J Biol Chem 283: 31005–31011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich D, Bettler B (2007) GABA(B) receptors: synaptic functions and mechanisms of diversity. Curr Opin Neurobiol 17: 298–303 [DOI] [PubMed] [Google Scholar]
- van Rijn RM, Whistler JL, Waldhoer M (2010) Opioid-receptor-heteromer-specific trafficking and pharmacology. Curr Opin Pharmacol 10: 73–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigot R, Barbieri S, Brauner-Osborne H, Turecek R, Shigemoto R, Zhang YP, Lujan R, Jacobson LH, Biermann B, Fritschy JM, Vacher CM, Muller M, Sansig G, Guetg N, Cryan JF, Kaupmann K, Gassmann M, Oertner TG, Bettler B (2006) Differential compartmentalization and distinct functions of GABAB receptor variants. Neuron 50: 589–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JF, Grodnitzky J, Louis JM, Trinh LB, Shiloach J, Gutierrez J, Northup JK, Grisshammer R (2007) Dimerization of the class A G protein-coupled neurotensin receptor NTS1 alters G protein interaction. Proc Natl Acad Sci USA 104: 12199–12204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JH, Wise A, Main MJ, Green A, Fraser NJ, Disney GH, Barnes AA, Emson P, Foord SM, Marshall FH (1998) Heterodimerization is required for the formation of a functional GABA(B) receptor. Nature 396: 679–682 [DOI] [PubMed] [Google Scholar]
- Whorton MR, Bokoch MP, Rasmussen SG, Huang B, Zare RN, Kobilka B, Sunahara RK (2007) A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proc Natl Acad Sci USA 104: 7682–7687 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.