Abstract
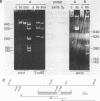
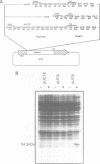
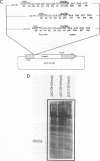
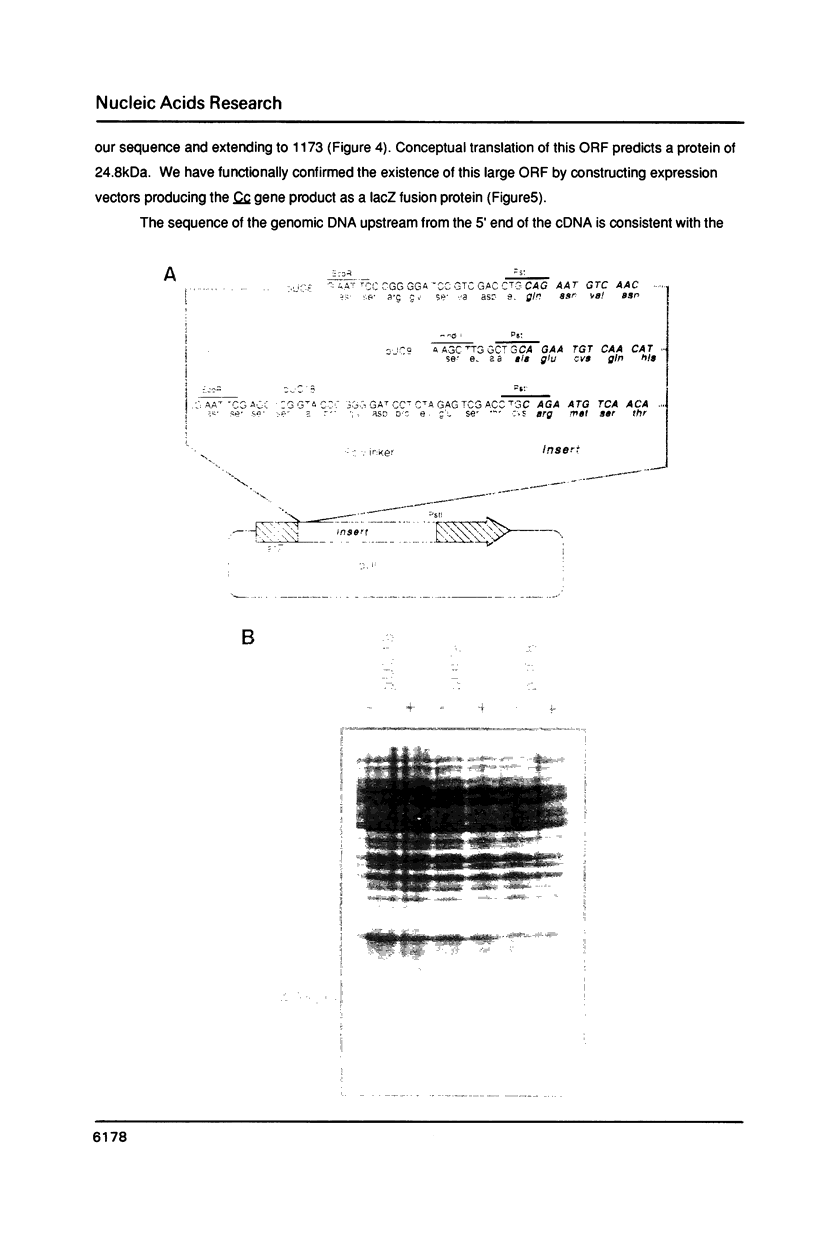
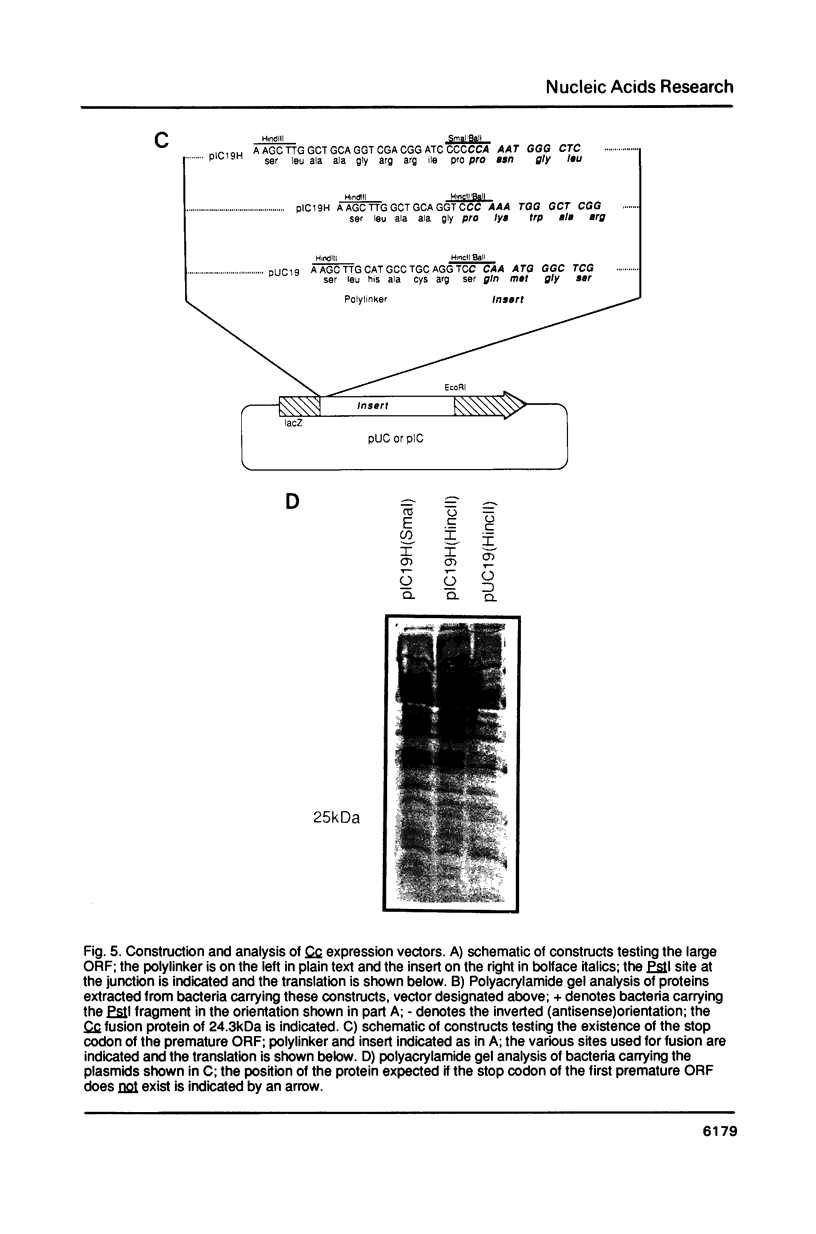
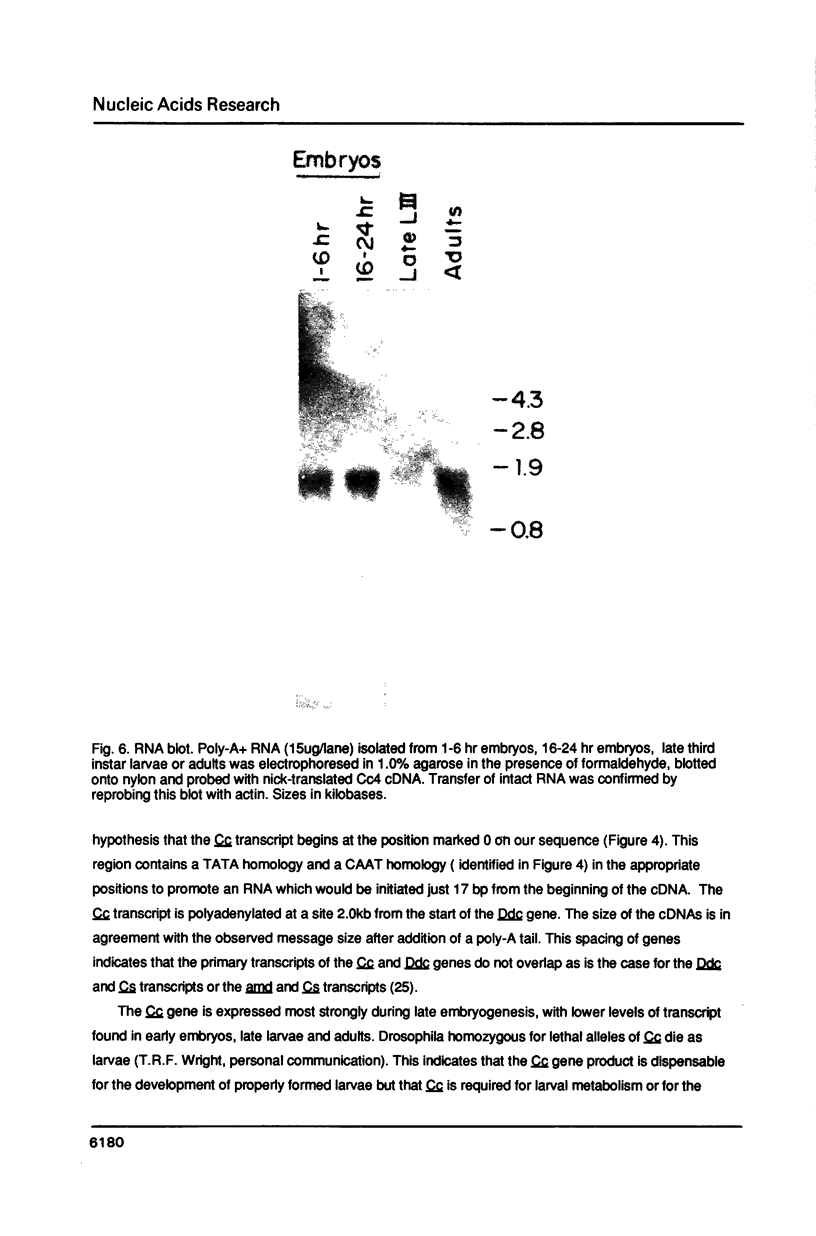
A transcript has been localized proximal to the dopa decarboxylase (Ddc) gene within a cluster of genes involved in cuticle formation and catecholamine metabolism in Drosophila. This gene, which has been identified as I(2)37Cc, maps 2.0kb from the 5' end of the Ddc gene and is transcribed in the same direction as Ddc. We describe a new deficiency which in conjunction with previous deficiencies localizes the I(2)37Cb and I(2)37Cc loci to the cytogenetic interval 5' to Ddc. We present the sequence of the Cc gene and corresponding cDNA. The Cc message contains several open reading frames 5' to the large open reading frame responsible for the lethal complementation group, suggesting that expression of Cc function may be regulated translationally. The Cc transcript is expressed in early embryos, late embryos, late third instar larvae and adults. We discuss the implications of these findings with respect to the gene organization in the region.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chandler V. L., Maler B. A., Yamamoto K. R. DNA sequences bound specifically by glucocorticoid receptor in vitro render a heterologous promoter hormone responsive in vivo. Cell. 1983 Jun;33(2):489–499. doi: 10.1016/0092-8674(83)90430-0. [DOI] [PubMed] [Google Scholar]
- Corces V., Holmgren R., Freund R., Morimoto R., Meselson M. Four heat shock proteins of Drosophila melanogaster coded within a 12-kilobase region in chromosome subdivision 67B. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5390–5393. doi: 10.1073/pnas.77.9.5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. L., Davidson N. Isolation of the Drosophila melanogaster dunce chromosomal region and recombinational mapping of dunce sequences with restriction site polymorphisms as genetic markers. Mol Cell Biol. 1984 Feb;4(2):358–367. doi: 10.1128/mcb.4.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Fink G. R. Translational control of transcription in eukaryotes. Cell. 1986 Apr 25;45(2):155–156. doi: 10.1016/0092-8674(86)90378-8. [DOI] [PubMed] [Google Scholar]
- Gietz R. D., Hodgetts R. B. An analysis of dopa decarboxylase expression during embryogenesis in Drosophila melanogaster. Dev Biol. 1985 Jan;107(1):142–155. doi: 10.1016/0012-1606(85)90383-5. [DOI] [PubMed] [Google Scholar]
- Gilbert D., Hirsh J., Wright T. R. Molecular mapping of a gene cluster flanking the Drosophila Dopa decarboxylase gene. Genetics. 1984 Apr;106(4):679–694. doi: 10.1093/genetics/106.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res. 1981 Oct 24;9(20):5233–5252. doi: 10.1093/nar/9.20.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marsh J. L., Erfle M., Wykes E. J. The pIC plasmid and phage vectors with versatile cloning sites for recombinant selection by insertional inactivation. Gene. 1984 Dec;32(3):481–485. doi: 10.1016/0378-1119(84)90022-2. [DOI] [PubMed] [Google Scholar]
- Marsh J. L., Gibbs P. D., Timmons P. M. Developmental control of transduced dopa decarboxylase genes in D. melanogaster. Mol Gen Genet. 1985;198(3):393–403. doi: 10.1007/BF00332929. [DOI] [PubMed] [Google Scholar]
- Marsh J. L., Wright T. R. Developmental relationship between dopa decarboxylase, dopamine acetyltransferase, and ecdysone in Drosophila. Dev Biol. 1980 Dec;80(2):379–387. doi: 10.1016/0012-1606(80)90412-1. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Parkhurst S. M., Corces V. G. Forked, gypsys, and suppressors in Drosophila. Cell. 1985 Jun;41(2):429–437. doi: 10.1016/s0092-8674(85)80016-7. [DOI] [PubMed] [Google Scholar]
- Pentz E. S., Wright T. R. A diphenol oxidase gene is part of a cluster of genes involved in catecholamine metabolism and sclerotization in Drosophila. II. Molecular localization of the Dox-A2 coding region. Genetics. 1986 Apr;112(4):843–859. doi: 10.1093/genetics/112.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell D. C., Higgins M. J., McMillan B. J., White B. N. Structural analysis of the three vitellogenin genes in Drosophila melanogaster. Nucleic Acids Res. 1981 Mar 25;9(6):1323–1338. doi: 10.1093/nar/9.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh L., Jones K. W. The use of heparin as a simple cost-effective means of controlling background in nucleic acid hybridization procedures. Nucleic Acids Res. 1984 Jul 25;12(14):5627–5638. doi: 10.1093/nar/12.14.5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M., Hunkapiller M., Yuen D., Silvert D., Fristrom J., Davidson N. Cuticle protein genes of Drosophila: structure, organization and evolution of four clustered genes. Cell. 1982 Jul;29(3):1027–1040. doi: 10.1016/0092-8674(82)90466-4. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spencer C. A., Gietz R. D., Hodgetts R. B. Analysis of the transcription unit adjacent to the 3'-end of the dopa decarboxylase gene in Drosophila melanogaster. Dev Biol. 1986 Mar;114(1):260–264. doi: 10.1016/0012-1606(86)90402-1. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Digan M. E., Mahowald A. P., Scott M., Craig E. A. Two clusters of genes for major chorion proteins of Drosophila melanogaster. Cell. 1980 Apr;19(4):905–914. doi: 10.1016/0092-8674(80)90082-3. [DOI] [PubMed] [Google Scholar]
- Wright T. R., Beermann W., Marsh J. L., Bishop C. P., Steward R., Black B. C., Tomsett A. D., Wright E. Y. The genetics of dopa decarboxylase in Drosophila melanogaster. IV. The genetics and cytology of the 37B10-37D1 region. Chromosoma. 1981;83(1):45–58. doi: 10.1007/BF00286015. [DOI] [PubMed] [Google Scholar]