Abstract
EMBO J 30 12, 2373–2387 (2011); published online May 06 2011
Genes on the inactive X chromosome (Xi) of female mammals are repressed in a remarkably stable manner and reactivation of transcription is generally not observed unless the cell is reprogrammed to an early embryonic type. In this issue of The EMBO Journal, Pasque et al (2011) use a reprogramming system in frog oocytes to study the stability of Xi chromatin in cells of different developmental stages and identify the histone variant macroH2A as a factor preventing transcriptional activation.
X inactivation provides dosage compensation between the sexes in mammals. One of the two X chromosomes is transcriptionally silent in female somatic cells and as a consequence a single X chromosome is active in males and females. In somatic cells, several mechanisms including DNA methylation and histone modifications are thought to contribute to maintaining silencing. As a result, genes on the Xi cannot be reactivated efficiently by interfering with epigenetic mechanisms. This contrasts the situation in embryogenesis. X inactivation is initiated in early female embryos by the non-coding Xist RNA. Pluripotent female mouse embryonic stem cells (ESCs) possess two active X chromosomes and initiate X inactivation upon entry into differentiation. Mouse epiblast-derived stem cells (EpiSCs) are also pluripotent but correspond to a stage when X inactivation has already been initiated. In later development gene repression on the Xi becomes progressively stabilized finally resulting in an irreversibly silenced chromosome.
The stability of gene repression on the Xi appears to reflect the differentiation state of a cell and in the case of ESCs marks their developmental plasticity. Consistent with this view is the observation that cell fusion of somatic female cells with mouse ESCs results in reactivation of the Xi (Takagi et al, 1983). Xi reactivation is also associated with reprogramming of somatic cells to induced pluripotent stem cells (iPSCs) in mice (Maherali et al, 2007). Xi reactivation thereby overlaps with the establishment of pluripotency. The mechanism for Xi reactivation has, therefore, attracted attention for its implications for understanding reprogramming.
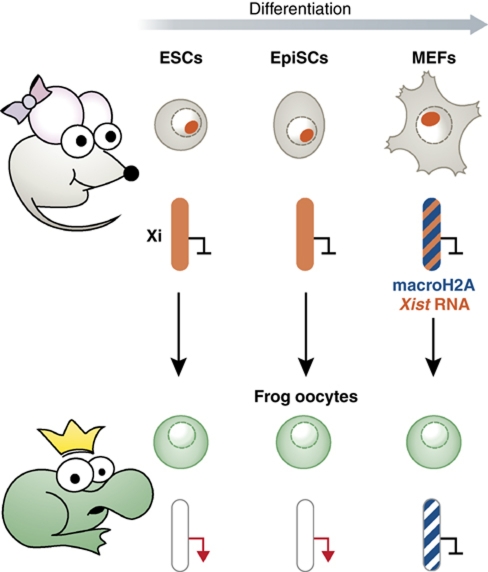
In this issue, Pasque et al (2011) introduce a new system for probing the stability of the Xi. Nuclear transfer into Xenopus oocytes is used to show that the Xi from female mouse embryonic fibroblasts (MEFs) remains repressed in frog oocytes. However, the Xi from differentiating ESCs and EpiSCs is reactivated (see Figure 1). Comparison of the chromatin composition of the Xi between the different donor cell types identifies the histone variant macroH2A as a key determinant of Xi stability. MacroH2A is present on the Xi in somatic cells but not in ESCs or EpiSCs. In addition, depletion of macroH2A from the somatic donor cells before nuclear transfer leads to not only elevated reactivation of the Xi but also reactivation of other genes associated with pluripotency including the transcription factors Oct4 and Sox2. This indicates a wider role of macroH2A in maintaining transcriptional repression of silent genes in somatic cells. Intriguingly, this study finds that other chromatin marks such as DNA methylation or histone methylation do not interfere with the reactivation of the Xi from EpiSCs.
Figure 1.
Reactivation of the Xi by nuclear transfer into frog oocytes. Nuclei from female mouse ESCs, EpiSCs and MEFs are transplanted into the reprogramming environment of frog oocytes. The activity of genes on the Xi was then analysed to assess reactivation of transcription. Whereas the Xi from differentiated ESCs and EpiSCs was reactivated the Xi derived from MEFs remained silent. Lack of reactivation correlated with the presence of mcaroH2A on the Xi in MEFs, which was maintained on the Xi after nuclear transfer.
MacroH2A has an unusual structure for a histone with a large non-histone domain added to its C-terminus which has been implicated in blocking transcription and as a binding domain (Takahashi et al, 2002; Karras et al, 2005). It was first discovered as a component of the Xi heterochromatin by its specific enrichment on the Xi in somatic cells (Costanzi and Pehrson, 1998). Previous work in MEFs has implicated macroH2A in Xi stability (Hernandez-Munoz et al, 2005). However, mutation of the macroH2A1 gene in mice is compatible with female development, suggesting that macroH2A1 contributes to stabilizing the Xi together with other mechanisms (Changolkar et al, 2007). Also, in MEFs macroH2A enrichment on the Xi appears to require Xist (Csankovszki et al, 1999). The fact that Xist was lost from the Xi after nuclear transfer into frog oocytes but macroH2A was maintained indicates differences between the frog and mouse systems. One important aspect is the absence of cell division during reprogramming in frog oocytes. Using appropriate controls early effects of the frog reprogramming environment on chromatin can be observed.
Regulation of macroH2A incorporation has recently been studied in mouse development. In particular, it has been shown that macroH2A is displaced from chromatin after fertilization, suggesting that exclusion of macroH2A from chromatin is associated with a period of genome-wide reprogramming in pre-implantation development (Nashun et al, 2011). Moreover, exchange of critical amino acids causing incorporation of macroH2A into chromatin in early embryos is detrimental for development. It is, therefore, likely that histone H2A variants have a major role in determining chromatin plasticity and developmental potential. Importantly, macroH2A might have a similar role in restricting gene expression for preventing tumourigenesis. A recent study has demonstrated that in aggressive melanoma loss of macroH2A expression correlates with the activation of known oncogenes (Kapoor et al, 2011). Insights into the Xi facultative heterochromatin obtained in the study by Pasque et al (2011) have, therefore, implications for understanding iPSC reprogramming and in tumourigenesis.
Footnotes
The author declares that he has no conflict of interest.
References
- Changolkar LN, Costanzi C, Leu NA, Chen D, McLaughlin KJ, Pehrson JR (2007) Developmental changes in histone macroH2A1-mediated gene regulation. Mol Cell Biol 27: 2758–2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzi C, Pehrson JR (1998) Histone macroH2A1 is concentrated in the inactive X chromosome of female mammals. Nature 393: 599–601 [DOI] [PubMed] [Google Scholar]
- Csankovszki G, Panning B, Bates B, Pehrson JR, Jaenisch R (1999) Conditional deletion of Xist disrupts histone macroH2A localization but not maintenance of X inactivation. Nat Genet 22: 323–324 [DOI] [PubMed] [Google Scholar]
- Hernandez-Munoz I, Lund AH, van der Stoop P, Boutsma E, Muijrers I, Verhoeven E, Nusinow DA, Panning B, Marahrens Y, van Lohuizen M (2005) Stable X chromosome inactivation involves the PRC1 Polycomb complex and requires histone MACROH2A1 and the CULLIN3/SPOP ubiquitin E3 ligase. Proc Natl Acad Sci USA 102: 7635–7640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A, Goldberg MS, Cumberland LK, Ratnakumar K, Segura MF, Emanuel PO, Menendez S, Vardabasso C, Leroy G, Vidal CI, Polsky D, Osman I, Garcia BA, Hernando E, Bernstein E (2011) The histone variant macroH2A suppresses melanoma progression through regulation of CDK8. Nature 468: 1105–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karras GI, Kustatscher G, Buhecha HR, Allen MD, Pugieux C, Sait F, Bycroft M, Ladurner AG (2005) The macro domain is an ADP-ribose binding module. EMBO J 24: 1911–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maherali N, Sridharan R, Xie W, Utikal J, Eminli S, Arnold K, Stadtfeld M, Yachechko R, Tchieu J, Jaenisch R, Plath K, Hochedlinger K (2007) Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell 1: 55–70 [DOI] [PubMed] [Google Scholar]
- Nashun B, Yukawa M, Liu H, Akiyama T, Aoki F (2011) Changes in the nuclear deposition of histone H2A variants during pre-implantation development in mice. Development (Cambridge, England) 137: 3785–3794 [DOI] [PubMed] [Google Scholar]
- Pasque V, Gillich A, Garrett N, Gurdon JB (2011) Histone variant macroH2A confers resistance to nuclear reprogramming. EMBO J 30: 2373–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi N, Yoshida MA, Sugawara O, Sasaki M (1983) Reversal of X-inactivation in female mouse somatic cells hybridized with murine teratocarcinoma stem cells in vitro. Cell 34: 1053–1062 [DOI] [PubMed] [Google Scholar]
- Takahashi I, Kameoka Y, Hashimoto K (2002) MacroH2A1.2 binds the nuclear protein Spop. Biochimica et biophysica acta 1591: 63–68 [DOI] [PubMed] [Google Scholar]