Abstract
EMBO J 30 12, 2325–2335 (2011); published online May 10 2011
The Hippo (Hpo) signalling pathway is an evolutionarily conserved pathway controlling tissue growth, but its upstream inputs remain poorly understood. In this issue of The EMBO Journal, Sansores-Garcia et al (2011) report that modulation of filamentous (F)-actin accumulation controls Hpo pathway activity. In a related study, Garcia-Fernandez et al (2011) confirm this finding, and also show that the Hpo pathway itself can modulate F-actin levels. Such actin-dependent regulation of Hpo pathway activity has relevance to the control of tissue growth by epithelial architecture and external tension cues.
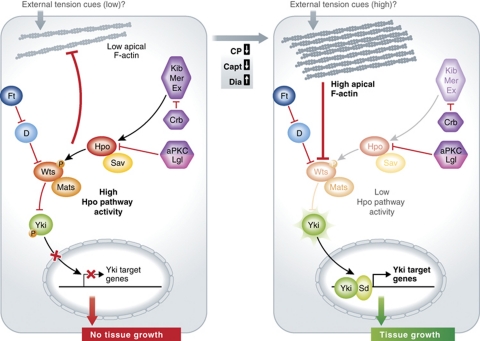
Over the last 10 years, the Hpo signalling pathway has emerged as a key negative regulator of tissue growth in Drosophila and mammalian systems (reviewed by Harvey and Tapon (2007)). Central to the Hpo pathway is the Hpo protein kinase and its adaptor, Salvador (Sav), along with the Warts (Wts) kinase and its adaptor Mats (see Figure 1). Activated Hpo phosphorylates and activates Wts, which in turn phosphorylates the transcriptional co-activator Yki, rendering it inactive and restricted to the cytoplasm. Upon inactivation of the Hpo pathway, Yki is dephosphorylated and can enter the nucleus where, in complex with the Scalloped (Sd) Tead-family transcription factor, it upregulates target genes, including the cell-cycle regulator Cyclin E and the inhibitor of apoptosis Diap1. The core Hpo kinase cassette is regulated by several upstream inputs, including the KEM complex, consisting of Expanded (Ex), Merlin (Mer) and Kibra (Kib), the atypical cadherin Fat (Ft), and the apico-basal polarity regulators, Crumbs (Crb), atypical protein kinase C (aPKC) and Lethal-giant-larvae (Lgl) (reviewed by Grusche et al (2010)). The KEM complex binds to and activates the core cassette. Crb controls Ex levels and localization, while the balance between aPKC and Lgl regulates Hpo localization. Ft acts by inhibiting the atypical Myosin, Dachs (D), which in turn regulates Wts stability. The recent findings of Sansores-Garcia et al (2011) and Garcia-Fernandez et al (2011) on the negative regulation of Hpo signalling by apical F-actin reveal a new layer of upstream regulation that may connect mechanical tension to tissue growth.
Figure 1.
Regulation of Hpo pathway signalling by F-actin. Under conditions of low apical F-actin, Hpo pathway activity is high, leading to inhibition of Yki activity and inhibition of tissue growth. When apical F-actin levels are high (upon activation of Dia or inhibition of Cp or Capt), Hpo pathway activity is inhibited leading to activation and nuclear import of Yki, and upregulation of Yki targets, thereby promoting tissue growth. Wts appears to be the key target of negative regulation of the Hpo pathway by apical F-actin. Active Hpo signalling also feeds back to block F-actin accumulation, at least partially independently of Yki function. Physiologically, F-actin accumulation may be regulated by external tension cues, thereby controlling tissue growth via the Hpo pathway.
The study from the Halder laboratory (Sansores-Garcia et al, 2011) discovered F-actin regulators as novel modulators of a Yki-responsive luciferase reporter in a genome-wide RNAi screen in Drosophila S2 cells. As negative regulators of Yki activity they identified the capping proteins, Cpa and Cpb (CP), which prevent addition of actin monomers to the barbed end of actin filaments; Capulet (Capt), which sequesters monomeric actin; and Cofilin (Twinstar, Tsr), which severs actin filaments and promotes dissociation of actin monomers from the pointed end of F-actin. They also found that knockdown of the positive regulators of actin polymerization, Wasp and Arc-p20, inhibited Yki activity in S2 cells. Consistent with F-actin being the essential regulator of Yki activity, the F-actin destablizing drug, Cytochalasin D, also inhibited Yki activity. Importantly, this connection was conserved in vivo, since knockdown of CP, or expression of a constitutively active version of the actin nucleation factor Diaphanous (DiaCA), in Drosophila larval epithelial tissues resulted in increased apical F-actin and increased tissue growth—in a Yki-dependent manner. This regulation was also observed in mammalian cells, where expression of mDiaCA in HeLa cells increased activity of the mammalian Yki homologue, Yap, while Cytochalasin D decreased Yap activity. These findings are consistent with previous studies already hinting at a link between actin and Hpo signalling: the mammalian Hpo homologue, Mst1 or 2, is activated by disruption of the actin cytoskeleton (Densham et al, 2009), and actin cytoskeleton disruption upregulates Merlin/NF2 and correlates with G1 cell-cycle arrest (Lohez et al, 2003).
The related study from the Janody laboratory (Garcia-Fernandez et al, 2011) agrees with the Halder study in that RNAi knockdown or mutant alleles of CP result in increased tissue growth and Yki target upregulation in the Drosophila larval wing epithelium. However, they found that, while CP and Capt—both of which restrict apical F-actin accumulation—regulate Hpo pathway signalling, Cofilin, which acts more globally on cortical F-actin, does not. Thus, they conclude that it is the apical pool of F-actin that is critical to Hpo pathway regulation. The Halder group identified Cofilin as a negative regulator of Yki activity in S2 cells, but did not analyse it in vivo. This difference in results may be explained by cell type (a non-adherent cell line derived from embryos versus larval polarized epithelial cells) or cell context (in vitro versus in vivo).
Garcia-Fernandez et al (2011) also found that ex, hpo, sav, mats or wts mutant clones upregulate F-actin, but overexpression of Yki did not. Consistent with this, knockdown of yki in cp mutant clones reduced the overgrowth phenotype, but did not prevent F-actin accumulation. Therefore, deregulation of the core Hpo pathway components affects F-actin via a Yki-independent mechanism. F-actin upregulation by Hpo pathway inactivation has been previously described in the pupal wing disc, but here Yki is involved (Fang and Adler, 2010). These differences may be due to the different tissue context, but provides cause for further investigation of this mechanism.
A key difference between the conclusions from the Halder and Janody laboratory studies concerns the issue of cell autonomy or non-autonomy. The Janody study clearly revealed that wild-type cells surrounding CP mutant clones also upregulate Yki targets. By contrast, the Halder study knocked down CP in clones using RNAi and co-expressed a dominant-negative Jun Kinase transgene (bskDN) in the clones to prevent cell death. In this situation, they did not observe non-autonomous effects. Given the recent involvement of JNK in compensatory proliferation (Warner et al, 2010; Sun and Irvine, 2011), it is possible that blocking JNK activity in cp mutant cells may prevent the non-cell autonomous activation of Yki and the compensatory proliferation that would normally occur.
Pertaining to possible mechanisms connecting F-actin to the Hpo pathway, the Halder laboratory showed that overexpression of Wts, but not Hpo or Ex, suppresses Yki target upregulation in DiaCA-expressing tissue, indicating that F-actin acts downstream or independently of Ex and Hpo, but upstream of Wts (Figure 1). In contrast to this, the Janody group claimed that overexpression of Ex or Hpo in cp-depleted tissue suppressed tissue overgrowth, although since the mutant tissue was not marked further analysis is needed. Furthermore, in this experiment, overexpression of Hpo reduced the level of F-actin in cp-depleted tissue, preventing conclusions about how F-actin accumulation and the Hpo pathway intersect. Thus, while these studies have revealed a novel regulatory connection between Hpo pathway activation and apical F-actin levels, the precise mechanism remains to be determined. Several key issues need to be addressed. Are the core Hpo pathway components assembled and activated on particular pools of F-actin? or does the level or state of apical F-actin relay information to the Hpo pathway via upstream regulators? If so, does apical F-actin act via existing upstream branches or via unknown regulators? Furthermore, is the upregulation of F-actin in hpo pathway mutant clones independent of the ability of actin regulators to modulate Hpo pathway activity, or linked in some way? Since Wts is regulated by Dachs (D), which was a Myosin-related protein would be expected to bind F-actin, one possibility is that D might respond to the state of apical F-actin to control Wts activity. Possible links between the Hpo pathway and actin in mammalian cells include Merlin/NF2 that requires interaction with the actin cytoskeleton to mediate cell-cycle arrest and can also inhibit actin polymerization (Curto and McClatchey, 2008), and Wts/Lats1 that can inhibit Limk1 (Yang et al, 2004), which induces actin polymerization via inhibition of Cofilin.
What is the physiological role of this connection of F-actin to the Hpo pathway? A possible role for this pathway involves the response to external tension cues (Figure 1). In Drosophila oogenesis, proliferation of the follicular epithelium is coupled to the force emanating from the growing germ-line cyst, and depends upon an intact actin cytoskeleton (Wang and Riechmann, 2007). Furthermore, in cultured mammalian cells, where the proliferative response to external mechanical forces has been well studied (reviewed by Assoian and Klein (2008) and Mammoto and Ingber (2009)), stiff extracellular matrix leads to increased stress fibre formation and promotes cell proliferation, dependent upon an intact actin cytoskeleton. There are important implications of these findings to cancer, since there is compelling evidence that mechanical forces within the tumour microenvironment are critical for the progression of the tumour (reviewed by Yu et al (2011)). Despite extensive research, these mechano-transduction studies have so far largely focused on how integrin, Rho-GTPase and Erk signalling impacts on the cell-cycle machinery, and have not examined connections to the Hpo pathway. Clearly, the work of Sansores-Garcia et al (2011) and Garcia-Fernandez et al (2011) highlighted here, pave the way for further investigation of the Hpo pathway and F-actin status in response to external tension cues in tissue growth control and cancer progression.
Acknowledgments
Thanks to Kieran Harvey, Nicola Grzeschik and Linda Parsons for helpful discussions, Nicola Grzeschik for preparing the figure, and Kieran Harvey and Nicola Grzeschik for critical comments on the manuscript. HER is supported by funding from an Australian National Health and Medical Research Council (NHMRC) Senior Research Fellowship and NHMRC grant #628401.
Footnotes
The author declares that she has no conflict of interest.
References
- Assoian RK, Klein EA (2008) Growth control by intracellular tension and extracellular stiffness. Trends Cell Biol 18: 347–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curto M, McClatchey AI (2008) Nf2/Merlin: a coordinator of receptor signalling and intercellular contact. Br J Cancer 98: 256–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Densham RM, O’Neill E, Munro J, Konig I, Anderson K, Kolch W, Olson MF (2009) MST kinases monitor actin cytoskeletal integrity and signal via c-Jun N-terminal kinase stress-activated kinase to regulate p21Waf1/Cip1 stability. Mol Cell Biol 29: 6380–6390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Adler PN (2010) Regulation of cell shape, wing hair initiation and the actin cytoskeleton by Trc/Fry and Wts/Mats complexes. Dev Biol 341: 360–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Fernandez B, Gaspar P, Bras-Pereira C, Jezowska B, Raquel-Rebelo S, Janody F (2011) Actin-capping protein and the Hippo pathway regulate F-actin and tissue growth in Drosophila. Development 138: 2337–2346 [DOI] [PubMed] [Google Scholar]
- Grusche FA, Richardson HE, Harvey KF (2010) Upstream regulation of the hippo size control pathway. Curr Biol 20: R574–R582 [DOI] [PubMed] [Google Scholar]
- Harvey K, Tapon N (2007) The Salvador-Warts-Hippo pathway—an emerging tumour-suppressor network. Nat Rev Cancer 7: 182–191 [DOI] [PubMed] [Google Scholar]
- Lohez OD, Reynaud C, Borel F, Andreassen PR, Margolis RL (2003) Arrest of mammalian fibroblasts in G1 in response to actin inhibition is dependent on retinoblastoma pocket proteins but not on p53. J Cell Biol 161: 67–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammoto A, Ingber DE (2009) Cytoskeletal control of growth and cell fate switching. Curr Opin Cell Biol 21: 864–870 [DOI] [PubMed] [Google Scholar]
- Sansores-Garcia L, Bossuyt W, Wada K-I, Yonemura S, Tao C, Sasaki H, Halder G (2011) Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J 30: 2325–2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, Irvine KD (2011) Regulation of Hippo signaling by Jun kinase signaling during compensatory cell proliferation and regeneration, and in neoplastic tumors. Dev Biol 350: 139–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Riechmann V (2007) The role of the actomyosin cytoskeleton in coordination of tissue growth during Drosophila oogenesis. Curr Biol 17: 1349–1355 [DOI] [PubMed] [Google Scholar]
- Warner SJ, Yashiro H, Longmore GD (2010) The Cdc42/Par6/aPKC polarity complex regulates apoptosis-induced compensatory proliferation in epithelia. Curr Biol 20: 677–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Yu K, Hao Y, Li DM, Stewart R, Insogna KL, Xu T (2004) LATS1 tumour suppressor affects cytokinesis by inhibiting LIMK1. Nat Cell Biol 6: 609–617 [DOI] [PubMed] [Google Scholar]
- Yu H, Mouw JK, Weaver VM (2011) Forcing form and function: biomechanical regulation of tumor evolution. Trends Cell Biol 21: 47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]