Abstract
The present work was carried out to investigate the hepatoprotective effect of ginger, chicory and their mixture against carbon tetrachloride intoxication in rats. Carbon tetrachloride treatment significantly elevated the alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase and gamma glutamyltransferase activities and the serum triglycerides and cholesterol concentration as compared to control group. It also increased RBCs counts and Hb concentration, total or differential leucocytes counts. However it decreased platelet counts, platelet distribution width, mean platelet volume, platelet larger cell ratio. Methanol extract of ginger (250 and 500 mg/kg) and chicory (250 and 500 mg/kg) given alone or mixed (1:1 wt/wt) significantly restored the carbon tetrachloride-induced alterations in the biochemical and cellular constituents of blood. No toxic symptoms were reported in doses up to 5 g/kg. Alkaloids and/or nitrogenous bases, carbohydrates and/or glycosides, tannins, flavonoids, saponins and unsaturated sterols and/or triterpenes are the main active constituents of their methanol extract. The hepatoprotective effect of ginger and chicory was also confirmed by the histopathological examination of liver tissue.
Keywords: Carbon tetrachloride, chicory, cholesterol, creatinine, ginger, hepatotoprotective, histopathological, liver function, triglycerides
Liver dysfunction caused mostly due to exposure to toxic chemicals, certain drugs and environmental pollutants, has been on the increase for the past few decades. In many countries hepatopathy is increasingly being managed using herbal treatment[1]. Ginger (Zingiber officinale Roscoe, Zingiberaceae), a commonly used flavoring agent and cooking spice is added as a herbal supplement. It has a large variety of pharmacological activities such as antibacterial, antioxidant, and antiinflammatory effect as well as attenuation of lipid peroxidation and oxidative stress[2,3]. It has been reported to improve various gastrointestinal illnesses[4,5] and immunologic dysfunction[6]. It also has been reported to exert antidiabetic activity, reduce liver damage[7] and possess anticarcinogenic effect[8]. It also has been reported to be used in hypertension and for exerting cardioprotective and antioxidant property[4,9].
Chicory (Cichorium intybus, Asteraceae) is a root vegetable whose green leafy tops are also used in salads or as a coffee supplement. Recently pharmacological actions of chicory have attracted the attention of many researchers. It was reported to induce hypocholesterolemia[10], protect against hepatocellular damage[11,12] and inhibit lipid peroxidation[13,14]. It also has an antihyperglycaemic effect[15], regulate appetite and carbohydrate and lipid metabolism[16]. It was the aim of this work to explore the hepatoprotective effect of the methanol extract of ginger and chicory when given alone or in a mixture.
MATERIALS AND METHODS
Zingiber officinale Roscoe, Zingiberaceae) rhizome and Cichorium intybus, Asteraceae) green plant were identified at the Herbarium of the department of Botany, Faculty of Science, Cairo University. A voucher sample was deposited in the department of Pharmacology, Faculty of Veterinary Medicine, Cairo University, Egypt. The air-dried plant material was moderately pulverized, and stored for further use. The powdered plant (200 g) was extracted and percolated in methanol 95% for 5 to 7 times till complete extraction. The methanol extracts was concentrated under reduced pressure using a rotary evaporator at temperature not more than 50°. The concentrated extract was kept at – 4° until used for phytochemical and pharmacological studies.
Animals:
Sprague Dawley rats (150-170 g) and Swiss mice weighing 20±5 g of both sexes were obtained from Laboratory Animal Colony, Helwan, Egypt. Animals were maintained in the Animal House of Pharmacology Department, Faculty of Veterinary Medicine, Cairo University under controlled hygienic conditions. Animals were fed on locally manufactured pellets and water was provided ad libitum. Animals were allowed to acclimatize to the laboratory environment for 7 days before initiating experiments. Rats were used for evaluating hepatoprotective effect and mice were used for the acute toxicity testing of the plant extracts studied.
Hepatoprotective effect:
The model described by Chakraborti and Handa[17] was used. Rats were divided into eight equal groups of six rats each. All groups received the administered doses orally by using stomach tube. Rats of group I (normal control) were given 2.5 ml/kg corn oil daily for 5 days and this group served as an environmental control. Rats of the 2nd group were used as positive control and were given 2.5 ml/kg carbon tetrachloride (50 % CCl4in corn oil). Rats of the 3rd and 4th groups were administered ginger extract at a dose of 250 and 500 mg/kg, respectively, while those of the 5th and 6th group were administered chicory extract in a dose of 250 and 500 mg/kg. The 7th group received 250 mg/kg of a 1:1 w/w mixture of ginger and chicory extracts. Doses were calculated depending on the yield of the methanol extract of an amount of the plants equivalent to human consumption. Rats of the 8th group were used as a standard treated group and received orally 25 mg/kg silymarin for the same period. All groups, except normal control group received 2.5 ml/kg CCl4 (50% of CCl4 in corn oil) on the 2nd and 3rd day. On the fifth day, blood samples were collected from the orbital plexus of all rats. Serum samples were separated and used for determination of different biochemical parameters. After collection of the blood samples, all rats were sacrificed to collect the liver for histopathological examination. Animals were handled according to the local rules and regulation of Experimental Animals, Cairo University.
Blood samples:
Blood samples were collected 24 h after CCl4administration through retro-orbital puncture. Blood samples were allowed to clot and centrifuged to separate clear serum. The activities of alanine aminotransferases (ALT), aspartate aminotransferase (AST), alkaline phosphatase (AP)[18], gamma glutamyltransferase (GGT)[19] and the level of glucose[20], triglyceride[21], total proteins, albumin and bilirubin[22], were determined using kits obtained from bioMérieux, France; while creatinine[23] and cholesterol[24] levels were determined using kits obtained from Diamond, Cairo, Egypt.
Pathological studies:
Liver tissue specimens were collected from all groups’ immediately after sacrifice of rats and fixed in 10% formol saline. Paraffin sections of 5µ thickness were prepared, stained by Hematoxylene and Eosin (H & E) and examined microscopically.
Acute toxicity study in mice:
The acute toxicity of plant extract was tested as described by Tanira et al.[25], using three doses (0.4, 1 and 2.5 g/kg orally). Control rats were kept under the same conditions without any treatments. Observation period extended up to 72 h post administration. In addition, the general behavior of animals, signs of discomfort, or any nervous manifestations were observed. The large dose was selected to represent the maximum dose in relation to the extract-dry plant yield.
Phytochemical screening:
The extracts were screened for the presence of tannins, saponins, sterols and or triterpenes, alkaloids, anthraquinones, flavonoids, lactones/esters, protein and or amino acids and carbohydrates and or glycosides with thin layer chromatography (TLC) as described by Stahl[26]. Thin layer plates precoated with Silica gel G (Merck, 0.25 mm thickness) were used. Development was carried out with different solvent systems, ethyl acetate:methanol:water (100:13.5:10 v/v), ethyl acetate:formic acid:acetic acid:water (100:11:11:27 v/v), chloroform:methanol:water (64:50:10 v/v), benzene:ethyl acetate (86:14 v/v) and ethyl acetate:methanol:water:acetic acid (65:15:15:10 v/v/v/v). After development in the solvents the plates were dried and sprayed with Dragendorrf's, AlCl3, Hydroxylamine-ferric chloride, and ninhydrin and antimony trichloride for the detection of alkaloids, flavonoids, lactones/esters, protein/amino acids and sterols/triterpenes. Detection of anthraquinones, saponins, tannins glycosides/ carbohydrate was carried out using KOH, anisaldehyde-sulphuric acid reagents, ferric chloride and naphthoresorcinol reagents respectively[27]. Detection was carried out by visualization in visible light and under UV light (λ366 nm).
Statistical analysis:
Results are expressed as mean±standard deviation (SD). Differences between means in different groups were tested for significance using a one-way analysis of variance (ANOVA) followed by Duncan's test and P value of 0.05 or less was considered significant.
RESULTS
Toxicity testing:
Oral administration of methanol extracts of either ginger or chicory in doses up to 5 g/kg revealed no symptoms of morbidity or mortality were reported. Therefore LD50 is more than 5 mg/kg indicating that the tested plants are safe for use.
Phytochemical study:
Preliminary phytochemical screening of the tested extracts revealed the presence of alkaloids and/or nitrogenous bases, carbohydrates and/or glycosides, tannins, flavonoides, saponins and unsaturated sterols and/or triterpenes in both ginger and chicory. Resins are reported only in ginger methanol extract.
Effect on serum biochemical constituents:
The CCl4 intoxication significantly elevated the ALT, AST, ALP and GGT activity as compared to control group. Ginger methanol extract in small or large dose decreased only the activity of ALP and GGT, however it did not significantly affect the level of ALT or AST. Methanol extract of chicory in both doses and mixture of both chicory and ginger (1:1; w/w) significantly decreased serum ALT, AST and ALP. Oral administration of silymarine (25 mg/kg) did not significantly alter the elevated serum liver enzymatic activity (fig. 1.).
Fig. 1.
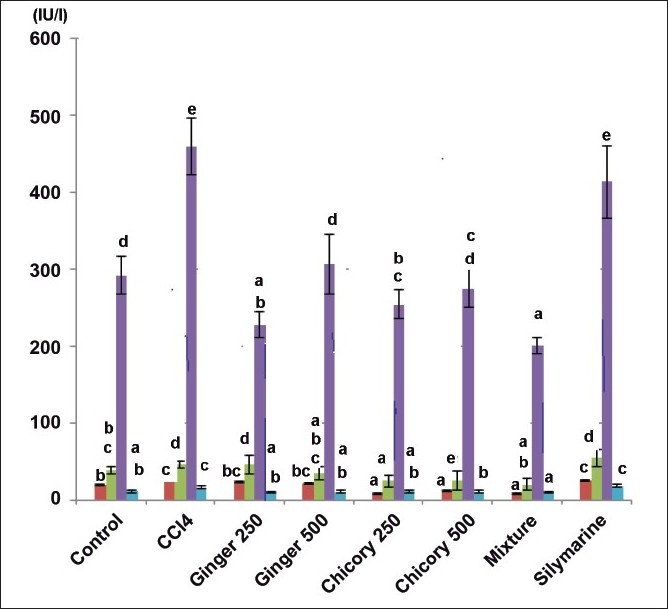
Effect of methanol extracts of ginger and chicory on serum enzymes in rats with CCl4-induced hepatotoxicity.
Effect of methanol extracts of ginger and chicory on serum enzymes in rats with CCl4-induced hepatotoxicity Different asterisks denote significant difference at P=0.05, n=6, values are mean±SD. ALT ( ), AST (
), AST ( ), ALP (
), ALP ( ) and GGT (
) and GGT ( )
)
CCl4 significantly increased serum trigycerides and cholesterol concentration. Methanol extract of chicory and ginger in both doses as well as their mixture (1:1; wt/wt) significantly decreased serum trigycerides and cholesterol concentration. Their lowering effect was more potent than that of silymarine. There was no significant effect on serum total bilirubin, total protein level or albumen concentration after administration of methanol extract of ginger, chicory in both doses as well as their mixture (Table 1).These results revealed that the CCl4 treatment at a dose of 2.5 ml /kg did not affect the serum glucose, urea or creatinine level compared to control group. Neither silymarine nor ginger and small dose of chicory affected serum glucose, urea or creatinine. Only large dose of chicory and mixture of both of ginger and chicory significantly decreased serum urea level (fig. 2).
TABLE 1.
EFFECT OF METHANOL EXTRACTS OF GINGER AND CHICORY ON SERUM BIOCHEMICAL CONSTITUENTS IN CARBON TETRACHLORIDE–INDUCED HEPATOTOXICITY
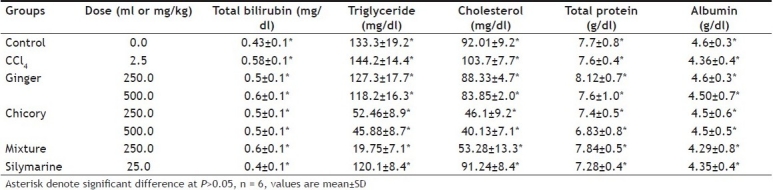
Fig. 2.
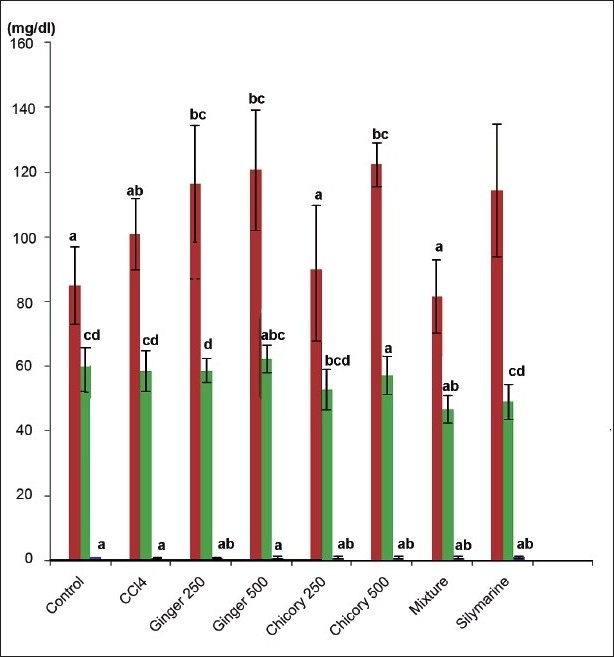
Effect of methanol extracts of ginger and chicory on some serum biochemical constituents in rats with carbon tetrachloride-induced hepatotoxicity.
Effect of methanol extracts of ginger and chicory on some serum biochemical constituents in rats with carbon tetrachloride-inducedhepatotoxicity, different asterisks denote signifi cant difference at P>0.05, n=6, values are mean±SD. Glucose ( ), Serum Urea (
), Serum Urea ( ), Serum Creatinine (
), Serum Creatinine ( )
)
Effect on RBCs and related indices:
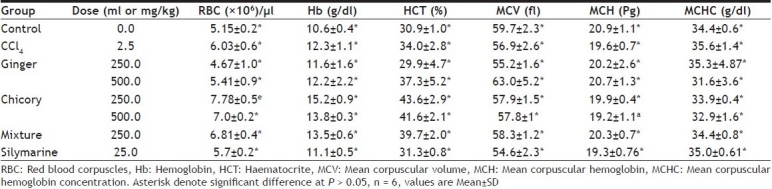
CCl4 significantly increased RBC counts and Hb concentration. Chicory in both doses and also the mixture of both ginger and chicory significantly increased the RBCs count, Hb and haematocrite concentration when compared to control or carbon tetrachloride- intoxicated group. Ginger did not affect any of the blood parameters (Table 2).
TABLE 2.
EFFECT OF METHANOL EXTRACT OF GINGER, CHICORY AND MIXTURE OF BOTH ON SOME HEMATOLOGICAL INDICES
RBC: Red blood corpuscles, Hb: Hemoglobin, HCT: Haematocrite, MCV: Mean corpuscular volume, MCH: Mean corpuscular hemoglobin, MCHC: Mean corpuscular hemoglobin concentration. Asterisk denote signifi cant difference at P = 0.05, n = 6, values are MeanŷSD
Effect on total and differential leucocytic counts:
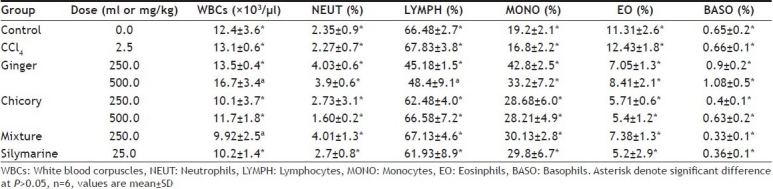
CCl4 did not significantly affect total or differential leucocytes counts. There was no significant difference in the total WBCs count between any of plant extract or silymarine- and CCl 4alone-treated groups. Lymphocytes and eosinophiles percent were significantly decreased. Monocyte percent was significantly increased in ginger treated rats. On the other hand monocytes were significantly increased and the eosinophile count was significantly decreased in chicory-, mixture of ginger and chicory-, and silymarine-treated rats. Basophiles percent were significantly decreased in mixture of ginger and chicory- and silymarine-treated rats (Table 3).
TABLE 3.
EFFECT OF METHANOL EXTRACT OF GINGER, CHICORY AND MIXTURE OF BOTH ON TOTAL AND DIFFERENTIAL LEUCOCYTES COUNT
Effect on blood platelets:
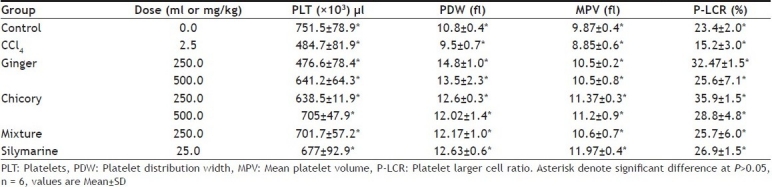
Results indicated that platelet counts (PLT)×103 / µl in control group was 751.5±78.87. In CCl4-intoxicated rats, platelet counts, platelet distribution width (PDW), mean platelet volume (MPV), platelet larger cell ratio (P-LCR) was significantly (P>0.05) decreased in comparison to normal control. Ginger in the large dose, chicory in both doses and mixture of both ginger and chicory as well as silymarine significantly increased platelets counts, platelet distribution width (PDW), mean platelet volume (MPV), platelet larger cell ratio (P-LCR) to levels nearly similar to that of normal control (Table 4).
TABLE 4.
EFFECT OF METHANOL EXTRACT OF GINGER, CHICORY AND MIXTURE OF BOTH ON PLT, MPV, P-LCR
Pathological examination:
Macroscopically, the livers in CCl4-treated group were enlarged, friable and pale in color. The livers of ginger-treated rats was slightly enlarged, moderate friable and mottled in appearance. Liver from rats treated with chicory was apparently normal in texture. Histopathological observation of CCl4-treated group showed very severe hepatotoxicity as evidenced by the appearance of centrilobular necrosis, ballooning degeneration in hepatocytes, congestion in the central vein and sinusoids, proliferation of Kupffer cells and mononuclear leucocytes inflammatory cells infiltration mainly surrounding the central vein (fig. 3b) compared to normal non treated rats (fig. 3a). In ginger and chicory-treated groups, moderate to mild degenerated and necrobiotic changes (centrolobular) in the hepatocytes were observed (fig. 3c and d, respectively). Similar picture was observed in silymarine- and mixture of ginger and chicory-treated groups (picture not shown).
Fig. 3.
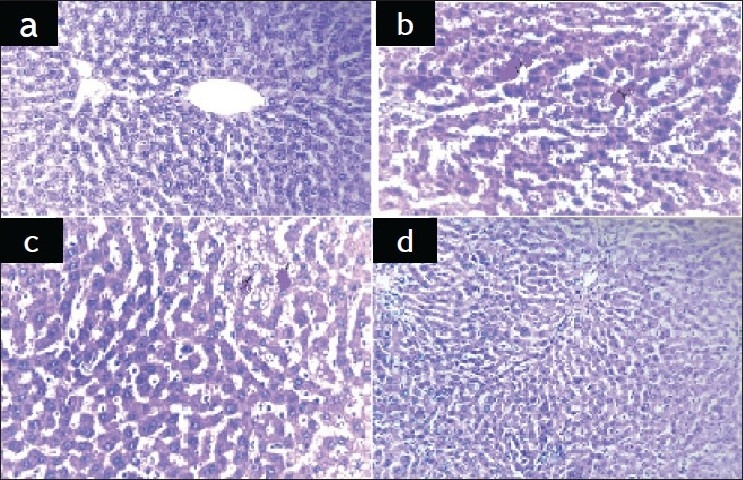
Photomicrographs of hepatocytes from normal and treated rat livers
(a) liver of normal non treated rat showing normal histological picture, (b) liver of CCl4-intoxicated rats showing severe centrilobular necrosis, degeneration in hepatocytes, congestion in the central vein and sinusoids, proliferation of Kupffer cells and mononuclear leucocytes infl ammatory cells. (c) liver of CCl4-intoxicated rats and treated with 250 mg/kg of methanol extract of ginger. (d) Liver of CCl4-intoxicated rats and treated with 250 mg/kg of methanol extract of chicory (H and E ×200).
DISCUSSION
Results of this study revealed that there were no symptoms of morbidity or mortality reported 48 h after oral administration of tested methanol extracts of both ginger and chicory in doses up to 5000 mg/kg in mice. These results indicated that both tested extracts were safe to be used and they are nontoxic. In this respect[28] reported that plants with LD50 more than 5000 mg/kg is considered nontoxic. A toxicological evaluation of a chicory root extract was also done by Schmidt et al.[29]. They reported that there were no observed adverse effects of chicory extract in their studies. The NOAEL (no observable effective level) for the extract is 1000 mg/kg when administered orally for 28 days. Moreover, the extract was nontoxic up to a dose of 1500 mg/kg[30].
Estimation of the activity of ALT, AST and GGT are good marker of assessment liver function. These enzymes are normally located in the cytosol of hepatocytes. When liver cells are damaged, these enzymes are released in the plasma and increased their activity in plasma is a useful marker of the extent and type hepatocellular damage[1]. Carbon tetrachloride administration to experimental animals induced acute pathological changes in the liver. This finding correlates with the marked increase in serum ALT, AST and GGT activities. Moreover, the increased triglyceride content in the blood is in correlation with the fatty degeneration of the liver induced by CCl4[31]. Biochemical mechanism for CCl4toxicity based on mitochondrial damage was previously described[32].
The present results clearly demonstrate the marked antihepatotoxic effects of methanol extract of ginger, chicory and their mixture (1:1 wt/wt) as compared to the reference drug, silymarine. This fact is based on the tendency of liver enzymes, glucose and triglycerides to return towards their respective levels in the normal control group. Previous studies have demonstrated that both ginger[7,33], or chicory[11,12] exhibited hepatoprotective effect. In this study additional evidence that not only methanol extract of ginger or chicory but also a mixture of both (1:1 wt/wt) exhibited a hepatoprotective effect which was more effective in reducing serum liver enzymes towards or even below the normal values than ginger or chicory alone. The decreased liver enzymes below the carbon tetrachloride-treated or normal rats are probably due to a membrane stabilizing effect.
Although the active compounds of the tested plants have not been well identified in the present study, preliminary phytochemical study revealed the presence of alkaloids and/or nitrogenous bases, carbohydrates and/or glycosides, tannins, flavonoids, saponins and unsaturated sterols and/or triterpenes as major active constituents. Saponins, flavonoids, and alkaloids have been previously reported in the crude extract of ginger[4]. The reduced lipid peroxidation in rat liver homogenates were reported to be due to total phenolics or flavonoids contents of some medicinal plants[13]. Therefore the protective effect of plant extract against CCl4 hepatotoxicity may be attributed to the presence of triterpenoids and steroids[16].
Many compounds known to be beneficial against CCl4-mediated liver injury exert their protective action by toxin-mediated lipid peroxidation either via decreased production of carbon tetrachloride derived free radicals or through the antioxidant activity of the protective agents themselves[34]. Phenolic compounds were also separated from ginger[5] and chicory[14]. These constituents were reported to exhibit strong antioxidant properties and cytoprotective actions in rats[5]. Therefore it may be suggested that the hepatoprotective effect of the tested plants is due at least partially to their content of flavonoids and or triterpenoids.
The triglyceride and cholesterol lowering effect could be related to the total lipid lowering effect by ginger[2,35]. The blood glucose lowering effect may be due to the presence of inulin and oligofructose[16,36].
Although no available literature could be obtained concerning the effect of the tested methanol extracts on blood cellular elements, however the increased RBCs count and Hb and HCT in chicory and mixture treated rats could be attributed to its protective effect on bone marrow[30,37]. The increased monocyte counts in blood of rats treated with methanol extract of ginger and /or chicory may be related to the antiinflammatory effect of their active constituents[38,39] and to their immunoprotectant effect[40,36]. The present data clearly demonstrate that oral administration of methanol extract of ginger or chicory has restored the platelets counts which were decreased after CCl4-induced intoxication.
Oral administration of the tested methanol extracts revealed that administration of mixture of both ginger and chicory improved of the degenerative histopathological changes induced by CCl4intoxication in liver. In this concern, it is reported that cichorium root extract therapy leads to normalization of some morphofunctional liver features (decreases glycogen content and cell necrosis) in rats with CCl4-induced hepatitis[12,41]. The antihepatotoxic activity of ginger extract may be due to its direct radical scavenging activity[33]. In conclusion, ginger or chicory were effective as antihepatotoxic and can be considered as good natural sources of hepatoprotective constituents.
Footnotes
Atta, et al.: Hepatoprotective effect of Zingiber officinale and Cichorium intybus
REFERENCES
- 1.Mitra SK, Venkataranganna MV, Sundaram R, Goupmadhavan S. Protective effect of HD-03, a herbal formulation, against various hepatotoxic agents in rats. J Ethnopharmacol. 1998;63:181–6. doi: 10.1016/s0378-8741(98)00088-9. [DOI] [PubMed] [Google Scholar]
- 2.Bhandari U, Kanojia R, Pillai KK. Effect of ethanolic extract of Zingiber officinale on dyslipidaemia in diabetic rats. J Ethnopharmacol. 2005;97:227–30. doi: 10.1016/j.jep.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Mallikarjuna K, Sahitya CP, Sathyavelu RK, Rajendra , W Ethanol toxicity: Rehabilitation of hepatic antioxidant defense system with dietary ginger. Fitoterapia. 2008;79:174–8. doi: 10.1016/j.fitote.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Ghayur MN, Gilani AH. Ginger lowers blood pressure through blockade of voltage-dependent calcium channels. Dig Dis Sci. 2005;50:1889–97. doi: 10.1097/00005344-200501000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Siddaraju MN, Dharmesh SM. Inhibition of gastric HC, HC-ATPase and Helicobacter pylori growth by phenolic antioxidants of Zingiber officinale. Mol Nutr Food Res. 2007;51:324–32. doi: 10.1002/mnfr.200600202. [DOI] [PubMed] [Google Scholar]
- 6.Liu H, Zhu Y. Effect of alcohol extract of Zingber officinale rose on immunologic function of mice with tumor. Wei Sheng Yan Jiu. 2002;31:208–9. [PubMed] [Google Scholar]
- 7.Yemitan OK, Izegbu MC. Protective effects of Zingiber officinale (Zingiberaceae) against carbon tetrachloride and acetaminophen-induced hepatotoxicity in rats. Phytother Res. 2006;20:997–1002. doi: 10.1002/ptr.1957. [DOI] [PubMed] [Google Scholar]
- 8.Manju V, Nalini N. Effect of ginger on bacterial enzymes in 1, 2-dimethylhydrazine induced experimental colon carcinogenesis. Eur J Cancer Prev. 2006;15:377–83. doi: 10.1097/00008469-200610000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Ansari MN, Bhandari U, Pillai KK. Ethanolic Zingiber officinale R. extract pretreatment alleviates isoproterenol-induced oxidative myocardial necrosis in rats. Indian J Exp Biol. 2006;44:892–7. [PubMed] [Google Scholar]
- 10.Kim M, Shin HK. The water-soluble extract of chicory influences serum and liver lipid concentrations, cecal short-chain fatty acid concentrations and fecal lipid excretion in rats. J Nutr. 1998;128:1731–6. doi: 10.1093/jn/128.10.1731. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed B, Al-Howiriny TA Siddiqui AB. Antihepatotoxic activity of seeds of Cichorium intybus. J Ethnopharmacol. 2003;87:237–40. doi: 10.1016/s0378-8741(03)00145-4. [DOI] [PubMed] [Google Scholar]
- 12.Krylova SG, Efimova LA, Vymiatina , ZK , Zueva EP. The effect of cichorium root extract on the morphofunctional state of liver in rats with carbon tetrachloride induced hepatitis model. Eksp Klin Farmakol. 2006;69:34–6. [PubMed] [Google Scholar]
- 13.Kery A, Blazovics A, Fejes S, Nagy E, Lugasi A, Kursinszki L, et al. Antioxidant activity of medicinal plants used in phytotherapy. Int J Hortcultural Sci. 2001;7:28–35. [Google Scholar]
- 14.Rossetto M, Lante A, Vanzani P, Spettoli , P , Scarpa M, Rigo A. Red chicories as potent scavengers of highly reactive radicals: A study on their phenolic composition and peroxyl radical trapping capacity and efficiency. J Agric Food Chem. 2005;53:8169–75. doi: 10.1021/jf051116n. [DOI] [PubMed] [Google Scholar]
- 15.Petlevski R, Hadzija M, Slijepcevic , M , Juretic D, Petrik J. Glutathione S-transferases and malondialdehyde in the liver of NOD mice on short-term treatment with plant mixture extract P-9801 091. Phytother Res. 2003;17:311–4. doi: 10.1002/ptr.1128. [DOI] [PubMed] [Google Scholar]
- 16.Kaur N, Gupta AK. Applications of inulin and oligofructose in health and nutrition. J Biosci. 2002;27:703–14. doi: 10.1007/BF02708379. [DOI] [PubMed] [Google Scholar]
- 17.Chakraborti KK, Handa SS. Antihepatotoxic investigations on Boerhavia repanda Willd. Ind Drugs. 1989;27:19–24. [Google Scholar]
- 18.Reitman S, Frankel S. Colorimetric determination of serum glutamic oxalactic and glutamic pyruvic transaminase. Am J Clin Path. 1957;28:56–8. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 19.Szasz G. A kinetic photometric method for serum gamma glutamyl transferase. Clin Chem. 1969;15:124–36. [PubMed] [Google Scholar]
- 20.Trinder P. Enzymatic methods for glucose determination. Ann Clin Biochem. 1969;6:24–8. [Google Scholar]
- 21.VanHendle E, Zilversmith , DB Determination of serum triglycerides. J Lab Clin Med. 1957;50:152–7. [PubMed] [Google Scholar]
- 22.Henary RJ, Cannon DC, Winkleman JW. Clinical Chemistry Principles and Techniques. 2nd ed. New York: Harper and Roe; 1974. [Google Scholar]
- 23.Husdan H, Rapoport A. Estimation of Creatinine by the Jaffe reaction. Clin Chem. 1968;14:222–38. [PubMed] [Google Scholar]
- 24.Fredrikson DS, Levi RL, Less RS. Determination of cholesterol. N Engl J Med. 1967;276:148–56. [Google Scholar]
- 25.Tanira MO, Shah AH, Mohsin A, Ageel AM, Qureshi S. Pharmacological and toxicological investigations on Foeniculum vulgare dried fruit extract in experimental animals. Phytother Res. 1996;10:33–6. [Google Scholar]
- 26.Stahl E. Thin Layer Chromatography. 2nd ed. New York: Springer-Verlag; 1969. [Google Scholar]
- 27.Wagner H, Bladt S, Zgainski EM. Drogen Analyse. Heidelberg: Springer Verlag; 1983. [Google Scholar]
- 28.Buck WB, Osweiter GD, Van Gelder AG. Clinical and Diagnostic Veterinary Toxicology. 2nd ed. Iowa City: Kendall, Hunt Publishing Company; 1976. [Google Scholar]
- 29.Schmidt BM, Ilić N, Poulev A, Raskin I. Toxicological evaluation of a chicory root extract. Food Chem Tox. 2007;45:1131–9. doi: 10.1016/j.fct.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jagetia GC, Baliga , MS Venkatesh P. Ginger (Zingiber officinale Rosc.), a dietary supplement, protects mice against radiation-induced lethality: Mechanism of action. Cancer Biother Radiopharm. 2004;19:422–35. doi: 10.1089/cbr.2004.19.422. [DOI] [PubMed] [Google Scholar]
- 31.Gergely J, Kulcsár A, Hársfalvi J. Changes in fat metabolism in acute carbon tetrachloride intoxication of rats. Acta Pharm Hung. 1995;65:3–4. [PubMed] [Google Scholar]
- 32.Judah JD. Biochemical disturbances in liver injury. Br Med Bull. 1969;25:274–7. doi: 10.1093/oxfordjournals.bmb.a070717. [DOI] [PubMed] [Google Scholar]
- 33.Ajith TA, Hema U, Aswathy MS. Zingiber officinale Roscoe prevents acetaminophen-induced acute hepatotoxicity by enhancing hepatic antioxidant status. Food Chem Toxicol. 2007;45:2267–72. doi: 10.1016/j.fct.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Gilani AH, Janbaz KH. Preventive and curative effects of Artemisia absinthium on acetaminophen and CCl4–induced hepatotoxicity. Gen Pharmacol. 1995;26:309–15. doi: 10.1016/0306-3623(94)00194-r. [DOI] [PubMed] [Google Scholar]
- 35.Nicoll R, Henein MY. Ginger (Zingiber officinale Roscoe): A hot remedy for cardiovascular disease? Int J Cardiol. 2009;131:408–9. doi: 10.1016/j.ijcard.2007.07.107. [DOI] [PubMed] [Google Scholar]
- 36.Islam MS, Choi H. Comparative effects of dietary ginger (Zingiber officinale) and garlic (Allium sativum) investigated in a type 2 diabetes model of rats. J Med Food. 2008;11:152–9. doi: 10.1089/jmf.2007.634. [DOI] [PubMed] [Google Scholar]
- 37.Thulasi GP, Dos PD. Prevention of radiation-induced chromosome damage in mouse bone marrow by aqueous leaf extract of Cichorium intybus. J Cell Mol Biol. 2007;6:59–64. [Google Scholar]
- 38.Lantz RC, Chen GJ, Sarihan M, Sólyom AM, Jolad SD, Timmermann BN. The effect of extracts from ginger rhizome on inflammatory mediator production. Phytomedicine. 2006;14:123–8. doi: 10.1016/j.phymed.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Levy AS, Simon O, Shelly J, Gardener M. 6-Shogaol reduced chronic inflammatory response in the knees of rats treated with complete Freund's adjuvant. BMC Pharmacol. 2006;1:6–12. doi: 10.1186/1471-2210-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grieshop CM, Flickinger EA, Bruce KJ, Patil AR, Czarecki-Maulden GL, Fahey GC. Gastrointestinal and immunological responses of senior dose to chicory and mannan-oligosaccharides. Arch Animal Nutr. 2004;58:483–93. doi: 10.1080/00039420400019977. [DOI] [PubMed] [Google Scholar]
- 41.Kim JH, Mun YJ, Woo WH, Jeon KS, An NH, Park JS. Effect of the ethanol extract of Cichorium intybus on the immunotoxicity by ethanol in mice. Int Immunopharmacol. 2002;2:733–44. doi: 10.1016/s1567-5769(02)00008-5. [DOI] [PubMed] [Google Scholar]


