Abstract
Substance P (SP) is a potent modulator of neuroimmunoregulation. We recently reported that human immune cells express SP and its receptor. We have now investigated the possible role that SP and its receptor plays in HIV infection of human mononuclear phagocytes. SP enhanced HIV replication in human blood-isolated mononuclear phagocytes, whereas the nonpeptide SP antagonist (CP-96,345) potently inhibited HIV infectivity of these cells in a concentration-dependent fashion. CP-96,345 prevented the formation of typical giant syncytia induced by HIV Bal strain replication in these cells. This inhibitory effect of CP-96,345 was because of the antagonism of neurokinin-1 receptor, a primary SP receptor. Both CP-96,345 and anti-SP antibody inhibited SP-enhanced HIV replication in monocyte-derived macrophages (MDM). Among HIV strains tested (both prototype and primary isolates), only the R5 strains (Bal, ADA, BL-6, and CSF-6) that use the CCR5 coreceptor for entry into MDM were significantly inhibited by CP-96,345; in contrast, the X4 strain (UG024), which uses CXCR4 as its coreceptor, was not inhibited. In addition, the M-tropic ADA (CCR5-dependent)-pseudotyped HIV infection of MDM was markedly inhibited by CP-96,345, whereas murine leukemia virus-pseudotyped HIV was not affected, indicating that the major effect of CP-96,345 is regulated by Env-determined early events in HIV infection of MDM. CP-96,345 significantly down-regulated CCR5 expression in MDM at both protein and mRNA levels. Thus, SP–neurokinin-1 receptor interaction may play an important role in the regulation of CCR5 expression in MDM, affecting the R5 HIV strain infection of MDM.
Keywords: neurokinin-1 receptor, CCR5, monocytes/macrophages
Substance P (SP), the most extensively studied and potent member of the tachykinin family, is a modulator of neuroimmunoregulation, in particular the immune functions of mononuclear phagocytes. SP specifically activates NF-κB, a transcription factor involved in the control of cytokine expression (1, 2), and stimulates human peripheral blood monocytes to produce inflammatory cytokines including IL-1, IL-6, IL-12, and tumor necrosis factor α (TNF-α) (3–5). These cytokines alter HIV expression in T cells and monocytes in vitro (6, 7).
SP has an autocrine regulation role in macrophage function (8, 9). For example, in the macrophage cell line P388D1, anti-SP antibody depletion of macrophage-secreted SP from the culture resulted in abrogation of SP-increased IL-1 production (8). The pretreatment of rats with the SP antagonist CP-96,345 inhibited Clostridium difficile toxin A-mediated TNF-α release from isolated lamina propria macrophages. Furthermore, lamina propria macrophages obtained from the toxin A-injected ileal loops incubated in vitro with CP-96,345 had reduced TNF-α release, indicating an autocrine stimulation of SP in the cytokine secretion (9). Autocrine regulation of SP also has been suggested in other cell types (10–12). SP autocrine regulation in monocyte-derived macrophages (MDM) is further supported by the presence of specific cell membrane SP receptors (13) and production of SP protein by macrophages (8, 9, 14–17). SP mRNA and protein are present in monocyte/macrophages and lymphocytes isolated from human peripheral blood (16, 18). We have also identified the presence of neurokinin-1 receptor (NK-1R) mRNA in these cells (16, 18). Because SP is secreted by human monocyte/macrophages and participates in immunoregulation in an autocrine fashion (8, 9, 14, 16, 19), it may have a role in the pathogenesis of immune-mediated diseases, including neuroimmunologic diseases and HIV/AIDS.
SP and its receptor, NK-1R, may be involved in the modulation of HIV infection both in vivo and in vitro. Azzari et al. (20), using a RIA, observed that HIV-positive children had higher plasma levels of SP compared with HIV-negative children. Annunziata et al. (21) showed that SP plays a critical role in HIV gp120-induced increases in permeability of rat brain endothelium cultures, and this effect of SP on gp120-induced increase in albumin permeability is abrogated by the SP antagonist (spantide) and/or anti-SP polyclonal antibody. Significant SP immunoreactivity has been observed in HIV gp120 transgenic mouse brain vessels in comparison with nontransgenic mouse brain vessels, suggesting that SP is involved in HIV gp120-induced changes in the vascular component of the blood–brain barrier (12). We previously reported that SP modulated HIV replication in human peripheral blood MDM in vitro (22). We have demonstrated elevated plasma levels of SP in HIV-positive men in comparison with high-risk HIV-negative men (unpublished data).
We, therefore, postulated that SP and its receptors are actively involved in HIV infection of human immune cells such as MDM. We further postulated that by interrupting the SP autocrine loop, SP antagonists inhibit HIV infection of these cells. We report here that the nonpeptide SP antagonist (CP-96,345) potently inhibits HIV infection of MDM.
Materials and Methods
Cells.
Peripheral blood was obtained from healthy normal adult donors. The blood samples were identified as HIV-1 antibody negative by anonymous testing with the ELISA method (Coulter). Monocytes were purified according to our previously described techniques (23, 24).
ACH-2 and U38 cell lines (25, 26) were obtained from the National Institutes of Health AIDS reagent program. DNA extracted from ACH-2 cells was used as a positive standard for HIV DNA PCR as reported (27). The U38 cell line contains stably integrated, silent copies of the HIV long-terminal repeat (LTR) promoter linked to the chloramphenicol acetyltransferase (CAT) gene and was used to study the effect of SP and/or CP-96,345 on the activation of HIV LTR promoter.
Reagents.
Fluorescein (FITC)-conjugated anti-CCR5, anti-CD4, anti-CD14, IgG1, and IgG2a were obtained from PharMingen. Anti-CXCR4-FITC was obtained from R & D Systems. SP, a rabbit anti-SP antibody, and lipopolysaccharide (LPS) were obtained from Sigma. Sodium azide was removed from the anti-SP antibody by using CHROMA SPIN STE-30 columns (CLONTECH). The nonpeptide SP antagonist, CP-96,345, and its 2R,3R inactive enantiomer, CP-96,344 (28), were generously provided by Pfizer Diagnostics. CP-96,345 and CP-96,344 were dissolved in FPLC grade H2O at the concentration of 10−3 M, filtered through a 0.22-μm filter (Millipore), and stored at −70°C.
HIV Strains.
Based on their differential use of the major HIV coreceptors, HIV isolates have been referred to as R5, X4, or R5X4 strains (29). The M-tropic R5 prototype strains (Bal and ADA), dual tropic strain (89.6), and X4 primary isolate (UG024) were obtained from the National Institutes of Health AIDS reagent program. Primary M-tropic isolates (BL-6 and CSF-6) were isolated from the blood and cerebrospinal fluid cells, respectively, of a subject with AIDS.
CP-96,345 Treatment and HIV Infection.
SP and CP-96,345 treatment.
Seven-day-cultured macrophages in 24-well plates (1 × 106 cells/well) were incubated with or without CP-96,345 or its inactive enantiomer, CP-96,344, for 2 h before infection with HIV. The cells were also incubated with SP and/or anti-SP antibody. In this case, SP was incubated with (1:1,000 dilution in 10% FCS DMEM) or without anti-SP antibody at room temperature for 10 min, and then the mixture was added to the cell cultures for 2 h. When the cells were treated with the combination of CP-96,345 and SP, or CP-96,345, SP and anti-SP antibody, CP-96,345 was incubated with the cell culture for 10 min; SP was then added to the MDM, or CP-96,345, SP, and anti-SP (1:1,000 in 10% FCS DMEM) were incubated for 10 min at room temperature, and the mixture was incubated with the MDM for 2 h before infection with HIV. Untreated MDM served as controls. The cells were treated before, during, and throughout the infection.
HIV infection.
After 2 h incubation with or without the reagents described above, the cells were infected with equal amounts of cell-free HIV based on p24 antigen content (20 ng/106 cells) overnight, and then washed to remove unbound virus. Fresh media containing SP and/or CP-96,345 or anti-SP antibody were added to the MDM as described above. The culture media and the reagents were replaced twice weekly. The culture supernatants were harvested for HIV reverse transcriptase (RT) activity determinations. After HIV infection, the MDM were incubated 8–12 days. At the termination of the experiments, cellular RNA was extracted for assessment of HIV gag gene expression by using RT-PCR or real-time RT-PCR assays.
HIV RT Assay.
The HIV RT activity assay was carried out based on the technique of Willey et al. (30) with modification (22).
Syncytia Formation.
To study the effect of CP-96,345 on HIV-induced syncytia formation in MDM, 7-day-cultured macrophages were incubated in the presence or absence of CP-96,345 (10−7 M) for 2 h and then infected with HIV Bal strain. MDM that was neither incubated with CP-96,345 nor infected with HIV was used as a cell culture control. The morphology of HIV-induced syncytia formation was observed and photographed by a light microscopy (400×) 12 days after HIV infection.
Pseudotype Reporter Virus Entry Assay.
Recombinant luciferase-encoding HIV virions pseudotyped with Env from the M-tropic ADA (CCR5-dependent) and amphitropic murine leukemia virus (MLV) (CCR5-independent) were used to study HIV infection of MDM incubated with or without CP-96,345. The Env-deleted luciferase reporter plasmid PNL-Luc-E-R+ (kindly provided by N. Landau, The Salk Institute for Biological Studies, La Jolla, CA) was cotransfected into 293T cells along with plasmids encoding the ADA or MLV Env genes as described (31). Supernatants were collected 48 h later, assayed for p24Gag antigen content, and frozen at −80°C. MDM (2.5 × 105/well in 48-well plates) were incubated overnight with or without CP-96,345 and then infected by using 20 ng of p24Gag antigen equivalent of each pseudotype HIV per well, in the presence of polybrene (7.5 μg/ml). At 72 h postinfection, the cells were lysed in 150 μl of 0.5% Triton-X-100 in PBS. Lysate (50 μl) was mixed with an equal volume of luciferase substrate (Promega), and luciferase activity was determined in a microtiter plate luminometer (Dynex Technologies, Chantilly, VA). The results were expressed as relative light units in CP-96,345-treated and infected MDM as a percentage of that in untreated and infected MDM.
CAT Activity Assay.
U38 cells (2 × 106 cells) were incubated with different concentrations of SP and/or CP-96,345 for 48 h. Untreated U38 cells were used as a background control. Cellular proteins were extracted by using 1× reporter lysis buffer (Promega). The lysates were incubated with a mixture containing 0.15 μCi of 14C-labeled chloramphenicol (DuPont) and 200 μg/ml n-butyryl CoA (Promega) in a total volume of 125 μl at 37°C for 3 h. The acetylated forms of chloramphenicol were extracted with xylene (Aldrich). CAT activity of each sample was quantified by using a liquid-scintillation counter (Packard). The results were expressed as the ratio of CAT activity (cpm) of the SP and/or CP-96,345-treated U38 to that of the background control.
Flow Cytometry.
To determine the expression of CCR5, CD4, CXCR4, and CD14 on CP-96,345-treated monocytes/macrophages, monocytes or MDM (106 cells) were incubated with or without CP-96,345 or its inactive enantiomer CP-96,344 at different concentrations for 24 h. The cells were removed from the culture plate and then resuspended in 100 μl of PBS. After incubation with 20 μl of anti-CCR5-FITC, anti-CXCR4-FITC, anti-CD4-FITC, or anti-CD14-FITC for 45 min at 4°C, the cells were washed twice with PBS and fixed with 1% paraformaldehyde in PBS. Fluorescein-conjugated control antibodies were isotype-matched IgG. Fluorescence was analyzed on an EPICS-Elite flow cytometer (Beckman Coulter).
RNA Isolation.
Total RNA was isolated from human peripheral blood MDM (106 cells) by using Tri-Reagent (Molecular Research Center, Cincinnati) as reported (16).
Reverse Transcription.
Total RNA (1 μg) was reverse transcribed by using the reverse transcription system (Promega) with the specific primers (antisense) for HIV gag (5′-TGACATGCTGTCATCATTTCTTC-3′) (27) or CCR5 (5′-CCTGTGCCTCTTCTTCTCATTTCG-3′) (32) for 1 h at 42°C. RT reactions were terminated by incubating the reaction mixture at 99°C for 5 min and then kept at 4°C. The resulting cDNA was used as a template for PCR amplification or real-time PCR quantification.
PCR Analysis.
PCR amplification of CCR5 and HIV gag cDNA was performed with one-tenth of the cDNA for 35 cycles (CCR5) (32) or 40 cycles (gag) (27) by using AmpliTaq Gold (Perkin–Elmer/Cetus) in a GeneAmp PCR System 2400 (Perkin–Elmer/Cetus). β-actin was used as a control to monitor the amount and integrity of RNA in each sample (CLONTECH). Real-time PCR was performed with one-tenth of cDNA by using the ABI Prism 7700 sequence detection system (Perkin–Elmer). The reaction mixture contained 0.25 mM dNTPs, AmpliTaq Gold (1.5 units), 5 mM MgCl2, 50 pmol of each of the two primers (SK38, 5′-ATAATCCACCTATCCCAGTAGGAGAAAT-3′; SK39, 5′-TTTGGTCCTGTCTTATGTCCAGAATGC-3′), 20 pmol of the molecular beacon probe (SK19, 5′-ATCCTGGGGATTAAATAAAATAGTAAGAATGTATAGCCCTAC-3′) labeled with FAM (a fluorophore) at its 5′ end and DABCYL (a quencher) at the 3′ end. The cycle conditions were 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The known amounts of HIV DNA isolated from ACH-2 cells were used as standard controls. All standards and samples were run in duplicates. HIV gag, CCR5, β-actin primer pairs, and the probe (SK19) were synthesized by Integrated DNA Technologies (Coralville, IA).
CP-96,345 Treatment and Cytokine Production.
To determine the role of CP-96,345 in the regulation of cytokine secretion, 7-day-cultured MDM were incubated with or without CP-96,345 (10−9 to 10−7 M) and/or LPS (1 ng/ml) for 24 h. The culture supernatants were collected to determine TNF-α and IL-6 levels by ELISA. The ELISA kits were purchased from Endogen (Cambridge, MA).
Statistical Analysis.
The data were analyzed by using the Student's t test for paired samples.
Results
Effect of SP and/or CP-96,345 on HIV Replication in MDM.
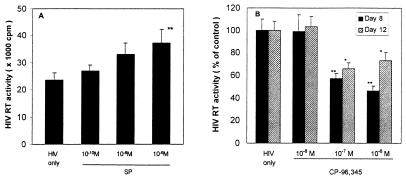
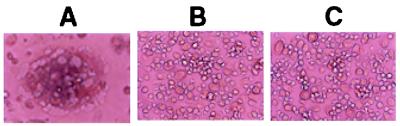
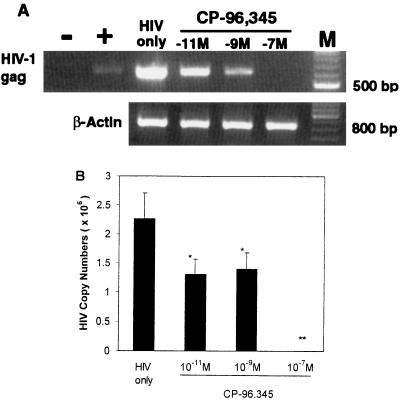
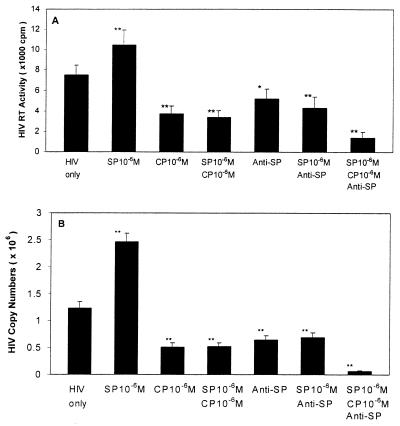
SP up-regulated HIV replication in MDM in a concentration-dependent fashion (Fig. 1A), which is consistent with our previous observation (22). HIV RT activity was increased by 14% (10−10 M), 40% (10−8 M), and 58% (10−6 M) (Fig. 1A). We thus determined whether the addition of CP-96,345 to MDM could inhibit HIV Bal strain infection of MDM. As shown in Fig. 1B, CP-96,345 inhibited HIV Bal strain infection of MDM in a concentration-dependent manner. The observed inhibition was 1% (10−8 M), 43% (10−7 M), and 54% (10−6 M) on day 8 after HIV infection. HIV Bal strain-infected MDM cultures (without CP-96,345 treatment) demonstrated characteristic giant syncytia formation, whereas the SP antagonist-treated MDM failed to develop the giant syncytia induced by HIV Bal strain infection (Fig. 2). To further examine the inhibition of CP-96,345 on HIV Bal strain at mRNA level, HIV gag gene expression was measured by using RT-PCR. HIV gag mRNA expression was potently inhibited by CP-96,345 treatment in a concentration-dependent fashion (Fig. 3A). To quantitatively measure mRNA copy numbers for HIV gag gene, we used real-time RT-PCR to quantify the same samples shown in Fig. 3A. Of note and most important to the findings reported here, CP-96,345 significantly suppressed HIV gag gene mRNA expression at all three concentrations tested (Fig. 3B).
Figure 1.
(A) SP up-regulated HIV Bal replication in MDM. MDM were incubated with different concentrations of SP as indicated and infected with HIV Bal strain. Untreated and HIV-infected MDM were used as an infection control (HIV only). HIV RT activity was determined in triplicates in the culture supernatants 12 days after infection. The data shown are presented as the mean ± SD of triplicate cultures and are representative of three experiments (**, P < 0.01, SP 10−6 M vs. untreated). (B) Effect of CP-96,345 on HIV infection of human MDM. The HIV Bal strain was used to infect MDM in the presence or absence of CP-96,345 (10−8 to 10−6 M). HIV RT activity was measured in the supernatants 8 and 12 days after infection. HIV RT activity in CP-96,345-treated and infected MDM culture supernatants were expressed as percentage of that of untreated and HIV-infected MDM controls.
Figure 2.
Effect of CP-96,345 on HIV-induced syncytium formation in MDM. The morphology of untreated and HIV-infected (A), CP-96,345-treated (10−7 M) and infected (B), and untreated and uninfected MDM (C) was observed and photographed under a light microscopy (×400) 12 days after infection.
Figure 3.
Effect of CP-96,345 on the levels of HIV gag mRNA. The HIV Bal strain was used to infect MDM in the presence or absence of CP-96,345 (10−11 to 10−7 M). HIV gag mRNA levels were determined by RT-PCR and real-time RT-PCR by using RNA extracted from the MDM 12 days after infection. β-actin was used as the control to monitor the amount and integrity of RNA in each sample. (A) RT-PCR: −, negative control; +, DNA isolated from ACH-2 cells was used as a PCR positive control (100 copies). HIV only: HIV Bal infected MDM as an infection control; MDM were infected with HIV Bal strain in the presence of CP-96,345 (10−11 M to 10−7 M) as indicated. M: 100-bp DNA ladder coelectrophoresed as markers. (B) Real-time RT-PCR: HIV gag mRNA levels were quantified by real-time RT-PCR by using the same RNA samples as indicated in A, and the data are expressed as HIV gag mRNA copy numbers per PCR. The data shown are presented as the mean ± SD of triplicate cultures and are representative of three experiments (*, P < 0.05; **, P < 0.01; CP-96,345 vs. HIV only).
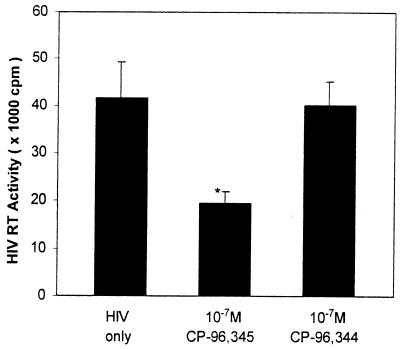
CP-96,345 Inhibited HIV Replication by Antagonism of NK-1R on MDM.
To determine whether inhibition of CP-96,345 on HIV infection of MDM is mediated by specific antagonism of NK-1R, MDM were incubated with CP-96,345 or its inactive enantiomer CP-96,344 for 2 h and then infected with HIV Bal strain. In comparison with the HIV infection control, viral replication was inhibited by CP-96,345 treatment (10−7 M), whereas CP-96,344 at the same concentration did not have the effect as determined by HIV RT activity (Fig. 4), demonstrating that CP-96,345 affected HIV replication through antagonism of NK-1R on MDM. To further test our hypothesis that SP–NK-1R interaction plays a role and is a necessary component in HIV replication in MDM, we incubated MDM with SP in the presence or absence of CP-96,345 or anti-SP antibody, or both for 2 h, and then infected the MDM with HIV Bal strain. As expected, SP alone enhanced HIV replication, whereas CP-96,345, anti-SP antibody, and the combination of both abrogated SP-enhanced HIV replication in MDM as determined by HIV RT activity and HIV gag mRNA levels (Fig. 5). Furthermore, treatment with CP-96,345 and/or anti-SP antibody down-regulated HIV replication to a level that was even lower than that of the HIV infection control (Fig. 5). The combination of anti-SP antibody and CP-96,345 had a synergistic effect on HIV replication in MDM, further demonstrating that both SP and SP receptors are involved in HIV infection of MDM and that interruption of SP autocrine loop resulted in inhibition of HIV replication in these cells (Fig. 5).
Figure 4.
Effect of CP-96,345 on HIV replication by antagonism of NK-1R on MDM. HIV Bal strain was used to infect MDM in the presence or absence of CP-96,345 (10−7 M) or CP-96,344 (10−7 M). HIV RT activity was determined in the culture supernatants collected 8 days after infection. RT activity in HIV-infected MDM was used as a positive control (HIV only), whereas that in HIV-infected MDM in the presence of CP-96,344 was used as a specificity control. The data shown are presented as the mean ± SD of triplicate cultures and are representative of three experiments (*, P < 0.05; CP-96,345 vs. HIV only).
Figure 5.
Effect of CP-96,345 and/or anti-SP antibody on SP-enhanced HIV replication. HIV Bal strain was used to infect MDM in the presence or absence of SP (10−6 M), CP-96,345 (10−6 M), or anti-SP antibody (1:1,000 dilution) as indicated. CP, CP-96,345. HIV only, untreated and HIV Bal strain-infected MDM was used as a control. HIV RT activity (A) in the culture supernatants was determined 12 days after infection, and HIV gag mRNA copy numbers (B) were quantified by using real-time RT-PCR 12 days after infection (B). The data shown are presented as the mean ± SD of triplicate cultures and are representative of three experiments (*, P < 0.05; **, P < 0.01; treatment vs. HIV only).
Effect of CP-96,345 on HIV Replication.
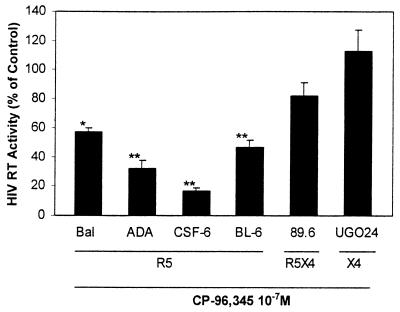
To determine the effect of CP-96,345 on different HIV tropic strains (R5, X4, and R5X4), MDM were incubated with or without CP-96,345 (10−7 M) and then infected with R5 strains (Bal, ADA, CSF-6, and BL-6), R5X4 strain (89.6), or X4 strain (UG024). In contrast to the prototype T cell line-adapted X4 strains, UG024 is capable of by using macrophage CXCR4 for infection (33), whereas 89.6 uses both macrophage CXCR4 and CCR5 (34). The replication of all R5 strains studied, including both prototype strains and primary isolates, was inhibited by CP-96,345 treatment, whereas the R5X4 strain (89.6) was inhibited to a lesser extent and the X4 strain (UG024) was not affected (Fig. 6).
Figure 6.
Effect of CP-96,345 on HIV infection of MDM. Different HIV strains were used to infect MDM in the presence or absence of CP-96,345 (10−7 M). HIV RT activity in the culture supernatants was determined 8 days after infection. HIV RT activity in the CP-96,345-treated and HIV-infected MDM were expressed as percentage of that of untreated and HIV (corresponding strains)-infected MDM controls, which were defined as 100%. The data shown are presented as the mean ± SD of triplicate cultures and are representative of three experiments (*, P < 0.05; **, P < 0.01; CP-96,345 vs. HIV only). R5, CCR5 tropic strains; X4, CXCR4 tropic strains; R5X4, dual tropic strains.
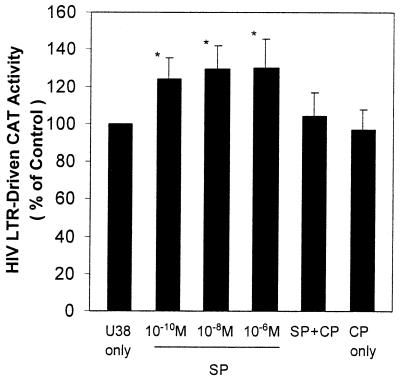
Effect of SP on Activation of HIV LTR-Driven CAT Activity in U38 Cell Line.
To determine whether SP activates HIV LTR promoter, we used U38 cells as a model for the experiments. SP significantly increased HIV LTR-driven CAT activity at the concentrations of 10−10 to 10−6 M (P < 0.05) in comparison with that of untreated control cells. This effect of SP (10−8 M) on HIV LTR was abrogated by CP-96,345 (10−6 M), indicating that SP activates HIV LTR through the interaction with NK-1R on the cell membrane (Fig. 7).
Figure 7.
Effect of SP on activation of HIV LTR-driven CAT activity in U38 cells. U38 cells were incubated with SP at different concentrations (10−10 to 10−6 M) or with CP-96,345 (10−6 M) and SP (10−8 M) (SP + CP) or with CP-96,345 (10−6 M) alone (CP only) or untreated (U38 only) for 48 h. The untreated U38 cells were used as baseline control. The CAT activity (cpm) of the treated U38 cells was expressed as percentage of that of untreated baseline control cells. The data shown are presented as the mean ± SD of triplicate cultures and are representative of three experiments (*, P < 0.05; SP-treated U38 vs. U38 only).
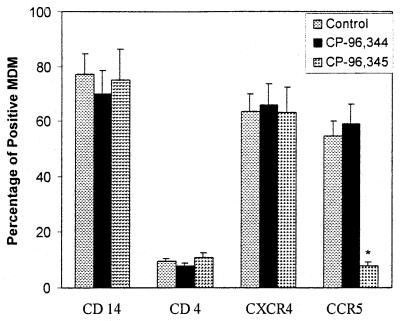
Effect of CP-96,345 on Macrophage Receptor Expression.
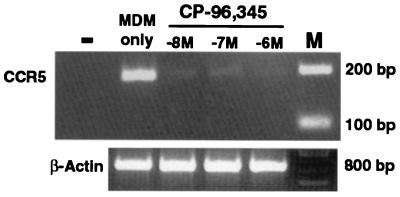
Because the replication of the HIV R5 strains but not the X4 strain was inhibited by CP-96,345, we hypothesized that the SP–NK-1R interaction participated in the regulation of HIV coreceptor CCR5 expression. To examine the effect of CP-96,345 on HIV receptor expression, MDM were incubated overnight with or without CP-96,345 or CP-96,344. Among the CD4, CCR5, CXCR4, and CD14 receptors, only CCR5 expression was down-regulated by CP-96,345, whereas CP-96,344 did not affect CCR5 receptor expression (Fig. 8). CCR5 mRNA was also down-regulated in MDM by CP-96,345 (10−8 to 10−6 M) (Fig. 9).
Figure 8.
Effect of CP-96,345 on macrophage receptor expression. MDM were incubated with or without CP-96,345 or CP-96,344 for 24 h, and the MDM receptor expression was determined by direct immunofluorescence. The results shown are the percentage of MDM positive for the receptors analyzed and are representative of three experiments. The data shown are presented as the mean ± SD of triplicate cultures and are representative of three experiments (*, P < 0.05; CP-96,345 treated vs. control).
Figure 9.
Effect of CP-96,345 on CCR5 mRNA expression in MDM. −, PCR negative control, which represents lack of PCR products when RT was omitted from the RT reaction by using RNA extracted from untreated MDM. MDM only, untreated MDM was used as a control. MDM were incubated with CP-96,345 (10−8 to 10−6 M) as indicated for 3 h, and CCR5 mRNA was then amplified by using RT-PCR. M, 100-bp DNA ladder coelectrophoresed as markers. β-actin was used to monitor the amount and integrity of RNA in each sample.
Effect of CP-96,345 on HIV Pseudotype Infection of MDM.
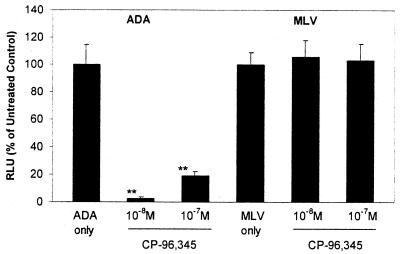
To examine the functional relevance of CP-96,345-mediated CCR5 down-regulation to HIV infection of MDM, the cells incubated with or without CP-96,345 were infected with luciferase-encoding HIV virions pseudotyped with either ADA Env (CCR5-dependent) or MLV Env (CCR5-independent). We observed that CP-96,345 inhibited M-tropic ADA infection of MDM as demonstrated by luciferase activity (Fig. 10). However, CP-96,345 failed to block MLV Env-pseudotyped HIV infection of MDM, indicating that the major effect of CP-96,345 inhibition is regulated by Env-determined early events in HIV entry into MDM.
Figure 10.
Effect of CP-96,345 on pseudotyped HIV infection of MDM. Recombinant luciferase-encoding HIV reporter viruses pseudotyped with ADA Env or MLV Env were used to infect untreated MDM (ADA only or MLV only) and CP-96,345-pretreated MDM (10−8 and 10−7 M overnight) as indicated. The data are expressed as relative light units of CP-96,345-treated cells to that of untreated control (ADA only or MLV only), which is defined as 100%. The data shown are presented as the mean ± SD of triplicate cultures and are representative of three experiments (**, P < 0.01; CP-96,345 treated vs. ADA only).
Effect of CP-96,345 on TNF-α Synthesis in MDM.
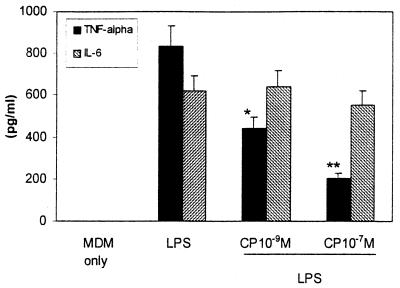
To determine whether SP participates in TNF-α synthesis in an autocrine fashion, the effect of CP-96,345 on LPS-stimulated TNF-α production in MDM was determined by ELISA. CP-96,345 attenuated LPS-stimulated TNF-α production in MDM in a concentration-dependent manner, whereas IL-6 production was not affected (Fig. 11), indicating that the SP is involved in the regulation of TNF-α synthesis and secretion in an autocrine fashion.
Figure 11.
Effect of CP-96,345 on cytokine production in MDM. MDM was incubated with or without CP-96,345 and/or LPS for 24 h. TNF-α and IL-6 levels in the culture supernatants were determined by ELISA. The treatment includes untreated MDM (MDM only), MDM incubated with LPS (1 ng/ml) only (LPS), and MDM incubated with LPS (1 ng/ml) plus CP-96,345 at the concentrations indicated. The data shown are presented as the mean ± SD of triplicate cultures and are representative of three experiments (*, P < 0.05; **, P < 0.01; LPS and CP-96,345 treated vs. LPS).
Discussion
In this communication, we have demonstrated that the SP antagonist, CP-96,345, potently inhibited HIV (R5 strains) replication in human peripheral blood MDM. Because SP is involved in the modulation of HIV infection of MDM (Fig. 1A) (22) and there is an autocrine regulation of SP (8–10) (Fig. 11), the observed inhibition is the consequence of the interruption of SP autocrine loop through antagonism of SP–NK-1R interaction on the cell membrane. The following data strongly support this hypothesis: (i) both CP-96,345 and anti-SP antibody inhibited SP-enhanced HIV Bal strain replication in MDM (Figs. 1B, 3, and 5); (ii) CP-96,345 inhibited HIV R5 strain replication in MDM, whereas its inactive enantiomer, CP-96,344, was ineffective (Fig. 4); and (iii) the HIV R5 (CCR5-dependent) strain replication was inhibited, whereas the R5X4 dual tropic strain (89.6) and the X4 (UGO24) strain were partially inhibited or not affected, respectively (Fig. 6).
Furthermore, we observed that CP-96,345 down-regulated CCR5 expression in MDM, whereas CP-96,344, the inactive enantiomer of CP-96,345, was ineffective (Figs. 8 and 9). Down-regulation of CCR5 is functionally relevant to the decreased susceptibility to HIV infection by MDM (Fig. 10). This observation is further supported by the findings that CP-96,345 inhibited the replication of HIV R5 strains (Bal, ADA, CSF-6, and BL-6) but not the X4 strain (UGO24) (Fig. 6) and that only ADA Env-pseudotyped HIV infection was inhibited (Fig. 10). These data strongly indicate that SP autocrine loop is involved in the regulation of CCR5 expression in MDM and that down-regulation of CCR5 expression on MDM by CP-96,345 is caused by an interruption of this autocrine loop in these cells. The precise mechanism(s) whereby SP–NK-1R interaction regulates CCR5 expression requires further investigation. SP activates NF-κB (1, 2, 35), which is a potent activator of the CCR5 promoter (36) and participates in the regulation of TNF-α expression and secretion in vitro and in vivo (9, 37–40). Pretreatment of mice with an SP antagonist down-regulated LPS-stimulated TNF-α transcription and secretion in vivo (39). Furthermore, our studies demonstrated that CP-96,345 attenuated LPS-stimulated TNF-α secretion in human MDM (Fig. 11). Recent studies have demonstrated that TNF-α up-regulates CCR5 expression in macrophages (41) and peripheral blood mononuclear cells (42) and altered HIV expression in monocytes in vitro (6, 7). Thus, CP-96,345-mediated TNF-α regulation may be partially responsible for the down-regulation of CCR5 expression in MDM. Further investigation is needed to determine the mechanism(s) whereby CP-96,345–NK-1R interaction regulates CCR5 expression in relation to HIV infection of human MDM.
Although the precise mechanism(s) by which CP-96,345 inhibits HIV R5 strain replication could not be ascertained from the present study, our data strongly support two coexisting potential mechanisms. One possibility is that CP-96,345 interrupts the SP autocrine loop, which may play a role in maintaining high-level CCR5 expression, permitting HIV R5 strain infection at the virus entry level. A second possibility is that CP-96,345 inhibits SP-stimulated HIV replication at the transcriptional level because the addition of SP to MDM cultures did not affect CCR5 expression (data not shown) but did enhance HIV replication (Figs. 1A and 5) and HIV LTR-driven CAT gene expression. This effect of SP was abrogated by preincubated MDM with CP-96,345 (Fig. 7). Moreover, we observed that CP-96,345 did not suppress HIV LTR-driven CAT activity below the control level (Fig. 7) as observed in Fig. 5 for HIV RT activity and gag mRNA copy numbers. This finding provides additional evidence that supports our hypothesis that the mechanism by which CP-96,345 inhibits HIV infection of MDM was mainly because of its suppressive effect on CCR5 expression. In addition, the neutralization of SP by anti-SP antibody resulted in down-regulation of HIV replication in MDM, and the combination of anti-SP antibody and CP-96,345 had a synergistic effect on HIV replication in MDM (Fig. 5). These findings indicate that both SP and its receptor (NK-1R) are indeed involved in HIV infection of MDM. Although the specific factors involved in SP-stimulated HIV replication in these immune cells are not yet known, our findings provide further evidence that there is an important link between neuropeptide SP and HIV infection of human immune cells.
Taken together, these in vitro effects of the SP antagonist CP-96,345, including attenuation of TNF-α production in MDM, down-regulation of CCR5 receptor expression on MDM, and inhibition of HIV R5 strain replication in MDM, may offer approaches to the design of new anti-HIV therapeutics. Studies of the mechanism(s) of SP action in the regulation of immune responses in HIV infection of MDM and the direct and/or indirect effect of SP–NK-1R interaction on HIV infection of and replication in human MDM and other immune cells will be beneficial.
Acknowledgments
We are grateful to Farida Shaheen of the Center for AIDS Research for technical assistance in optimizing real-time PCR for the HIV gag gene. This work was supported by National Institutes of Health Grants MH 49981, DA12815, and P30 AI45008.
Abbreviations
- SP
substance P
- MDM
monocyte-derived macrophages
- TNF
tumor necrosis factor
- LTR
long-terminal repeat
- CAT
chloramphenicol acetyltransferase
- LPS
lipopolysaccharide
- MLV
murine leukemia virus
- RT
reverse transcriptase
- NK-1R
neurokinin-1 receptor.
References
- 1.Marriott I, Mason M J, Elhofy A, Bost K L. J Neuroimmunol. 2000;102:163–171. doi: 10.1016/s0165-5728(99)00182-4. [DOI] [PubMed] [Google Scholar]
- 2.Lieb K, Fiebich B L, Berger M, Bauer J, Schulze-Osthoff K. J Immunol. 1997;159:4952–4958. [PubMed] [Google Scholar]
- 3.Lotz M, Vaughan J H, Carson D A. Science. 1988;241:1218–1221. doi: 10.1126/science.2457950. [DOI] [PubMed] [Google Scholar]
- 4.Laurenzi M A, Persson M A, Dalsgaard C J, Haegerstrand A. Scand J Immunol. 1990;31:529–533. doi: 10.1111/j.1365-3083.1990.tb02801.x. [DOI] [PubMed] [Google Scholar]
- 5.Kincy-Cain T, Bost K L. J Immunol. 1997;158:2334–2339. [PubMed] [Google Scholar]
- 6.Rosenberg Z F, Fauci A S. Immunol Today. 1990;11:176–180. doi: 10.1016/0167-5699(90)90070-p. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg Z F, Fauci A S. FASEB J. 1991;5:2382–2390. doi: 10.1096/fasebj.5.10.1676689. [DOI] [PubMed] [Google Scholar]
- 8.Pascual D W, Bost K L. Immunology. 1990;71:52–56. [PMC free article] [PubMed] [Google Scholar]
- 9.Castagliuolo I, Keates A C, Qiu B, Kelly C P, Nikulasson S, Leeman S E, Pothoulakis C. Proc Natl Acad Sci USA. 1997;94:4788–4793. doi: 10.1073/pnas.94.9.4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cioni C, Renzi D, Calabro A, Annunziata P. J Neuroimmunol. 1998;84:76–85. doi: 10.1016/s0165-5728(97)00235-x. [DOI] [PubMed] [Google Scholar]
- 11.Bae S, Matsunaga Y, Tanaka Y, Katayama I. Biochem Biophys Res Commun. 1999;263:327–333. doi: 10.1006/bbrc.1999.1285. [DOI] [PubMed] [Google Scholar]
- 12.Toneatto S, Finco O, van der Putten H, Abrignani S, Annunziata P. AIDS. 1999;13:2343–2348. doi: 10.1097/00002030-199912030-00005. [DOI] [PubMed] [Google Scholar]
- 13.Lucey D R, Novak J M, Polonis V R, Liu Y, Gartner S. Clin Diagn Lab Immunol. 1994;1:330–335. doi: 10.1128/cdli.1.3.330-335.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bost K L, Breeding S A, Pascual D W. Reg Immunol. 1992;4:105–112. [PubMed] [Google Scholar]
- 15.De Giorgio R, Tazzari P L, Barbara G, Stanghellini V, Corinaldesi R. J Neuroimmunol. 1998;82:175–181. doi: 10.1016/s0165-5728(97)00201-4. [DOI] [PubMed] [Google Scholar]
- 16.Ho W Z, Lai J P, Zhu X H, Uvaydova M, Douglas S D. J Immunol. 1997;159:5654–5660. [PubMed] [Google Scholar]
- 17.Lai J P, Douglas S D, Rappaport E, Wu J M, Ho W Z. J Neuroimmunol. 1998;91:121–128. doi: 10.1016/s0165-5728(98)00170-2. [DOI] [PubMed] [Google Scholar]
- 18.Lai J P, Douglas S D, Ho W Z. J Neuroimmunol. 1998;86:80–86. doi: 10.1016/s0165-5728(98)00025-3. [DOI] [PubMed] [Google Scholar]
- 19.Castagliuolo I, Riegler M, Pasha A, Nikulasson S, Lu B, Gerard C, Gerard N P, Pothoulakis C. J Clin Invest. 1998;101:1547–1550. doi: 10.1172/JCI2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azzari C, Rossi M E, Resti M, Caldini A L, Lega L, Galli L, Fico E, Vierucci A. Pediatr Med Chir. 1992;14:577–581. [PubMed] [Google Scholar]
- 21.Annunziata P, Cioni C, Toneatto S, Paccagnini E. AIDS. 1998;12:2377–2385. doi: 10.1097/00002030-199818000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Ho W Z, Cnaan A, Li Y H, Zhao H, Lee H R, Song L, Douglas S D. AIDS Res Hum Retroviruses. 1996;12:195–198. doi: 10.1089/aid.1996.12.195. [DOI] [PubMed] [Google Scholar]
- 23.Hassan N F, Campbell D E, Douglas S D. J Immunol Methods. 1986;95:273–276. doi: 10.1016/0022-1759(86)90415-1. [DOI] [PubMed] [Google Scholar]
- 24.Hassan N F, Cutilli J R, Douglas S D. J Immunol Methods. 1990;130:283–285. doi: 10.1016/0022-1759(90)90058-4. [DOI] [PubMed] [Google Scholar]
- 25.Clouse K A, Powell D, Washington I, Poli G, Strebel K, Farrar W, Barstad P, Kovacs J, Fauci A S, Folks T M. J Immunol. 1989;142:431–438. [PubMed] [Google Scholar]
- 26.Felber B K, Pavlakis G N. Science. 1988;239:184–187. doi: 10.1126/science.3422113. [DOI] [PubMed] [Google Scholar]
- 27.Collin M, Gordon S. Virology. 1994;200:114–120. doi: 10.1006/viro.1994.1169. [DOI] [PubMed] [Google Scholar]
- 28.Snider R M, Constantine J W, Lowe J A d, Longo K P, Lebel W S, Woody H A, Drozda S E, Desai M C, Vinick F J, Spencer R W, et al. Science. 1991;251:435–437. doi: 10.1126/science.1703323. [DOI] [PubMed] [Google Scholar]
- 29.Berger E A, Doms R W, Fenyo E M, Korber B T, Littman D R, Moore J P, Sattentau Q J, Schuitemaker H, Sodroski J, Weiss R A. Nature (London) 1998;391:240. doi: 10.1038/34571. [DOI] [PubMed] [Google Scholar]
- 30.Willey R L, Smith D H, Lasky L A, Theodore T S, Earl P L, Moss B, Capon D J, Martin M A. J Virol. 1988;62:139–147. doi: 10.1128/jvi.62.1.139-147.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Connor R I, Chen B K, Choe S, Landau N R. Virology. 1995;206:935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- 32.Huang Y, Paxton W A, Wolinsky S M, Neumann A U, Zhang L, He T, Kang S, Ceradini D, Jin Z, Yazdanbakhsh K, et al. Nat Med. 1996;2:1240–1243. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- 33.Yi Y, Isaacs S N, Williams D A, Frank I, Schols D, De Clercq E, Kolson D L, Collman R G. J Virol. 1999;73:7117–7125. doi: 10.1128/jvi.73.9.7117-7125.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yi Y, Rana S, Turner J D, Gaddis N, Collman R G. J Virol. 1998;72:772–777. doi: 10.1128/jvi.72.1.772-777.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quinlan K L, Naik S M, Cannon G, Armstrong C A, Bunnett N W, Ansel J C, Caughman S W. J Immunol. 1999;163:5656–5665. [PubMed] [Google Scholar]
- 36.Liu R, Zhao X, Gurney T A, Landau N R. AIDS Res Hum Retroviruses. 1998;14:1509–1519. doi: 10.1089/aid.1998.14.1509. [DOI] [PubMed] [Google Scholar]
- 37.Ho W Z, Kaufman D, Uvaydova M, Douglas S D. J Neuroimmunol. 1996;71:73–80. doi: 10.1016/s0165-5728(96)00132-4. [DOI] [PubMed] [Google Scholar]
- 38.Ho W Z, Stavropoulos G, Lai J P, Hu B F, Magafa V, Anagnostides S, Douglas S D. J Neuroimmunol. 1998;82:126–132. doi: 10.1016/s0165-5728(97)00175-6. [DOI] [PubMed] [Google Scholar]
- 39.Dickerson C, Undem B, Bullock B, Winchurch R A. J Leukocyte Biol. 1998;63:602–605. doi: 10.1002/jlb.63.5.602. [DOI] [PubMed] [Google Scholar]
- 40.Lee H R, Ho W Z, Douglas S D. Clin Diagn Lab Immunol. 1994;1:419–423. doi: 10.1128/cdli.1.4.419-423.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wahl S M, Greenwell-Wild T, Peng G, Hale-Donze H, Doherty T M, Mizel D, Orenstein J M. Proc Natl Acad Sci USA. 1998;95:12574–12579. doi: 10.1073/pnas.95.21.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patterson B K, Czerniewski M, Andersson J, Sullivan Y, Su F, Jiyamapa D, Burki Z, Landay A. Clin Immunol. 1999;91:254–262. doi: 10.1006/clim.1999.4713. [DOI] [PubMed] [Google Scholar]