Abstract
The purpose of the present work was to design and optimize floating drug delivery systems of acyclovir using psyllium husk and hydroxypropylmethylcellulose K4M as the polymers and sodium bicarbonate as a gas generating agent. The tablets were prepared by wet granulation method. A 32 full factorial design was used for optimization of drug release profile. The amount of psyllium husk (X1) and hydroxypropylmethylcellulose K4M (X2) were selected as independent variables. The times required for 50% (t50%) and 70% (t70%) drug dissolution were selected as dependent variables. All the designed nine batches of formulations were evaluated for hardness, friability, weight variation, drug content uniformity, swelling index, in vitro buoyancy, and in vitro drug release profile. All formulations had floating lag time below 3 min and constantly floated on dissolution medium for more than 24 h. Validity of the developed polynomial equation was verified by designing two check point formulations (C1 and C2). The closeness of predicted and observed values for t50% and t70% indicates validity of derived equations for the dependent variables. These studies indicated that the proper balance between psyllium husk and hydroxypropylmethylcellulose K4M can produce a drug dissolution profile similar to the predicted dissolution profile. The optimized formulations followed Higuchi's kinetics while the drug release mechanism was found to be anomalous type, controlled by diffusion through the swollen matrix.
Keywords: Acyclovir, factorial design, gastro retentive drug delivery, hydroxypropylmethylcellulose, psyllium husk
Floating drug delivery systems (FDDS) have been developed for drugs that act either locally in the stomach or absorbed from it, for those drugs that are either poorly soluble at alkaline pH or unstable in the intestinal or colonic environment[1]. These systems help in completely releasing the drug in the vicinity of the absorption window to enhance bioavailability. Several approaches that are currently made to prolong gastric retention time include, floating drug delivery systems, swelling and expanding systems, bioadhesive systems, high density systems and other delayed gastric emptying devices[2–5].
Acyclovir is a guanine analogue used in the treatment of viral diseases. The reported oral bioavailability is 10-20% with a plasma elimination half life of 1-2 h[6]. Acyclovir has its absorption window in the duodenum and small intestine[7]. After per oral administration, only 20% of the drug is absorbed with the remaining 80% of the drug excreted in the feces. After repeated per oral dosing of small amounts of acyclovir the bioavailability can be enhanced[8,9]. These facts indicate that increasing gastric residence time may enhance bioavailability of acyclovir. Therefore, acyclovir was selected as a model drug for the design of a FDDS with a view to improve its oral bioavailability.
Belgamwar and Surana[10] formulated and optimized floating bioadhesive drug delivery systems using psyllium husk and hydroxypropylmethylcellulose (HPMC) K15M for retarding the release and crossprovidone as a swelling agent. In vitro drug release was found to have followed the Higuchi kinetics and release mechanism to be non-Fickian type. Rao et al.[11] developed and optimized FDDS of cephalexin by using HPMC K4M, xanthan gum, guar gum, sodium bicarbonate and tartaric acid. In this study, influence of independent variables like polymer, polymer ratio, polymer type and tartaric acid on floating lag time and cephalexin release profile were studied. The mechanism of drug release for optimized formulation was found to be anomalous type. Prajapati et al.[12] studied the formulation and in vitro evaluation of floating matrix tablets of domperidone by using different combinations of HPMC K4M, Carbopol 934P and sodium alginate. Carbopol 934P showed negative effect on floating properties but was found to be helpful to control the release rate of drug.
In the present work, it was attempted to formulate FDDS of acyclovir using HPMC K4M and psyllium husk as the polymers and sodium bicarbonate as gas generating agent, in order to deliver the drug at a controlled rate to its absorption site so that its oral bioavailability can be enhanced. After preliminary studies, optimization of designed FDDS was performed using 32 full factorial design by conducting experiments to evaluate all the nine batches of acyclovir FDDS. The validity of the derived polynomial equations for the dependent variables (dissolution parameters) was verified by designing and evaluating two extra check point formulations.
MATERIALS AND METHODS
Acyclovir and psyllium husk were gift samples from M/s Modern Laboratories Pvt. Ltd., Indore, India. HPMC K4M was obtained as a gift sample from Colorcon Asia Pvt. Ltd., Goa, India. Microcrystalline cellulose (MCC) was gifted by Motiff Labs., Goa, India. Polyvinyl pyrolidone K30 (PVP K30) and sodium bicarbonate were purchased from S. D. Fine Chemicals, Mumbai, India. All other ingredients used throughout the study were of analytical grade and were used as received.
Full factorial design:
The factorial design is a technique that allows identification of factors involved in a process and assesses their relative importance. In addition, any interaction between factors chosen can be identified. Construction of a factorial design involves the selection of parameters and the choice of responses. Optimization has been done by using 32 full factorial design, where amount of psyllium husk (X1) and amount of HPMC K4M (X2) were taken as independent variables and the time required for 50% (t50%) and 70% (t70%) drug dissolution as dependent variables. Step-wise backward linear regression analysis was used to develop polynomial equations for the dependent variables t50%and t70% values by using PCP Disso 2000 V3 software. The validity of the developed polynomial regression equations was verified by preparing two check point formulations (C1 and C2)[13,14].
Preparation of acyclovir floating drug delivery system:
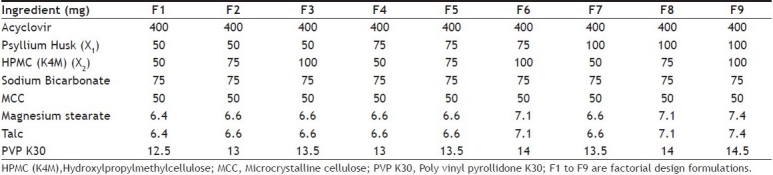
The tablets of acyclovir were prepared by wet granulation method by using psyllium husk and HPMC K4M as the matrix forming polymers, sodium bicarbonate as a gas generating agent and PVP K30 as a binding agent. Each formulation was composed of drug and excipients in various proportions as shown in Table 1. For formulation of tablets, acyclovir, psyllium husk, HPMC K4M, sodium bicarbonate and MCC were sifted through mesh (# 40) and were collected in an octagonal blender and mixed well to get a uniform mixture. The paste of PVP K30 in isopropyl alcohol was used as a granulating agent. The prepared granules (40 mesh sieve) were dried in a conventional hot air oven (Yorko India Pvt. Ltd., Mumbai, India) at 45° and dried granules were further passed through sieve (# 40). Magnesium stearate and talc (1%) were added as a lubricant and the granules were compressed into tablets using a single stroke tablet machine (Rimek, Ahmedabad, India).
TABLE 1.
COMPOSITION OF ACYCLOVIR FACTORIAL DESIGN FORMULATIONS
Hardness and friability test:
The crushing strength of tablets was determined by using Monsanto type hardness tester (Rolex, Chandigarh, India). In all the cases, mean of six replicate determinations were taken. Friability was determined by weighing 10 tablets after dusting, placing them in the friabilator (Riche-Rich Pharma, Bangalore, India) and rotating the plastic cylinder vertically for 100 revolutions[3]. After dusting, the total remaining weight of the tablets was recorded and the percent friability (PF) was calculated using the formula, PF= [(weightinitial–weight final)/weightinitial]×100.
Uniformity of weight and drug content:
Uniformity of weight was determined by dusting of each tablet and placing in the electronic balance (Shimadzu BL-220H, Japan). The weight data from the tablets were analyzed for sample mean and percent deviation. Uniformity of drug content was determined by taking 5 tablets in a glass mortar and powdered, 100 mg of this powder was placed in a 100 ml stoppered conical flask. The drug was extracted with 0.1 N HCl and shaking vigorously on a mechanical gyratory shaker (100 rpm) for 5 h, filtered into 50 ml volumetric flask through cotton wool and filtrate was made up to the mark by passing more 0.1 N HCl through filter. Further appropriate dilution were made and absorbance was measured at 256 nm using 0.1 N HCl as blank solution by UV/Vis double beam spectrophotometer (UV-1800, Shimadzu, Japan).
In vitro buoyancy study:
The in vitro buoyancy was characterized by floating lag time and total floating time. The test was performed using a USP XXIII type 2 dissolution test apparatus (Electrolab, India) using 900 ml of 0.1 N HCl at paddle rotation of 50 rpm at 37°±0.5°. The time required for the tablet to rise to the surface of the dissolution medium and the duration of time, the tablet constantly floated on the dissolution medium were noted as floating lag time and floating time, respectively (n=3).
Swelling index:
The individual tablets were weighted accurately and kept in 50 ml of water. Tablets were taken out carefully after 60 min blotted with filter paper to remove the water present on the surface and weighed accurately. Percentage swelling index (SI) was calculated by using the formula[15], SI = [(wet weight–dry weight)/dry weight]×100
In vitro dissolution study:
The in vitro dissolution studies of FDDS of acyclovir were carried out in USP XXIII type 2 dissolution test apparatus, employing a paddle stirrer at 50 rpm using 900 ml of 0.1 N HCl as dissolution medium. At predetermined time intervals, 5 ml of the samples were withdrawn by means of a syringe fitted with a prefilter. The volume withdrawn at each interval was replaced with same quantity of fresh dissolution medium maintained at 37±0.5°. The samples were analyzed for drug release by measuring the absorbance at 256 nm using UV/Vis double beam spectrophotometer after suitable dilutions. The determinations were performed in triplicate.
Kinetic modeling of drug release:
The dissolution profile of all the batches was fitted to zero-order (Eqn. 1), first-order (Eqn. 2), Higuchi (Eqn. 3) and Korsemeyer-peppas (Eqn. 4) models to ascertain the kinetic modeling of drug release[16–19].
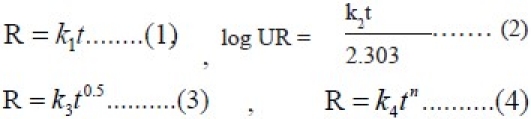
where R and UR are the released and unreleased percentages, respectively, at time (t), k1, k2, k3, and k4, are the rate constants of zero order, first order, Higuchi matrix, and Peppas-Korsmeyer model, respectively.
RESULTS AND DISCUSSION
The prepared FDDS tablets were evaluated for hardness, friability, uniformity of weight, uniformity of drug content, swelling index, floating lag time, in vitro buoyancy behavior, in vitro dissolution, short-term stability and drug-polymer interaction. The evaluation data is shown in Table 2. The hardness of the prepared FDDS of acyclovir was found to be in the range of 4.42 to 4.72 kg/cm2. The friability of all tablets was less than 1% i.e., in the range of 0.53 to 0.68%. The percentage deviation from the mean weights of all the batches of prepared FDDS was found to be within the prescribed limits. The low values of standard deviation indicate uniform drug content in all the batches (Table 2).
TABLE 2.
EVALUATION OF ACYCLOVIR FACTORIAL DESIGN FORMULATIONS
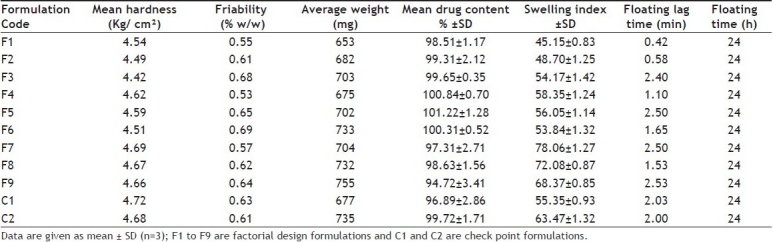
The floating lag time and floating time was noted visually. When an optimum concentration of gas generating agent sodium bicarbonate (75 mg/tablet) was used, the floating lag time was found to be in between 0.42–2.53 min with a floating time of 24 h (Table 2). It was observed that the floating lag time of formulation F9 was maximum which may be due to high drug polymer ratio among all formulations. Results were found in agreement with the earlier studies that the batches without HPMC and gum alone, failed to form a gel with sufficient strength, while tablets with HPMC K4M produced tablets with good gel strength, entrapping carbon dioxide gas and imparts stable and persistent buoyancy[20].
The swelling index of factorial design formulations F1 to F9 were found to be in the range of 45.15–78.06 (Table 2). The swelling index was found to be maximum for F7. The swelling index of the formulation increases with an increase in the concentration of psyllium husk. Cumulative percent drug release of factorial design formulation F1 to F9 in 10 h were found to be in the range of 52.70 to 102.42% (fig. 1). Formulation F2 containing psyllium husk (50 mg) and HPMC K4M (75 mg) showed promising dissolution parameters (t50% = 1.55 h, t70% = 5.30 h and t90% = 8.35 h) with desired floating properties. From the result it was clear that the release rate was higher for formulation containing low level of HPMC K4M or psyllium husk compared with other formulations containing higher level. This may be owing to the release retarding effect of HPMC K4M and psyllium husk at higher concentration because drug may have entrapped within a polymer matrix causing a decrease in the rate of drug release. Therefore, required release rate of drug can be obtained by manipulating the composition of HPMC K4M and psyllium husk.
Fig. 1.
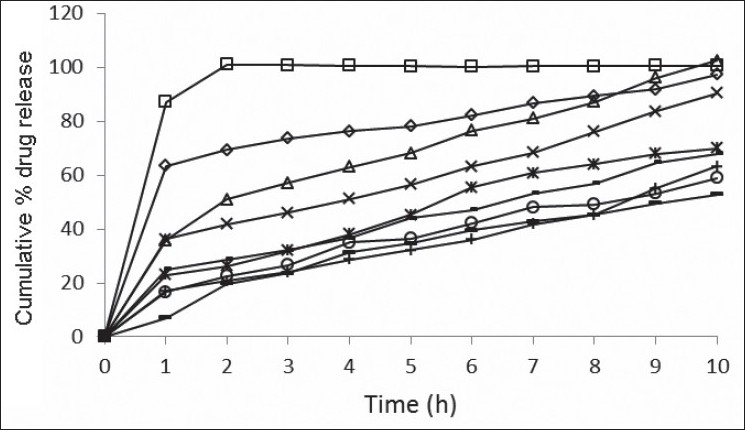
In vitro drug release studies of factorial F1 to F9 formulations Release profi les of acyclovir from formulations containing varying psyllium husk: HPMCK4M content; (—□—) F1, 50 mg:50 mg; (—Δ—) F2, 50 mg:75 mg; (—-—) F3, 50 mg:100 mg; (—◊—) F4, 75 mg:50 mg; (—×—) F5, 75 mg:75 mg; (—○—) F6, 75 mg:100 mg; (—*—) F7, 100 mg:50 mg; (—-—) F8, 100 mg:75 mg; (—+—) F9, 100 mg:100 mg. Each data represents mean±SD (n=3).
The results found supports the earlier data that the release profile was biphasic with initial burst effect followed by a polymer controlled slow release in the second phase. The difference in burst effect of the initial time is a result of the difference in the viscosity of the polymeric mixtures[21]. Dortunc and Gunal have reported that increased viscosity resulted in a corresponding decrease in the drug release, which might be due to the result of thicker gel layer formulation[22]. Our data were in good agreement with the studies of Belgamwar and Surana[10], Rao et al.[11] and Prajapati et al.[12].
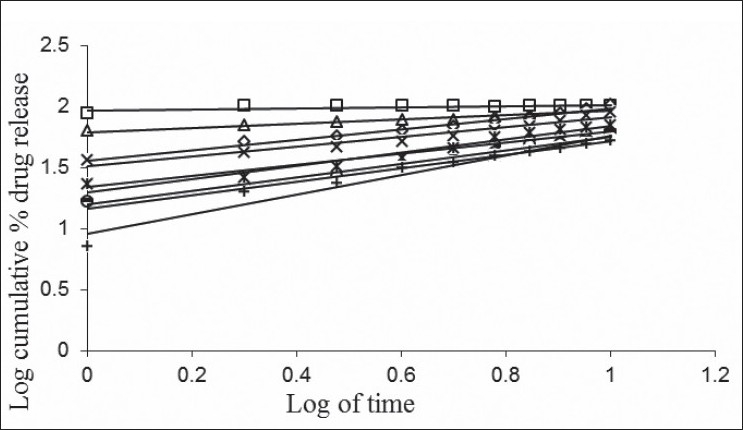
The in vitro dissolution data of acyclovir FDDS was subjected to goodness of fit test by linear regression analysis according to zero order and first order kinetic equations, Higuchi's and Korsmeyer-Peppas models to ascertain the mechanism of drug release. The results of linear regression analysis including regression coefficients are summarized in Table 3and plots shown in figs. 1] to 3. It was observed from the above data that all formulations have displayed zero order release kinetics (r values are in the range of 0.9714 to 0.9333). The values of r of factorial formulations for Higuchi's equation was found to be in the range of 0.95 to 0.99, which shows that the data fitted well to Higuchi's square root of time equation confirming the release followed diffusion mechanism. Kinetic data also treated for Peppas equation, the slope (n) values ranges from 0.432 to 0.800 (except for formulation F1 = 0.213) that shows non-Fickian diffusion mechanism.
TABLE 3.
REGRESSION ANALYSIS DATA OF 32 FACTORIAL DESIGN FORMULATIONS OF ACYCLOVIR
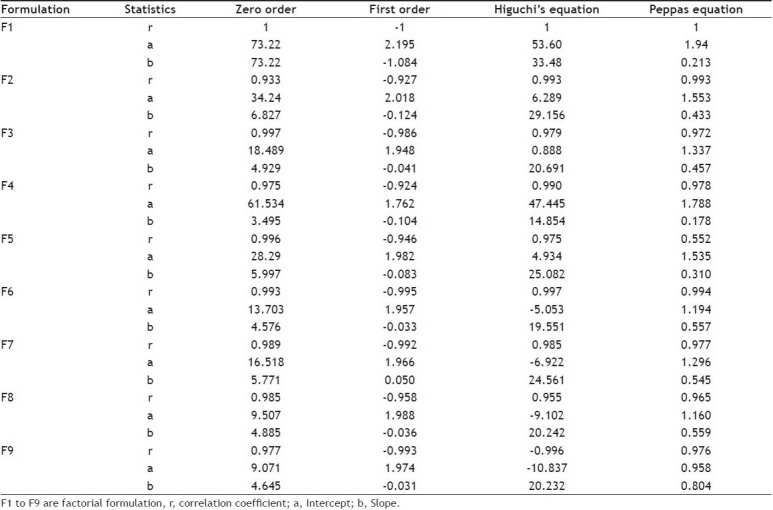
Fig. 3.
Log cumulative percent drug released Vs Log time (Peppas plots) of F1 to F9 formulations.
Peppas plots for F1 to F9 formulations containing varying psylliumhusk:HPMCK4M content; (—□—) F1, 50 mg:50 mg; (—Δ—) F2, 50 mg:75 mg; (—-—) F3, 50 mg:100 mg; (—◊—) F4, 75 mg:50 mg; (—×—) F5, 75 mg:75 mg; (—○—) F6, 75 mg:100 mg; (—*—) F7, 100 mg:50 mg; (—-—) F8, 100 mg:75 mg; (—+—) F9, 100 mg:100 mg. Each data point represents mean ±SD(n=3).
Fig. 2.
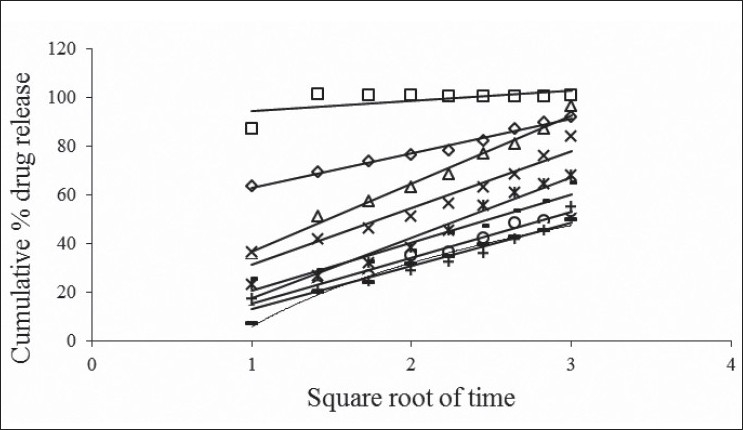
Cumulative percent drug released Vs square root of time (Higuchi's plots) of F1 to F9 formulations.
Higuchi's plots for F1 to F9 formulations containing varying psyllium husk:HPMCK4M content; (—□—) F1, 50 mg:50 mg; (—Δ—) F2, 50 mg:75 mg; (—-—) F3, 50 mg:100 mg; (—◊—) F4, 75 mg:50 mg; (—×—) F5, 75 mg:75 mg; (—○—) F6, 75 mg:100 mg; (—*—) F7, 100 mg:50 mg; (—-—) F8, 100 mg:75 mg; (—+—) F9, 100 mg:100 mg. Each data point represents mean ±SD (n=3).
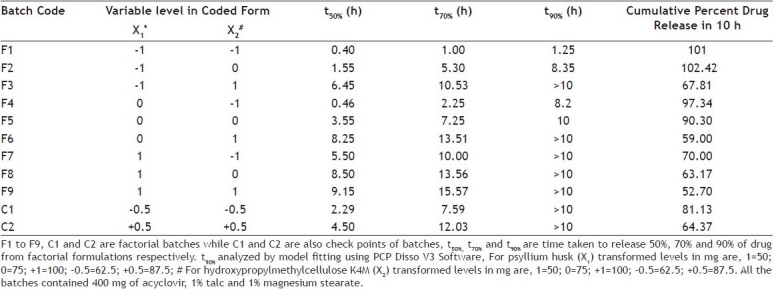
Formulation optimization[23,24] has been done by using 32 full factorial design to determine the effect of amount of psyllium husk (X1) and amount of HPMC K4M (X2) on independent variables t50%and t70%. Polynomial equations were derived for t50% and t70% values by backward stepwise linear regression analysis using PCP Disso 2000 V3 software. Validity of the derived equations was verified by preparing two check point formulations of intermediate concentration (C1 and C2). Factorial design batches of acyclovir FDDS and coded values and actual values for the independent variables given in Table 4] and 5, respectively. The dissolution data of factorial formulations F1 to F9 are shown in Table 6. Polynomial equation for 32 full factorial designs is given in Eqn. 5, Y = b0+b1 X1+b2 X2+b12 X1 X2+b11 X12+b22 X22…(5), where, Y is dependent variable, b0 arithmetic mean response of nine batches, and b1 estimated coefficient for factor X1. The main effects (X1 and X2) represent the average result of changing one factor at a time from its low to high value. The interaction term (X1 X2) shows how the response changes when two factors are simultaneously changed. The polynomial terms (X12 and X22) are included to investigate non-linearity. The equations for t50% and t70% developed as follows, Y1 =4.94+2.392X1+2.915 X2..(6) and Y2=8.774+3.817X1+4.493 X2...(7), respectively. The positive sign for coefficient of X2in Y1and Y2 equations indicates that as the concentration of HPMC K4M increases, t50%and t70% value increases.
TABLE 4.
FACTORIAL DESIGN BATCHES OF ACYCLOVIR FDDS

TABLE 5.
CODED VALUES AND ACTUAL VALUES FOR THE INDEPENDENT VARIABLES
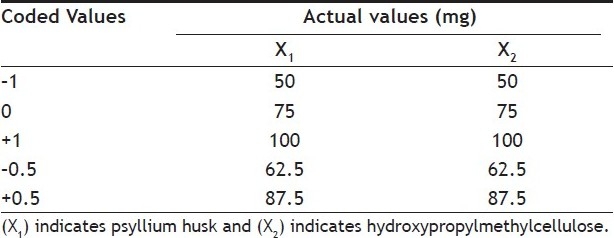
TABLE 6.
DISSOLUTION PARAMETERS FOR 32 FULL FACTORIAL DESIGN BATCHES
Validity of the above equations was verified by designing two check point formulations (C1 and C2) taking values of the independent variables for prefixed value of t50% and t70% and studying the drug release profiles. The dissolution parameters predicted from the equations derived and those observed from experimental results are summarized in the Table 7. The closeness of predicted and observed values for t50%and t70% indicates validity of derived equations for the dependent variables. Response surface plots are presented to show the effects of X1and X2on t50%and t70%. Good agreement was observed between the predicted and observed data of dissolution profiles is also a reflection of good applicability of the selected formulation. The computer generated (using ‘PCP Disso 2000 V3 software) response surfaces graph for the effect of factorial variables on t50%and t70% are shown in figs 4] and 5, respectively. The data demonstrate that both X1(amount of psyllium husk) and X2(amount of HPMC K4M) affect the drug release (t50%and t70%). As the amount of the polymer in the formulations increase, the drug release rate decreases. It can be concluded that the drug release pattern may be changed by appropriate selection of the X1 and X2 levels.
TABLE 7.
DISSOLUTION PARAMETERS FOR PREDICTED AND OBSERVED VALUES
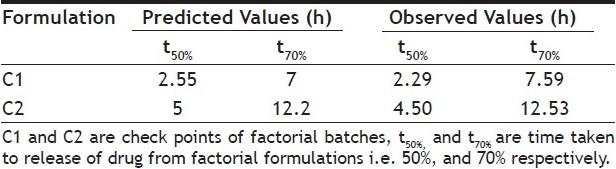
Fig. 4.
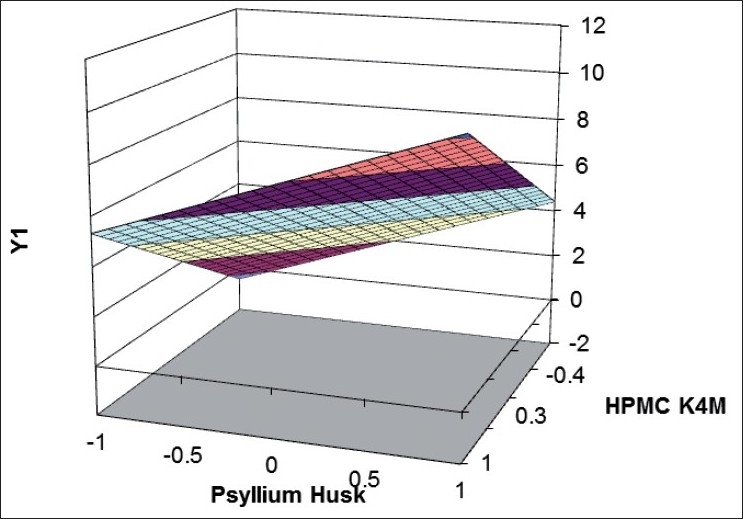
Response surface plot showing effect of factorial variables on t50%.
HPMC indicates hydroxypropylmethylcellulose; Y1 indicates dissolution data at t70%. Time taken to dissolve 50% of drug from factorial formulations (t50%) were ( ) 0-2; (
) 0-2; ( ) 2-4; (
) 2-4; ( ) 4-6; (
) 4-6; ( ) 6-8; (
) 6-8; ( ) 8-10; (
) 8-10; ( ) 10-12.
) 10-12.
Fig. 5.
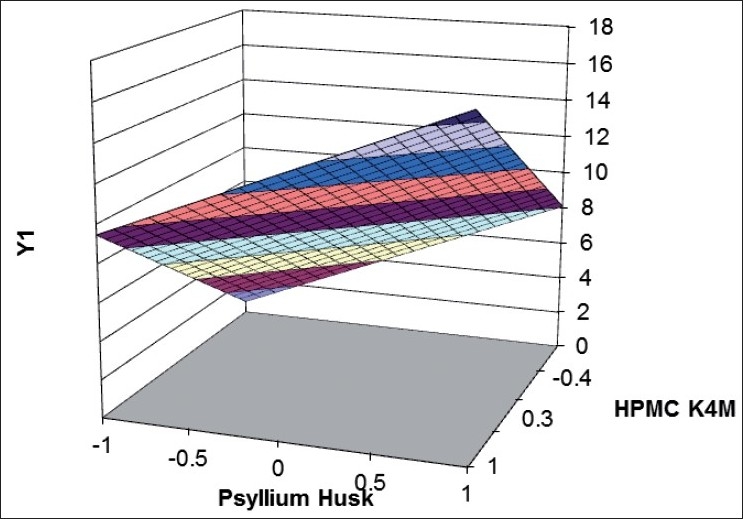
Response surface plot showing effect of factorial variables on t70%.
HPMC indicates hydroxypropylmethylcellulose; Y1 indicates dissolution data at t70%. Time taken to dissolve 70% of drug from factorial formulations (t50%) were ( ) 0-2; (
) 0-2; ( ) 2-4; (
) 2-4; ( ) 4-6; (
) 4-6; ( ) 6-8; (
) 6-8; ( )8-10; (
)8-10; ( ) 10-12; (
) 10-12; ( )12-14; (
)12-14; ( ) 14-16; (
) 14-16; ( ) 16-18.
) 16-18.
This study reflected that the hydrophilic polymer such as HPMC K4M and psyllium husk plays an important role for the formulation of FDDS. As the amount of the polymer in the formulations increase, the drug release rate decreases. The optimized formulations followed Higuchi's kinetics while the drug release mechanism was found to be anomalous type, controlled by diffusion through the swollen matrix. Both the polymers can be used in combination and do not interact with the drug. On the basis of evaluation parameters, the optimized formulation F2 may be used once a day administration in the management of viral diseases.
Acknowledgments
The authors would like to thank Modern laboratories, Indore, India for providing funding support for the project.
Footnotes
Kharia, et al.: Floating Drug Delivery System of Acyclovir
REFERENCES
- 1.Patil JM, Hirlekar RS, Gide PS, Kadam VJ. Trends in floating drug delivery systems. J Sci Ind Res. 2006;65:11–21. [Google Scholar]
- 2.Kumar R, Philip A. Gastroretentive dosage forms for prolonging gastric residence time. Int J Pharm Med. 2007;21:157–71. [Google Scholar]
- 3.Arora S, Ali J, Ahuja A, Khar RK, Baboota S. Floating drug delivery systems: A Review. AAPS PharmSciTech. 2005;06:E372–90. doi: 10.1208/pt060347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain SK, Awasthi AM, Jain NK, Agrawal GP. Calcium Silicate Based Microspheres of Repaglinide for Gastroretentive Floating Drug Delivery: Preparation and in vitro Characterization. J Control Release. 2005;107:300–9. doi: 10.1016/j.jconrel.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Jain SK, Agrawal GP, Jain NK. Evaluation of porous carrier-based floating microspheres for gastric delivery. AAPS PharmSciTech. 2006;7:E1–7. doi: 10.1208/pt070490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sweetman SC, editor. Martindale: The Complete Drug Reference. 33rd ed. London: Pharmaceutical Press; 2002. p. 612. [Google Scholar]
- 7.Groning R, Berntgen M, Geogarakis M. Acyclovir serum concentrations following peroral administration of magnetic depot tablets and the influence of extra corporal magnet to control gastrointestinal transit. Eur J Pharm Biopharm. 1996;46:285–91. doi: 10.1016/s0939-6411(98)00052-6. [DOI] [PubMed] [Google Scholar]
- 8.Lewis L, Fowle A, Bittiner S, Bye A, Isaacs P. Human gastrointestinal absorption of acyclovir from tablet, duodenal infusion and sipped solution. Br J Clin Pharmacol. 1986;21:459–62. doi: 10.1111/j.1365-2125.1986.tb05223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vergin H, Kikuta C, Mascher H, Metz R. Pharmacokinetics and bioavailability of different formulations of acyclovir. Arzneim Forsch. 1995;45:508–15. [PubMed] [Google Scholar]
- 10.Belgamwar VS, Surana SJ. Floating bioadhesive drug delivery system using novel effervescent agents. Asian J Pharm. 2009;2:156–60. [Google Scholar]
- 11.Rao BP, Kottan NA, Snehith VS, Ramesh C. Development of gastroretentive drug delivery system of cephalexin by using factorial design. Ars Pharma. 2009;50:8–23. [Google Scholar]
- 12.Prajapati ST, Patel LD, Patel DM. Studies on formulation and in vitro evaluation of floating matrix tablet of domperidone. Indian J Pharm Sci. 2009;71:19–23. doi: 10.4103/0250-474X.51944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shoufeng L, Senshang L, Chein YW, Daggy BP, Mirchandani HL. Statistical optimization of gastric floating system for oral controlled delivery of calcium. AAPS PharmSciTech. 2001;2:1–12. doi: 10.1208/pt020101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gohel MC, Parikh RK, Amin AF, Surati AK. Preparation and formulation optimization of sugar crosslinked gelatin microspheres of diclofenac sodium. Indian J Pharm Sci. 2005;67:575–81. [Google Scholar]
- 15.Chavanpatil MD, Jain P, Chaudhari S, Shear R, Vavia PR. Development of sustained release gastrorentantive drug delivery system for ofl oxacin: in vitro and in vivo evaluation. Int J Pharm. 2005;304:178–84. doi: 10.1016/j.ijpharm.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Siber BM, Bialer M, Yacobi A. Controlled drug delivery fundamentals and applications. In: Robinson JR, Lee VHL, editors. 22nd ed. New York: Marcel Dekker Inc; 2005. pp. 213–51. [Google Scholar]
- 17.Notari RE. Biopharmaceutics and clinical pharmacokinetics. 2nd ed. New York: Marcel Dekker Inc; 1987. pp. 6–21. [Google Scholar]
- 18.Higuchi T. Mechanism of sustained-action medication.Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci. 1963;51:1145–9. doi: 10.1002/jps.2600521210. [DOI] [PubMed] [Google Scholar]
- 19.Peppas NA. Analysis of Fickian and non-Fickian drug release from polymers. Pharm Acta Helv. 1985;60:110–1. [PubMed] [Google Scholar]
- 20.Dave BS, Amin AE, Patel MM. Gastroretentive drug delivery system of ranitidine HCl: Formulation and in vitro evaluation. AAPS PharmSciTech. 2004;5:E34. doi: 10.1208/pt050234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel VF, Patel NM. Intragastric floating drug delivery system of cefuroxime axetil: in vitro evaluation. AAPS PharmSciTech. 2006;7:E17. doi: 10.1208/pt070233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dortunc B, Gunal N. Release of acetazolamide from swellable Hydroxypropylmethylcellulose matrix tablets. Drug Dev Ind Pharm. 1997;23:1245–9. [Google Scholar]
- 23.Bolton S, Bon C. Pharmaceutical statistics: Practical and clinical applications. 4th ed. New York: Marcel Dekker Inc; 2004. pp. 506–39. [Google Scholar]
- 24.Schwartz JB, Connor RE, Schnaare RL. Optimization techniques in pharmaceutical formulation and processing. In: Banker GS, Rhodes CT, editors. Modern pharmaceutics. New York: Marcel Dekker Inc; 2002. pp. 607–26. [Google Scholar]


