Abstract
Thiazolidin-4-one fused pyrimidines, [1,5]-benzodiazepines and their oxygen substituted hydroxylamine derivatives have been screened for antibacterial, antifungal and antimalarial activity. Bacillus subtilis, Escherichia coli, Proteus mirabilis and Salmonella typhi were used for antibacterial screening. Aspergillus fumigatus and Candida albicans were used for antifungal screening and Plasmodium species were used for antimalarial screening. The antibacterial and antifungal activities are expressed in terms of zone of inhibition and antimalarial activity is expressed in IC50 value. Fifteen compounds 2Xa, 2Xb, 2Xc, 2Xs, 3IV, 3Va, 3Vc, 3VIIIa, 3VIIIh, 3IXa, 3IXb, 3IXc, 3Xa, 4IXa and 4Xa were tested for antibacterial as well as antifungal activity and seven compounds 2IXb, 2Xb, 3VIIIc, 3Xc, 4IXa, 4Xa and 4IXw were tested for antimalarial activity. Streptomycin, griseofulvin and chloroquine were taken as standard drugs in antibacterial, antifungal and antimalarial activity, respectively. The compound 2Xs was found significant antimicrobial against Bacillus subtilis, E. coli, Aspergillus fumigatus and Candida albicans as well as compound 3Xa was significant antimicrobial against Bacillus subtilis, E. coli, Salmonella typhi, Aspergillus fumigatus and Candida albicans. The compound 2Xb showed significant antimalarial activity.
Keywords: [1,5]-benzodiazepines; IC50 value; oxygen substituted hydroxylamine derivatives; pyrimidines; quantitative structure activity relationship (QSAR); Thiazolidin-4-ones; zone of inhibition
Literature survey revealed that thiazolidin-4-ones and their derivatives, which have been synthesized and screened for antimicrobial activity, are found biologically active with anticonvulsant[1], antitubercular[2], antifungal[3], local anesthetic/antiHIV[4], and antiinflammatory activity[5] with dual cyclooxygenase/lipooxygenase inhibition[6]. Literature survey also revealed that thiazolidin-4-ones have been used as new SHP-2[7], and protein tyrosine phosphatase-1B inhibitors[8]. Like thiazolidin-4-ones, pyrimidines also play a vital role in many biological processes and have potential anticancer, antiinflammatory and analgesic activities[9,10]. Some pyrimidines and their derivatives are found to possess antiHIV activity[11]. It has also been found that 2-phenylaminopyrimidines possess protein tyrosine kinase inhibitor activity[12].[1,5]-benzodiazepines are widely used as sedatives, sleep inducers, anesthetics, anticonvulsants, muscle relaxants and also as tranquilizers since 1960 when chlordiazepoxide was introduced as a tranquilizer.
Dilazep, a non-nucleoside reverse transcriptase inhibitor (NNRTI) has also been evaluated for the treatment of kidney disease such as chronic nephritis and its tablets (containing dilazep HCl, lactose, starch and cellulose-20:20:50:5) are used for improving hematopoiesis. Some arylpyridodiazepine and thidiazepine derivatives are highly selective HIV-1 inhibitors[13]. The antiarrhythmic activity of derivatives of dibenzazepine have been studied and found that the most active compounds are those in which carbethoxyamine groups in position-3 in combination with dimethylamino or diethylaminoacetyl groups in position-5 of dibenzazepine ring are present[14]. Literature survey revealed that hydroxylamines and their oxygen-substituted derivatives are reversible inhibitors of allinase. These are also potent inhibitors of aminobutyrate and aminotransferase in Pseudomonas.
The aminooxy compounds possess broad spectrum antibacterial activity. Aminooxy compounds have also been known for some time as potent inhibitors of pyridoxal-5-phosphate dependent enzymes such as aminotransferase, serine hydroxymethyltransferase, tyrosine decarboxylase, cystathionase and ornithine decarboxylase[15,16]. It is mostly true that even minor changes in structure of a molecule with appropriate substituent may change its pharmacological profile appreciably. Keeping in mind this concept, thiazolidin-4-one condensed pyrimidines, thiazolidin-4-one condensed [1,5]-benzodiazepines and their oxygen substituted hydroxylamine derivatives have been synthesized[17–19] and screened for antimicrobial activity to study the quantitative structure activity relationship between substituents present on the basic nucleus and activity alteration.
MATERIALS AND METHODS
Antibacterial activity:
Nutrient agar medium was used for culture of bacteria in which beef extract (3 g), peptone (5 g), sodium chloride (5 g) and agar-agar (15 g) were mixed in 1000 ml distilled water. The nutrient agar medium was sterilized by autoclaving at 15 psi and 121° for 20 min. The medium was poured in Petri dishes and left for some time to solidify. These Petri dishes were inoculated with 0.2 ml suspension of organisms using the cup-plate method[20]. Four wells were made in the medium with the help of a sterile borer and subsequently these wells were filled with four different concentrations (250, 500, 750 and 1000 ppm) of the synthesized compounds using well diffusion method[21]. Streptomycin (250, 500, 750 and 1000 ppm) was used as reference drug. The Petri dishes were incubated at 37° in an incubator and examined for the zone of inhibition[22] after 24-48 h. Bacillus subtilis (Org. 1), Escherichia coli (Org. 2), Proteus mirabilis (Org. 3) and Salmonella typhi (Org. 4) were taken for antibacterial activity.
Antifungal activity:
Sabouraud agar medium was used for culture of fungi in which glucose (20 g), peptone (10 g) and agar-agar (15 g) were mixed in 1000 ml distilled water. The procedure explained in the antibacterial activity was followed. Aspergillus fumigatus (Org. 5) and Candida albicans (Org. 6) were used for antifungal activity.
In vitro antimalarial activity:
Plasmodium falsiparum species were grown, adapted and maintained in vitro routinely. For conducting the experiment, the P. falciparum culture was synchronized to ring stage and diluted with fresh human erythrocytes to adjust the level of parasitaemia between 1000 to 80 000/΅l of blood. The in vitro assay of inhibition of schizont maturation was followed for screening the antimalarial activity of the compounds. The experiment was done in 24 well microtire plates in the presence of various concentrations of drugs followed by standard method[23,24]. The control wells were without drug. The growth was monitored after 24-36 h of culture when the parasites would have developed to schizont stage. Thick smear was prepared from each well. The schizont maturation in experimental well was compared with control well. Percent inhibition was calculated according to the following formula: % inhibition=100-(No. of schizonts or 200 asexual parasites in test well/ No. of schizonts or 200 asexual parasites in control well)×100.
In vivo antimalarial screening (Blood schizontocidal activity):
Healthy Swiss albino mice (4-6 weeks old) and P. berghei, sensitive to chloroquine, were used in the study. Peter's 4-day test[25] was followed to evaluate the blood schizontocidal action against P. berghei in Swiss albino mice. The animals were divided into groups consisting of 5 mice each. All the groups were injected P. berghei infected red blood cells in a volume of 0.1 ml (diluted in phosphate buffer solution, PBS) on day 0 intraperitoneally. All the experimental groups received the drugs in different concentration on day 0 to day 3. Control group received only PBS and one group was given chloroquine (IC50 value= 0.03 mg/ml) 3 mg/kg as a standard antimalarial drug. On day 4, thin blood smears were prepared from the tail vein to monitor the parasitaemia. Percent inhibition of the parasitaemia is calculated against the control group. The chloroquine recipients group was negative throughout.
RESULTS AND DISSCUSION
Results of antibacterial activity are summarized in Table 1. The zone of inhibition was measured in mm for each concentration. All of the screened compounds were found to have moderate to significant antibacterial activity. Compound 2Xa showed very significant activity against Bacillus subtilis and Proteus mirabilis but moderate activity against E. coli and Salmonella typhi. Compounds 2Xb, 2Xc and 2Xs were found to exhibit very significant activity against Bacillus subtilis and moderate activity against E. coli, Proteus mirabilis and Salmonella typhi. It was also observed that antibacterial activity was increased with introducing chloro group at para-position in phenylimino moiety but decreased with nitro group at same position. Antibacterial activity was also increased when number of carbon atoms increased in alkoxyphthalimide moiety. Compounds 3IV, 3Va, 3Vc, 3VIIIa, 3VIIIh, 3IXa, 3IXb and 3IXc showed significant antibacterial activity while compound 3Xa was found to show more significant antibacterial activity than standard drug. It was also observed that when alkoxyphthalimide group was introduced in thiazolidinone unit as well as number of carbon atoms was increased in alkoxyphthalimide group, the antibacterial activity was also increased. Introduction of arylidene unit at position-5 in thiazolidinone moiety also increases the activity but hydroxyl group at p-position in arylidene unit the decreases activity. Thiazolidinopyrimidines were found to show higher activity than 5-arylidene-4-thiazolidinones. Compounds 4IXa and 4Xa were exhibited moderate to significant activity against all of the organisms. Activity was also increased with introduction of alkoxyphthalimide group in the [1,5]-benzodiazepines.
TABLE 1.
ANTIBACTERIAL ACTIVITY
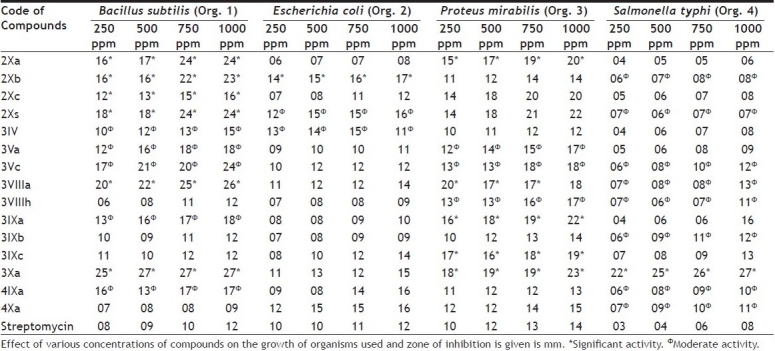
Results of antifungal activity are given in Table 2. The zone of inhibition was measured in mm for each concentration. All of the tested compounds were found to exhibit very significant antifungal activity against Aspergillus fumigatus and Candida albicans. Growth of mycelia was inhibited maximum at 1000 ppm while minimum at 250 ppm. Antifungal activity increased with introduction of chloro group at arylimino moiety whereas decreased when nitro group was introduced at the same position. Although all compounds were exhibited strong antifungal activity yet [1,5]-benzodiazepines were found to show lower activity than thiazolidinopyrimidines.
Table 2.
ANTIFUNGAL ACTIVITY
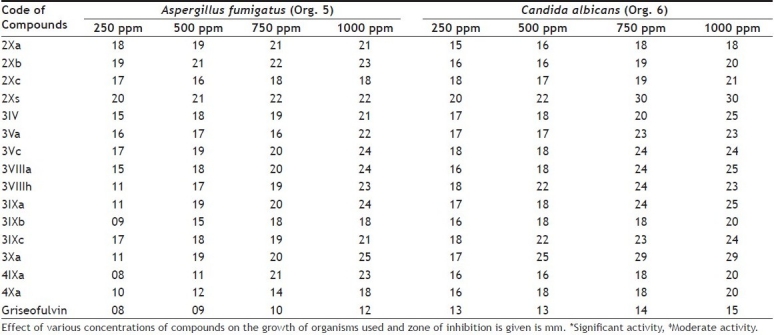
Results for antimalarial activity are mentioned in Table 3. Compounds 2Xb and 3Xc have been found to show significant antimalarial activity than the chloroquine. Other compounds have showed mild to moderate activity. It may further conclude that introduction of alkoxyphthalimide group in thiazolidinopyrimidine moiety increases to antimalarial activity. It was also observed that antimalarial activity was slightly increased on increasing length of alkyl chain in alkoxyphthalimide unit. Presence of chloro group at the para-position also increases to activity. Thiazolidinopyrimidines have been found to possess higher activity than 5-arylidene-4-thiazolidinones.
TABLE 3.
ANTIMALARIAL ACTIVITY
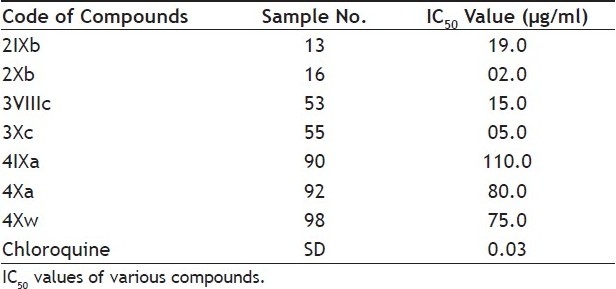
Alkoxyphathalimide derivative of thiazolidinop-yrimidines were found to have significant antifungal, antibacterial and antimalarial activity. Activity was increased with introduction of chloro group at the para-position in arylimino moiety and with increasing of length of alkyl chain in alkoxyphthalimide group whereas it is decreased when hydroxyl group was introduced. Structures of compounds 2Xa, 2Xb, 2Xc and 2Xs are shown in fig. 1. The antibacterial and antifungal activities were increased when the arylidene unit was introduced in thiazolidinone nucleus at position-5 but activity was decreased when hydroxyl group was present at para-position in arylidene unit. Structures of the compounds are shown in figs. 2 and 3. Compounds 2Xs, 3Xa and 4Xa were found to exhibit significant antimicrobial activities. These results suggest that antimicrobial activities are strongly affected by the presence or absense of alkoxyphthalimide moiety and hence compound 3Xa and 4Xa showed significant activity than compounds 3IXa and 4IXa (comparison in figs. 3] and 4). Although [1,5]-benzodiazepines have strong antibacterial and antifungal activity but it was found to show slightly lower activity than alkoxyphthalimide derivatives of thiazolidinopyrimidines.
Fig. 1.
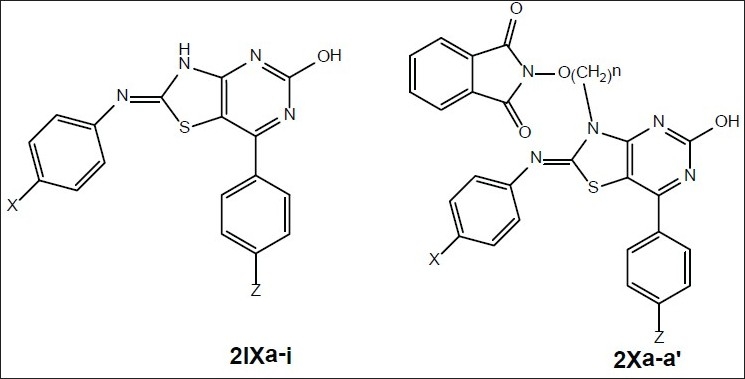
General structure of compounds synthesized[17] 2Xa (X=H, Z=H, n=2), 2Xb (X=Cl, Z=H, n=2), 2Xc (X=NO2, Z=H, n=2) and 2Xs (X=H, Z=H, n=4)
Fig. 2.
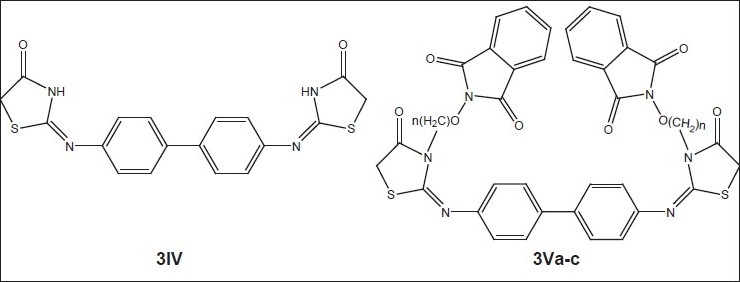
General structure of compounds synthesized[18] 3Va (n=2), 3Vb (n=2) and 3Vc (n=4)
Fig. 3.
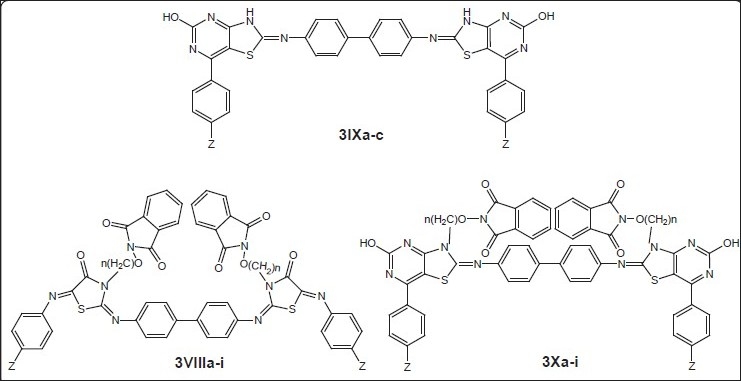
General structure of compounds synthesized[18] 3VIIIa (Z=H, n=2), 3VIIIh (Z=OH, n=4), 3IXa (Z=H), 3IXb (Z=OH),3IXc (Z=OCH3) and 3Xa (Z=H, n=2)
Fig. 4.
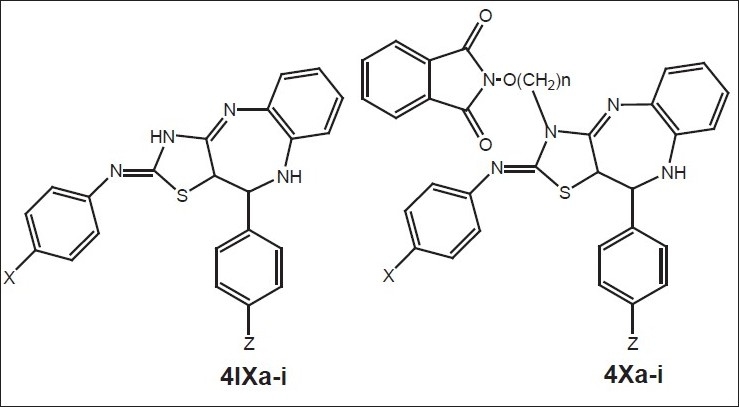
General structure of compounds synthesized[19] 4IXa (X=H, Z=H), 4Xa (X=H, Z=H, n=2) and 4Xw (X=Cl, Z=OH, n=4)
The compounds 2Xb, 2Xs, 2Vc and 2Xa were found to possess significant antibacterial activity while compounds 2Xa, 3Va, 3VIIIa, 3IXa, 3IXc, 4IXa and 4Xa showed moderate activity against Bacillus subtilis. The compound 2Xs possessed strong activity but other compounds showed moderate to good activity against E. coli. All of the examined compounds were found to show moderate activity against Proteus mirabilis. The compound 3Xa showed significant activity against Salmonella typhi whereas other compounds possessed moderate to good activity. All compounds were found to possess very strong activity against Aspergillus fumigatus and Candida albicans.
Based on the above study, it can be concluded that the compound 2Xs i.e. 7-N-(4-butoxyphthalimido)-2-hydroxy-4-phenyl-6-phenyliminothiazolidino[2,3-b]pyrimidine was found to have significant antimicrobial activity against Bacillus subtilis, E. coli, Aspergillus fumigatus and Candida albicans as well as the compound 3Xa i.e. p-Bis-(7-{2-ethoxyphthalimido)-2-hydroxy-4-phenyl-6-iminothiazolidino[2,3-b]pyrimidine-N2-yl)biphenyl was found to possess significant antimicrobial activity against Bacillus subtilis, E. coli, Salmonella typhi, Aspergillus fumigatus and Candida albicans. The compound 2Xb i.e. 7-N-(4-ethoxyphthalimido)-2-hydroxy-4-phenyl-6-(4-cholrophenyl)iminothiazolidino[2,3-b]pyrimidine was found to possess significant antimalarial activity fig. 5.
Fig. 5.
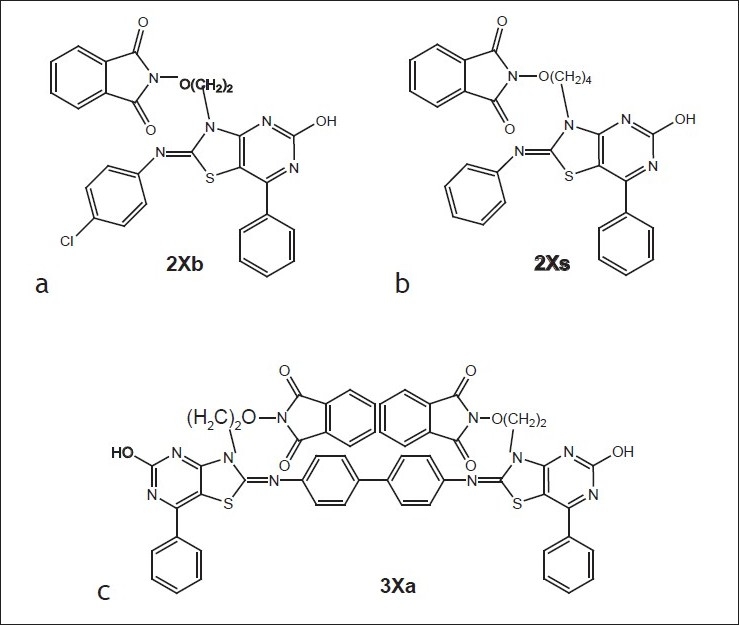
Compounds with signifi cant antimicrobial activity (a) Antimalarial compound, (b and c) antibacterial and antifungal compounds
Acknowledgments
The authors are thankful to the Head, Department of Chemistry, M. L. Sukhadia University, Udaipur (Rajasthan) for providing laboratory facilities to synthesize the compounds, to the Head, Department of Botany, M. L. Sukhadia University, Udaipur (Rajasthan) for providing laboratory facilities to screen the microbiological activity and Dr. C. Usha Devi, Scientist, Malaria Research Centre, Indian Council of Medical Research, Delhi for screening of antimalarial activity of the synthesized compounds.
Footnotes
Singh, et al.: Studies of antimicrobial activities of pyrimidines, [1,5]-benzodiazepines
REFERENCES
- 1.Aysel G, Nalan T. Synthesis and isolation of new regioisomeric 4-thiazolidinones and their anticonvulsant activity. Turk J Chem. 2005;29:247–54. [Google Scholar]
- 2.Khadse BG, Lokhande SR, Bhamaria RP, Prabhu SR. Synthesis and antitubercular activity of 4-(5-nitro-2-furyl/2-pyrazinyl/1-adomantyl)-2-aryl/alkyl/arylimino)thiazoles. Indian J Chem. 1987;26B:856–60. [Google Scholar]
- 3.Lakhan R, Singh RL. Synthesis and evaluation of 2-imino-3-(4-arylthiazol-2-y1)-4-thiazolidinones and their 5-arylidene derivatives as potential fungicidest. J Agric Chem. 1991;39:580–3. [Google Scholar]
- 4.Rawal RK, Tripathi R, Kulkarni S, Paranjape R, Katti SB, Pannecouque C, et al. 2-(2,6-Dihalo-phenyl)-3-heteroaryl-2-ylmethyl-1, 3-thiazolidin-4-ones: Anti-HIV agents. Chem Biol Drug Des. 2008;72:147–54. doi: 10.1111/j.1747-0285.2008.00683.x. [DOI] [PubMed] [Google Scholar]
- 5.Ottana R, Maccari R, Barreca ML, Bruno G, Rotondo A, Rossi A, et al. 5-Arylidene-2-imino-4-thiazolidinones: Design and synthesis of novel antiinflammatory agents. Bioorg Med Chem. 2005;13:4243–52. doi: 10.1016/j.bmc.2005.04.058. [DOI] [PubMed] [Google Scholar]
- 6.Geronikaki AA, Lagunin AA, Hadjipavlou-Litina DI, Eleftheriou PT, Filimonov DA, Poroikov VV, et al. Computer-aided discovery of anti-inflammatory thiazolidinones with dual cyclooxygenase/lipoxygenase inhibition. J Med Chem. 2008;51:1601–9. doi: 10.1021/jm701496h. [DOI] [PubMed] [Google Scholar]
- 7.Geronikaki A, Eleftheriou P, Vicini P, Alam I, Dixit A, Saxena AK. 2-Thiazolylimino/heteroarylimino-5-arylidene-4-thiazolidinones as new agents with SHP-2 inhibitory action. J Med Chem. 2008;51:5221–8. doi: 10.1021/jm8004306. [DOI] [PubMed] [Google Scholar]
- 8.Combs AP, Zhu W, Crawley ML, Glass B, Polam P, Sparks RB, et al. Potent benzimidazole sulfonamide protein tyrosine phosphatase 1B inhibitors containing the heterocyclic (S)-isothiazolidinone phosphotyrosine mimetic. J Med Chem. 2006;49:3774–89. doi: 10.1021/jm0600904. [DOI] [PubMed] [Google Scholar]
- 9.Sondhi SM, Johar M, Singhal N, Dastidar SG, Shukla R, Raghubir R. Synthesis and anticancer, antiinflammatory and analgesic activity evaluation of some sulfa drug and acridine derivatives. Monastsh Chem/Chemical Monthly. 2000;131:511–20. [Google Scholar]
- 10.Sondhi SM, Johar M, Rajvanshi S, Dastidar SG, Shukla R, Raghubir R, et al. anticancer, antiinflammatory and analgesic activity evaluation of heterocyclic compounds synthesized by the reaction of 4-isothiocyanato-4-methylpentan-2-one with substituted o-phenylenediamines, o-diaminopyridine. Aust J Chem. 2001;54:69–74. [Google Scholar]
- 11.Sondhi SM, Verma RP, Sharma VK, Singhal N, Kraus JL, Camplo M, et al. Synthesis and anti-HIV screening of some heterocyclic compounds. Phosphorus Sulfur Silicon. 1997;122:215–25. [Google Scholar]
- 12.Buchdunger E, Zimmermann J, Mett H, Meyer T, Müller M, Druker BJ, et al. Inhibition of the Abl protein-tyrosine kinase in vitro and in vivo by a 2-phenylaminopyrimidine derivative. Cancer Res. 1996;56:100–4. [PubMed] [Google Scholar]
- 13.Bellarosa D, Antonelli G, Bambacioni F, Giannotti D, Viti G, Nannicini R, et al. New arylpyrido-diazepine and thiodiazepine derivatives are potent and highly selective HIV-1 inhibitors targeted at the reverse transcriptase. Antiviral Res. 1996;30:109–24. doi: 10.1016/0166-3542(95)00906-x. [DOI] [PubMed] [Google Scholar]
- 14.Phillips OA, Murthy KS, Fiakpui CY, Knaus EE. Synthesis of 5-phenyl-10-methyl-7H- pyrimido[4,5-f][1,2,4]triazolo[4,3-a][1,4]diazepine and its evaluation as an anticonvulsant agent. Can J Chem. 1999;77:216–22. [Google Scholar]
- 15.Fuller AT, King H. Some alkoxy and alkylenedioxy-diamines and alkoxy and alkylenedioxy-diguanidines. J Chem Soc. 1947:963–9. doi: 10.1039/jr9470000963. [DOI] [PubMed] [Google Scholar]
- 16.Berger BJ. Antimalarial activities of amino-oxy compounds. Antimicrob Agents Chemother. 2000;44:2540–2. doi: 10.1128/aac.44.9.2540-2542.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh B, Mehta D, Baregama LK, Talesara GL. Synthesis and biological evaluation of 7-N-(n-alkoxyphthalimido)-2-hydroxy-4-aryl-6-aryliminothiazolidino [2,3-b]pyrimidines and related compounds. Indian J Chem. 2004;43:1306–13. [Google Scholar]
- 18.Singh B, Baregama LK, Sharma R, Ahmed M, Talesara GL. Synthesis of alkoxyphthalimide derivatives of p-bis- (2-hydroxy-4-aryl-6-iminothiazolidino [4,5-b]pyrimidin-N2-yl) biphenyls. J Indian Chem Soc. 2005;82:337–41. [Google Scholar]
- 19.Singh B, Baregama LK, Ahmed M, Dixit N, Talesara GL. Synthesis of 10-aryl-2-aryliminothiazolidino [4,5-b][1,5] benzodiazepines and their alkoxyphthalimide derivatives. Indian J Chem. 2005;44:1243–7. [Google Scholar]
- 20.Barry AL. Philadelphia: Illuslea and Febiger; 1976. The antimicrobial susceptibility tests: Principle and Practice; p. 180. [Google Scholar]
- 21.Santra SC, Chaterjee TP, Das AP. College Practical Botany. Vol. 2. Calcutta: New Central Book Agency; 1993. [Google Scholar]
- 22.Mukherjee PK, Balasubramanian P, Saha K, Saha BP, Pal M. Antibacterial Efficiency of Nelumbo nucifera (Nymphaeaceae) rhizomes extract. Indian Drugs. 1995;32:274–6. [Google Scholar]
- 23.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–5. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 24.In vitro microtest (Mark II) for the assessment of the response of Plasmodium flaciparum to chloroquine, mefloquine, quinine, sulfodoncine, pyrimethamine and amodiaquine. World Health Organization (WHO) 1990;MAP/87:1–21. [Google Scholar]
- 25.Peters W. Chemotherapy and drug resistance in malaria. 1980;Vol. 1:1133. [Google Scholar]


