Abstract
The syntheses are described of two types of linker molecule useful for the specific attachment of non-radioactive labels such as biotin and fluorophores to the 5' terminus of synthetic oligodeoxyribonucleotides. The linkers are designed such that they can be coupled to the oligonucleotide as a final step in solid-phase synthesis using commercial DNA synthesis machines. Increased sensitivity of biotin detection was possible using an anti-biotin hybridoma/peroxidase detection system.
Full text
PDF



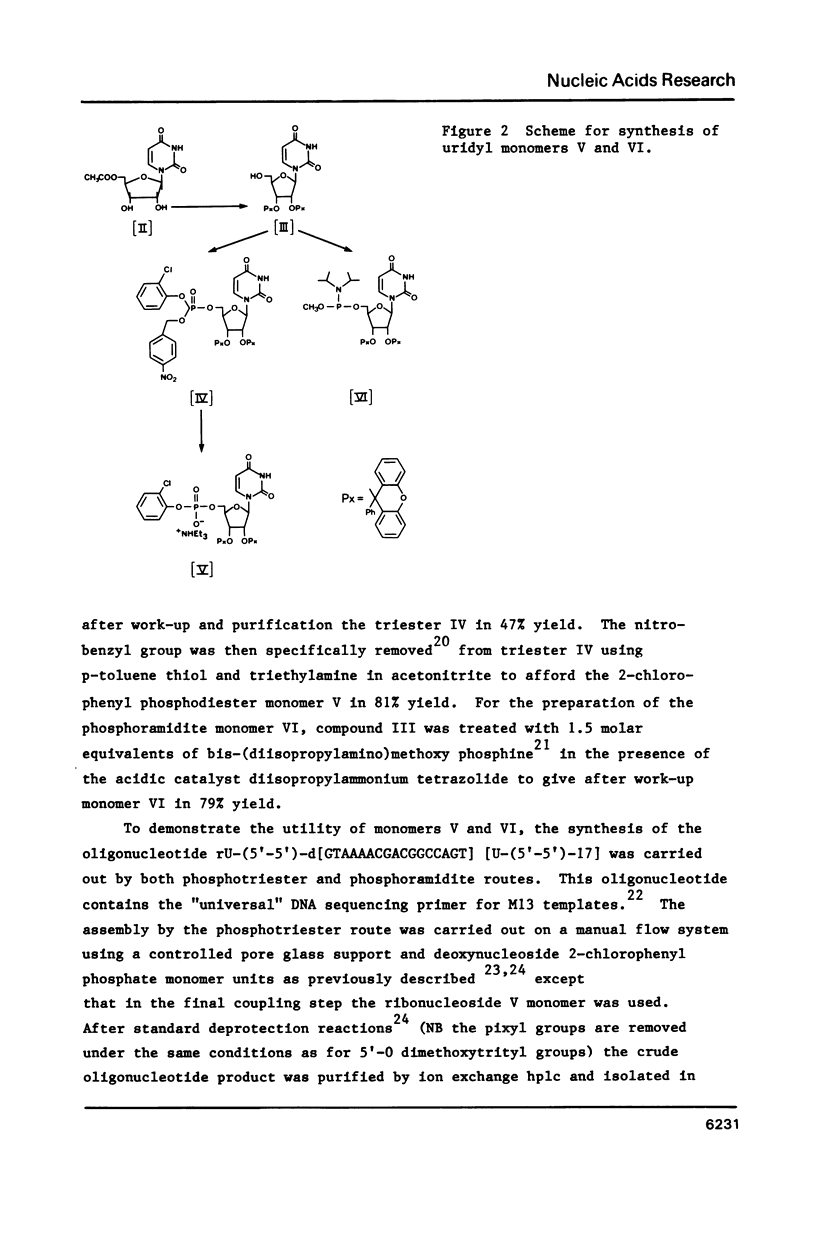
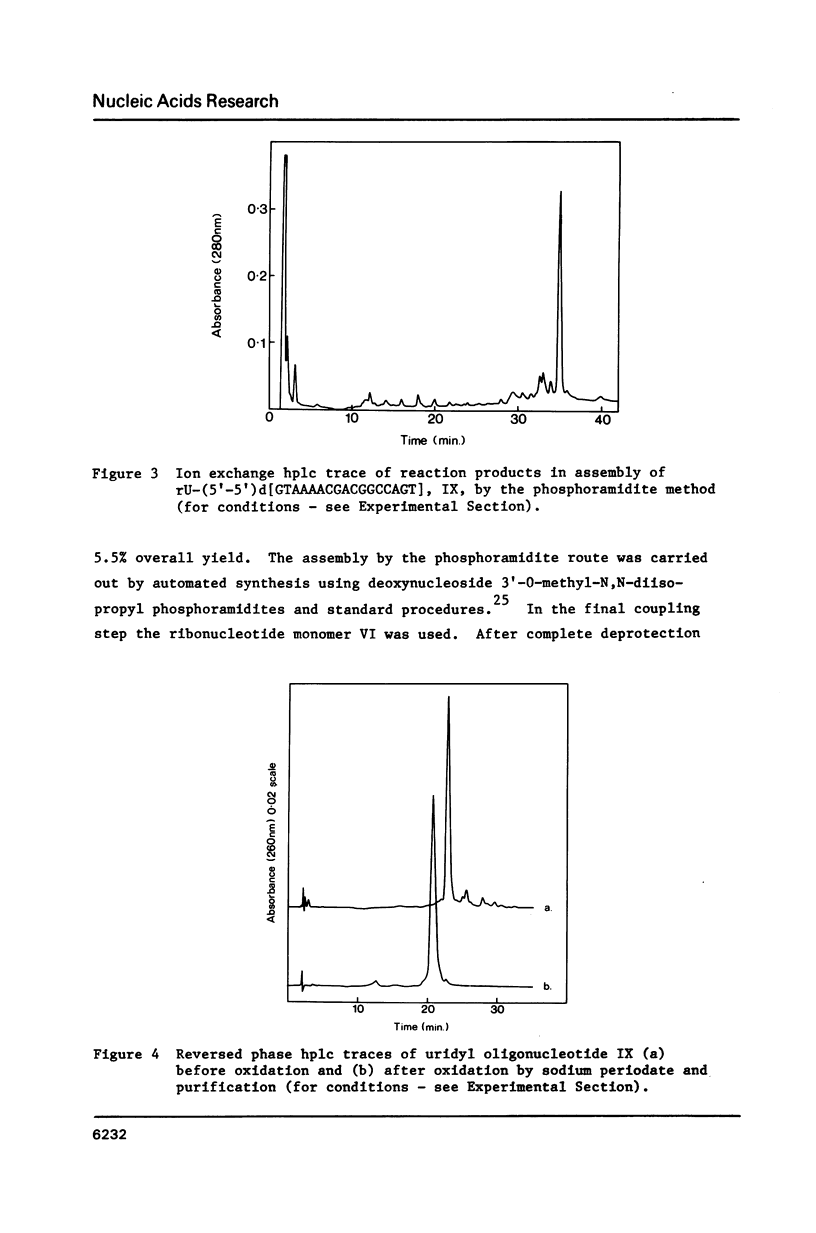
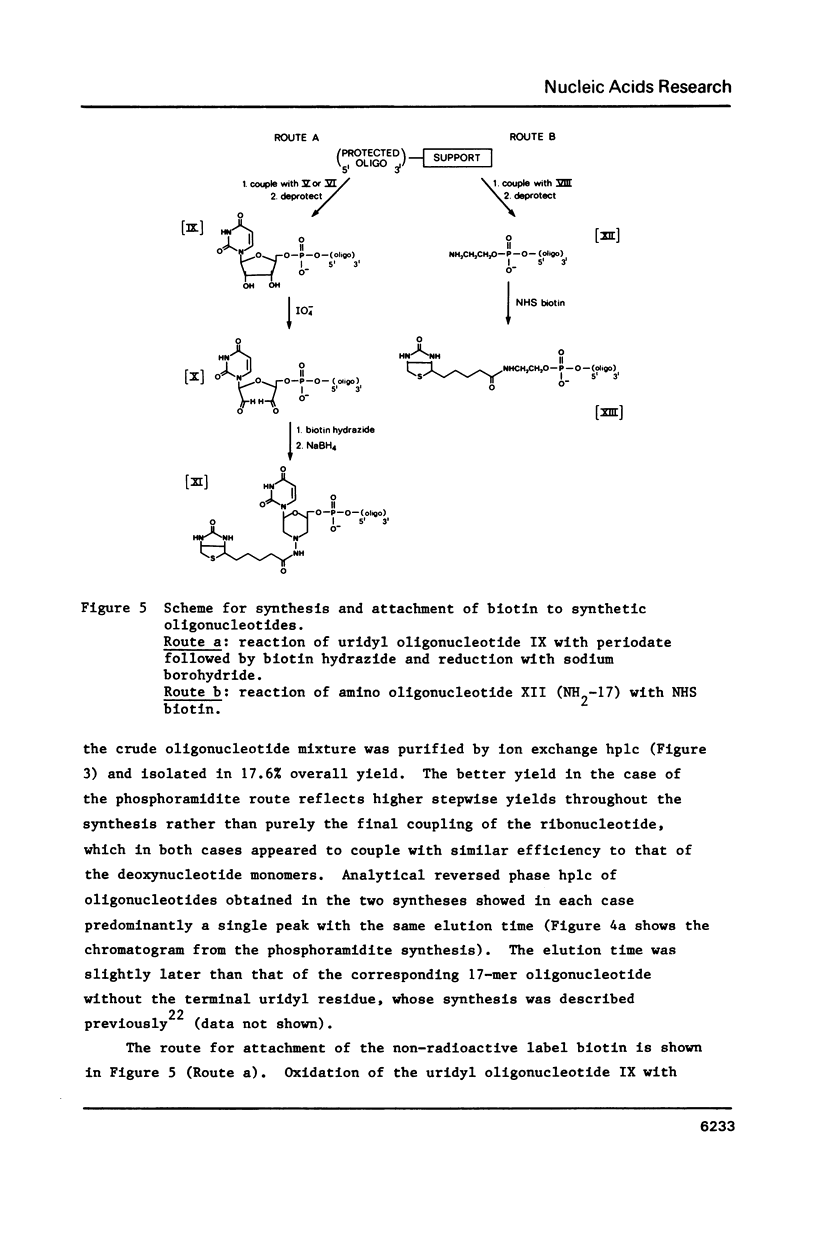
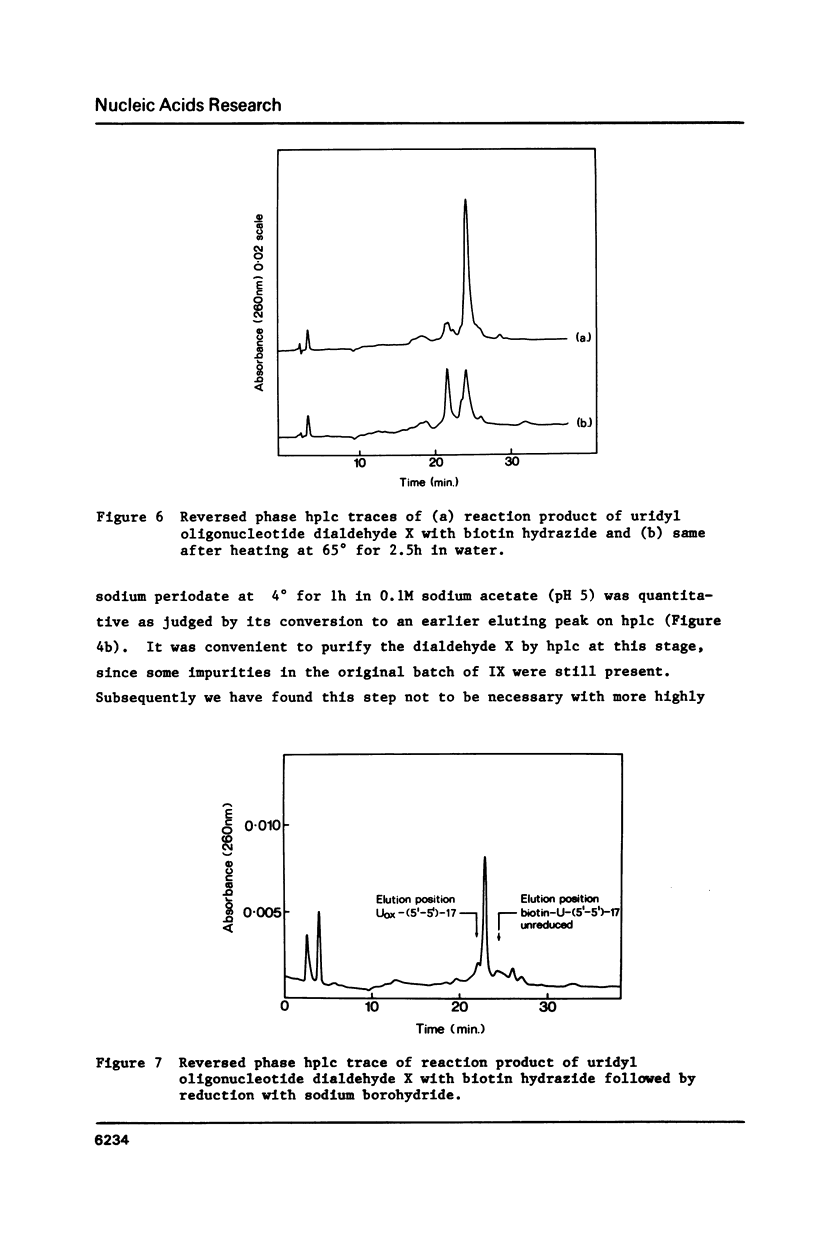

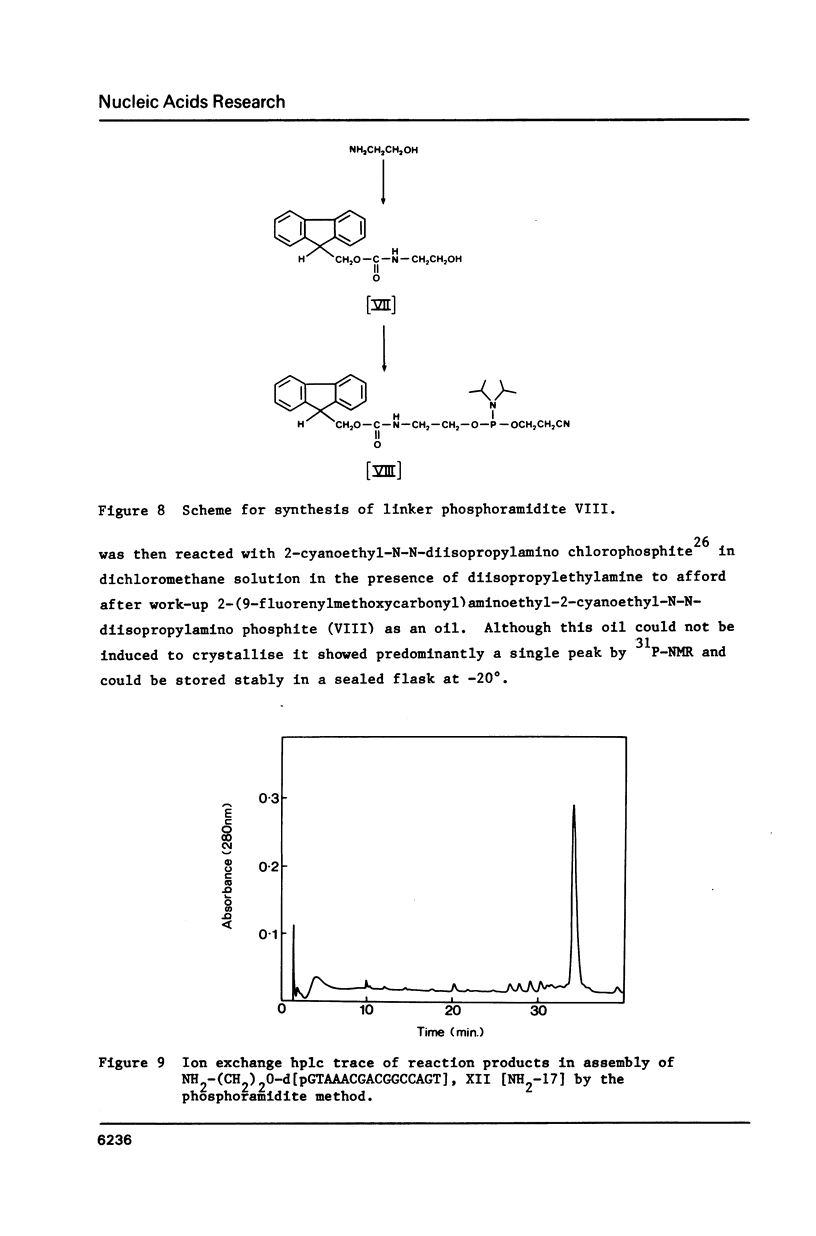
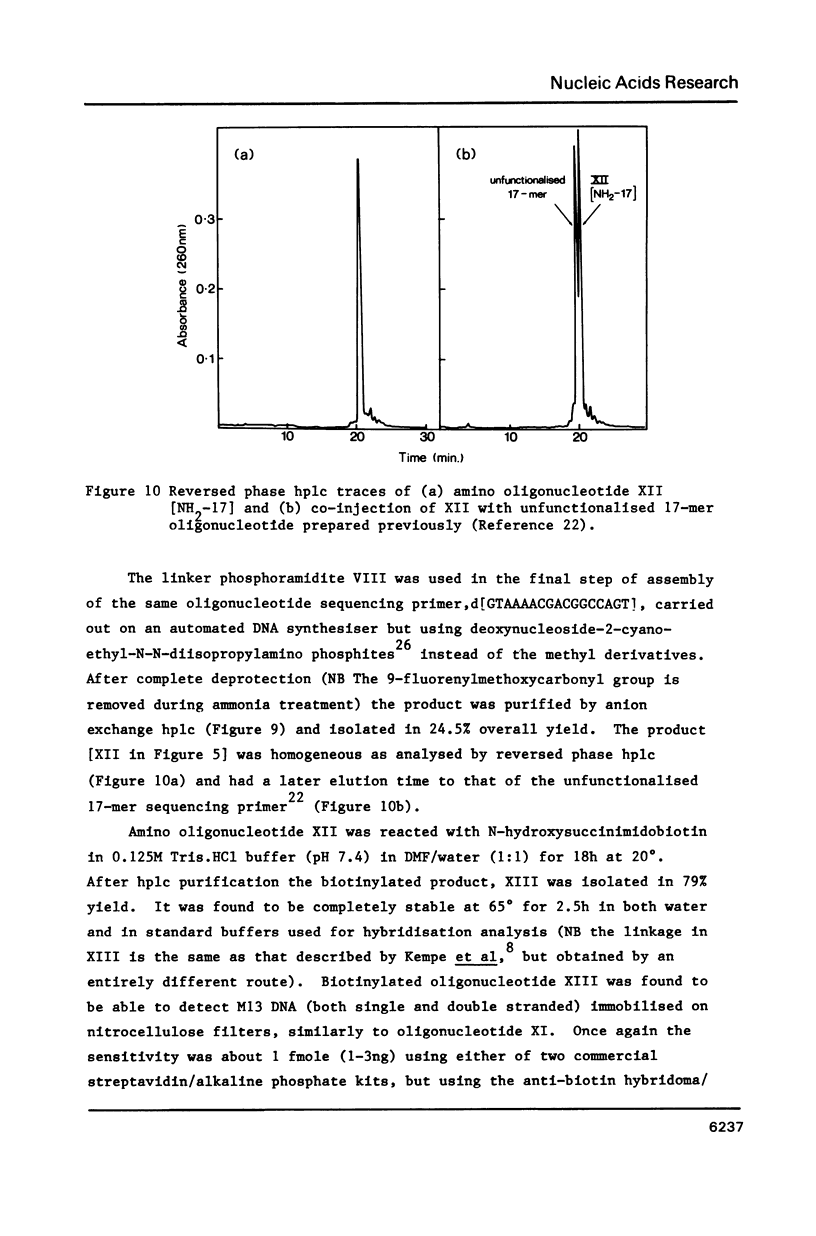








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barone A. D., Tang J. Y., Caruthers M. H. In situ activation of bis-dialkylaminophosphines--a new method for synthesizing deoxyoligonucleotides on polymer supports. Nucleic Acids Res. 1984 May 25;12(10):4051–4061. doi: 10.1093/nar/12.10.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann P., Narasimhachari Identification of N,N-dimethyltryptamine, 5-methoxy-N,N-dimethyltryptamine and bufotenin by cellulose TLC. J Chromatogr. 1973 Nov 7;86(1):269–273. doi: 10.1016/s0021-9673(01)81270-x. [DOI] [PubMed] [Google Scholar]
- Bayer E. A., Skutelsky E., Wilchek M. The avidin-biotin complex in affinity cytochemistry. Methods Enzymol. 1979;62:308–315. doi: 10.1016/0076-6879(79)62235-8. [DOI] [PubMed] [Google Scholar]
- Becker J. M., Wilchek M., Katchalski E. Irreversible inhibition of biotin transport in yeast by biotinyl-p-nitrophenyl ester. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2604–2607. doi: 10.1073/pnas.68.10.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broker T. R., Angerer L. M., Yen P. H., Hershey N. D., Davidson N. Electron microscopic visualization of tRNA genes with ferritin-avidin: biotin labels. Nucleic Acids Res. 1978 Feb;5(2):363–384. doi: 10.1093/nar/5.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chollet A., Kawashima E. H. Biotin-labeled synthetic oligodeoxyribonucleotides: chemical synthesis and uses as hybridization probes. Nucleic Acids Res. 1985 Mar 11;13(5):1529–1541. doi: 10.1093/nar/13.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu B. C., Orgel L. E. Detection of specific DNA sequences with short biotin-labeled probes. DNA. 1985 Aug;4(4):327–331. doi: 10.1089/dna.1985.4.327. [DOI] [PubMed] [Google Scholar]
- Connolly B. A., Rider P. Chemical synthesis of oligonucleotides containing a free sulphydryl group and subsequent attachment of thiol specific probes. Nucleic Acids Res. 1985 Jun 25;13(12):4485–4502. doi: 10.1093/nar/13.12.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth M. L., Gait M. J., Goelet P., Hong G. F., Singh M., Titmas R. C. Rapid synthesis of oligodeoxyribonucleotides VI. Efficient, mechanised synthesis of heptadecadeoxyribonucleotides by an improved solid phase phosphotriester route. Nucleic Acids Res. 1981 Apr 10;9(7):1691–1706. doi: 10.1093/nar/9.7.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epe B., Woolley P., Steinhäuser K. G., Littlechild J. Distance measurement by energy transfer: the 3' end of 16-S RNA and proteins S4 and S17 of the ribosome of Escherichia coli. Eur J Biochem. 1982 Dec;129(1):211–219. doi: 10.1111/j.1432-1033.1982.tb07042.x. [DOI] [PubMed] [Google Scholar]
- Forster A. C., McInnes J. L., Skingle D. C., Symons R. H. Non-radioactive hybridization probes prepared by the chemical labelling of DNA and RNA with a novel reagent, photobiotin. Nucleic Acids Res. 1985 Feb 11;13(3):745–761. doi: 10.1093/nar/13.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromageot H. P., Griffin B. E., Reese C. B., Sulston J. E. The synthesis of oligoribonucleotides. 3. Monoacylation of ribonucleosides and derivatives via orthoester exchange. Tetrahedron. 1967 May;23(5):2315–2331. doi: 10.1016/0040-4020(67)80068-1. [DOI] [PubMed] [Google Scholar]
- Itakura K., Rossi J. J., Wallace R. B. Synthesis and use of synthetic oligonucleotides. Annu Rev Biochem. 1984;53:323–356. doi: 10.1146/annurev.bi.53.070184.001543. [DOI] [PubMed] [Google Scholar]
- Kempe T., Sundquist W. I., Chow F., Hu S. L. Chemical and enzymatic biotin-labeling of oligodeoxyribonucleotides. Nucleic Acids Res. 1985 Jan 11;13(1):45–57. doi: 10.1093/nar/13.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer P. R., Waldrop A. A., Ward D. C. Enzymatic synthesis of biotin-labeled polynucleotides: novel nucleic acid affinity probes. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6633–6637. doi: 10.1073/pnas.78.11.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary J. J., Brigati D. J., Ward D. C. Rapid and sensitive colorimetric method for visualizing biotin-labeled DNA probes hybridized to DNA or RNA immobilized on nitrocellulose: Bio-blots. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4045–4049. doi: 10.1073/pnas.80.13.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minganti C., Ganesh K. N., Sproat B. S., Gait M. J. Comparison of controlled pore glass and Kieselguhr-polydimethylacrylamide composite as supports for solid-phase synthesis of 23-residue oligodeoxyribonucleotides in milligram amounts. Anal Biochem. 1985 May 15;147(1):63–74. doi: 10.1016/0003-2697(85)90009-0. [DOI] [PubMed] [Google Scholar]
- Murasugi A., Wallace R. B. Biotin-labeled oligonucleotides: enzymatic synthesis and use as hybridization probes. DNA. 1984 Jun;3(3):269–277. doi: 10.1089/dna.1.1984.3.269. [DOI] [PubMed] [Google Scholar]
- Nadeau J. G., Singleton C. K., Kelly G. B., Weith H. L., Gough G. R. Use of ribonucleosides as protecting groups in synthesis of polynucleotides with phosphorylated terminals. Biochemistry. 1984 Dec 4;23(25):6153–6159. doi: 10.1021/bi00320a040. [DOI] [PubMed] [Google Scholar]
- Reines S. A., Cantor C. R. New fluorescent hydrazide reagents for the oxidized 3'-terminus of RNA. Nucleic Acids Res. 1974 Jun;1(6):767–786. doi: 10.1093/nar/1.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha N. D., Biernat J., McManus J., Köster H. Polymer support oligonucleotide synthesis XVIII: use of beta-cyanoethyl-N,N-dialkylamino-/N-morpholino phosphoramidite of deoxynucleosides for the synthesis of DNA fragments simplifying deprotection and isolation of the final product. Nucleic Acids Res. 1984 Jun 11;12(11):4539–4557. doi: 10.1093/nar/12.11.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. M., Fung S., Hunkapiller M. W., Hunkapiller T. J., Hood L. E. The synthesis of oligonucleotides containing an aliphatic amino group at the 5' terminus: synthesis of fluorescent DNA primers for use in DNA sequence analysis. Nucleic Acids Res. 1985 Apr 11;13(7):2399–2412. doi: 10.1093/nar/13.7.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh M. R., Milstein C. A direct antigen-binding assay to screen hybridoma supernatants. Anal Biochem. 1985 Nov 15;151(1):192–195. doi: 10.1016/0003-2697(85)90071-5. [DOI] [PubMed] [Google Scholar]
- Weiss B., Richardson C. C. End-group labeling of nucleic acids by enzymatic phosphorylation. Cold Spring Harb Symp Quant Biol. 1966;31:471–478. doi: 10.1101/sqb.1966.031.01.061. [DOI] [PubMed] [Google Scholar]
- Zamecnik P. C., Stephenson M. L., Scott J. F. PARTIAL PURIFICATION OF SOLUBLE RNA. Proc Natl Acad Sci U S A. 1960 Jun;46(6):811–822. doi: 10.1073/pnas.46.6.811. [DOI] [PMC free article] [PubMed] [Google Scholar]



