Abstract
The advent of powerful neuroimaging tools such as functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) has begun to redefine how we diagnose, define, and understand disorders of consciousness such as the vegetative and minimally conscious states. In my paper, I review how research using these methods is both elucidating these brain states and creating diagnostic dilemmas related to their classification as the specificity and sensitivity of traditional behavior-based assessments are weighed against sensitive but not yet fully validated neuroimaging data. I also consider how these methods are being studied as potential communication vectors for therapeutic use in subjects who heretofore have been thought to be unresponsive or minimally conscious. I conclude by considering the ethical challenges posed by novel diagnostic and therapeutic neuroimaging applications and contextualize these scientific developments against the broader needs of patients and families touched by severe brain injury.
DISCORDANCE, NEUROIMAGING, AND THE HISTORY AND PHYSICAL EXAMINATION IN DISORDERS OF CONSCIOUSNESS
Since the advent of medical technology, physicians have used technology to augment their ability to observe the human body and make more precise diagnoses of disease. The introduction of the stethoscope in 1816 enabled our predecessors to expand the art of physical diagnosis to listen more astutely to heart sounds (1), as Abraham Verghese so wonderfully reminded us (2).
Our era is no different. But instead of listening to the heart, we are looking at the brain as it is now represented in images rendered through methods like functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) (3). To be sure, this is not physical diagnosis, but neither is it technology divorced from the bedside examination. Instead, it is an evolving story of medical advance that promises to transform our diagnostic categorization of severe brain injury from a descriptive nosology to a schema founded upon a more expansive notion of the underlying physiology of injury and recovery (4).
This advance of technology has all the usual seductions that pull us from the bedside and discount the importance of the history and physical examination. One of my themes is the importance of these bedside competencies to the assessment of patients with severe brain injury and to the advance of scientific discovery. Indeed, reconciling the discordance between what can be seen on bedside examination and what patients may demonstrate on sophisticated neuroimaging will be essential to our understanding of these conditions and their amelioration.
BRAIN DEATH
This discordance goes both ways. Let us consider patients who are brain dead, as defined by the 1968 Harvard Criteria of whole brain death (5). Even though no brainstem functions are preserved and these patients do not exhibit spontaneous respiration when challenged with an apnea examination, on superficial assessment they appear to be alive. They are perfused and have heartbeats on cardiac monitoring. They may even demonstrate the aptly named lazarus reflex and seem to pray as their cervical spinal reflexes bring the hands together toward the midline, with the outlines of a steeple.
I recall an intensivist who had, after having completed apnea testing and confirmed a lack of spontaneous breathing and a pC02 that had risen the requisite 20 mmHg, witnessed a lazarus reflex (6). Despite his laboratory and intellectual assessment of the patient's status, he reached into his pocket to retrieve his stethoscope and confirm via a bedside examination that this seemingly vital patient was in fact dead.
The picture is different and far more objectively dire when brain death is viewed via imaging. When these patients are placed into a scanner for a perfusion study, the result is the “black hole” of a brain with an empty vault sign beneath a perfused cranium (7). That is one sort of discordance between the clinical examination and neuroimaging data: here, the brain-dead patient looks “better,” as it were, than the patient's neuroimage.
THE VEGETATIVE STATE
Now consider a patient in the vegetative state (VS), that politically contentious biological state exemplified by the Schiavo case (8), which was first described by Bryan Jennett, the Scottish neurosurgeon known as well for the Glasgow Coma Scales, and by my late teacher Fred Plum, the American neurologist who needs no introduction here (9). Jennett and Plum, in a landmark 1972 paper in The Lancet, described the VS as a state of “wakeful unresponsiveness,” a parsimonious phrase if there ever was one (10). What they meant for their “syndrome without a name” was an eyes-opened state, in contradistinction to the eyes-closed and self-limited state of coma, in which there is no awareness of self, others, or the environment.
Classically, vegetative patients have an intact brainstem without higher cortical function. They breathe spontaneously, as Karen Anne Quinlan did for years—when she was removed from her ventilator in that significant right-to-die case (11), and they must—for if there is no brainstem function and spontaneous respiration, brain death would be the correct diagnosis.
Dr. Plum once told me the story of his examination of Ms. Quinlan, as a court-appointed neurologist (12). He confessed that he knew she would breathe when the court ordered the removal of her ventilator. I asked him how he knew. He told me that he had removed her from the ventilator as part of his judicially sanctioned neurological examination in order to distinguish her diagnosis from brain death (12).
During most of our clinical lives, the VS was another one of those brain states in which the patient looked better than his/her scan. The image of the vegetative brain in my mind was that of an old, grainy CT scan of massive hyrocephalus ex vacuo in which the thinning cortex was replaced by massive ventricles. In my mind's eye, I imagined a gelatinous gel (13), and not much else, bolstered by the Quinlan autopsy results in The New England Journal of Medicine, which showed that her brain weighed half as much as a normal brian (14) and also by cultural forces that so closely linked the right to die in America to the utter futility of this brain state (15). The Quinlan case was a pivotal case in bioethics and in my own early work in clinical ethics and palliative care. That landmark case enfranchised us with a right to die and acculturated a whole generation of physicians to look at severe brain injury as warranting a societal neglect syndrome (16).
But even as I was working in palliative care and medical ethics at life's end (17), the old truths about the VS were beginning to come into question. Although the original description of the VS was the persistent vegetative state abbreviated as “PVS,” the Multi-Society Taskforce Report on the vegetative state published in 1994 in The New England Journal of Medicine was starting to refine the nomenclature (18). Presciently, it noted that the persistent VS was a diagnosis, while the permanent VS was a prognosis. Somehow, the report had to account for the rare but disturbing patient who regained consciousness after being pronounced persistently vegetative.
More sophisticated functional neuroimaging further complicated matters—or more approximated the biologic reality of the VS—by illustrating that the vegetative was itself heterogeneous with significant variability in the neuroimages that might be consistent with that state. Work at Weill Cornell Medical College, led by my colleague, the neurologist and neuroscientist Nicholas D. Schiff, revealed this heterogeneity in a paper published in Brain (19).
Variations in neuroimages among patients who were vegetative resulted from the etiology of injury and/or its localization. Patients who sustain anoxic brain injury have lower overall rates of metabolism and more pervasive injury because the entire brain is exposed to the offending anoxia.* In contrast, patients with multi-focal traumatic brain injury varying had degrees of injury. Strategic injury to key structures like the thalamus can be devastating and result in the VS even as broader swaths of cortex are preserved. This heterogeneity points to the peril of making any diagnosis devoid of the clinical context of an injury and the patient's history (20).
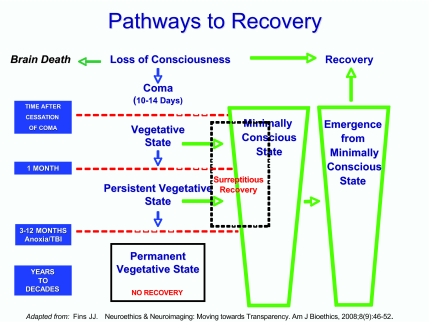
This variation also helps explain the time line to the permanent VS as 3 or 12 months, as articulated by the Multi-Society Task Force report. The anoxic brain with diffuse injury and a pervasive decline in metabolic function takes 3 months to become permanently vegetative. Traumatic brain injury can take up to a year to reach permanence, in that its pathology is more discrete although potentially—over time—as dire. We see the salience of mechanism of injury in the schematic diagram in Figure 1, which illustrates the time line for recovery, as well as how patients progress out of the VS if they move toward recovery (21).
Fig. 1.
Pathways to recovery in disorders of consciousness.
Patients who are vegetative or persistently vegetative have the potential to move into the minimally conscious state (MCS). The MCS is a newly described disorder of consciousness entering the medical lexicon in the Aspen Criteria formulated in 2002 (22). Patients in the MCS have definitive evidence of consciousness, demonstrating intention, attention, memory, and awareness of the self, others, or the environment. The challenge is that these behaviors are episodic and intermittent and not reproducible, making these patients indistinguishable from vegetative patients to the untrained eye, especially in an isolated single examination.
DIAGNOSTIC ERRORS: DIFFERENTIATING VS FROM MCS
Recent studies have demonstrated that the diagnostic error rate in cases of patients diagnosed as vegetative but, who are in fact minimally conscious is up to 40%, depending on the study cited (23–26), an error rate that would be unconscionable in any other field of medicine. The reasons for this error rate are multifactorial (27). The behaviors that patients manifest in consciousness are episodic and intermittent but definite. They are not captured in a single examination, and if a family sees a behavior and only reports it to a nursing home staff, the staff are likely to discount the observation, chalking it up to denial, especially when the patient has come from an academic medical center in which the patient was definitively diagnosed as being vegetative. Clinical staff members in these settings often fail to appreciate that the diagnosis is not fixed until the aforementioned temporal markers are reached, and that patients often surreptitiously move unnoticed into an MCS.
The most prominent of such cases was that of Terry Wallis, a 39-year-old Arkansan who was in a motor-vehicle accident in 1984 and was diagnosed as vegetative until he had what was described as a “miracle awakening” in 2003, when he began to speak spontaneously and consistently, thus reaching the level of emergence from MCS conscious state (28). Mr. Wallis continues to improve and diffusion tensor fMRI imaging of his brain has revealed dynamic changes, with axonal sprouting and pruning two decades after his injury (29). Whether these changes account for his improvement has to remain a speculation, because no antecedent functional imaging data were recorded until he emerged from MCS. Nonetheless, the question is a pointed one and indicates the need to study these patients systematically and longitudinally, as suggested by an exploratory panel that gathered at the Institute of Medicine (30).
However long it takes for us to recognize patients who are in the MCS, this recognition and differentiation of the MCS from the VS has biological and ethical salience (31). Biologically, because these patients' brains have the capability for integrative function, which is the basis for consciousness, in contradistinction to vegetative patients.
This distinction is seen in a paper on PET by Steven Laureys and his colleagues at Liege showing the response of vegetative patients to pain (32). Unlike controls, this first-order activation was “functionally disconnected” from secondary somatosensory areas and higher-level associative areas. Contrast this to what my colleague Nicholas Schiff and co-investigators found in subjects who were minimally conscious (33). Using a passive language paradigm, they found that spoken narratives read by patients' family members resulted in large-scale network activation or integration. This activity was indistinguishable from that in controls. Notably, network activation did not occur in subjects when the identical narratives, with the same frequency spectrum, were played in reverse without lingusitic content.
Ethically, these findings suggested the capability of patients in the MCS to process language, grammar, and speech. These findings were spine-chilling, as it were, because they indicated, at least to me, that these patients' in the MCS—heretofore conflated with vegetative patients—were not only conscious and responsive to their environment, but also able to process language and perhaps experience the profound isolation of being able to understand but not respond. This was a grave ethical concern because, as I have already noted, these patients are often shunted to chronic care facilities where they are misdiagnosed and treated as if they are neither present nor part of the human community, a community which, if nothing else, is marked by shared communication. If even part of these concerns were true, it would suggest a strong fiduciary obligation for a proper diagnosis and appropriate efforts at remediation of an historic legacy of neglect (34).
QUESTIONING AN OLD NOSOLOGY
But this was only the beginning of the complexity brought forward by neuroimaging. In 2006, Adrian Owen and colleagues at the University of Cambridge and at Liege demonstrated the capability of a vegetative patient to respond to active language paradigms (35). Published in Science, they asked a 25-year-old woman with a traumatic brain injury to imagine playing tennis in her head, walking through her home, and disambiguating two linguistically similar words. The results were spectacular. Activations in motor, spacial, and language networks were identical to those of controls, even though the woman was vegetative by established behavioral criteria.
In response to the Owen paper, my colleague and I suggested a new category of non-behavioral MCS (36), asserting that the patient was no longer vegetative if she was responsive and had not exceeded the temporal boundaries of the VS, in that she was only 5 months into her injured state. Indeed, by 11 months, she met behavioral criteria for MCS, fixating on a mirror presented at a 45-degree angle (37).
How to reconcile physical findings with ones on neuroimaging is akin to reconciling genotype and phenotype before one has a theory of inheritance (38). It is a pressing problem that we have begun to examine, urging caution about premature dissemination of this technology—outside of the research context—before test characteristics are better understood (39). As a group, many of us urged caution at a gathering at Stanford University in 2007 that examined the thorny question of how to reconcile behavioral and neuroimaging data (40).
BEDSIDE IMAGING AT THE CUSP OF CONSCIOUSNESS
The final paper that I want to present in this review is the most compelling, a paper by Martin Monti that built upon the active language paradigm of Owen to toggle yes/no responses to willful modulation of brain activity (41). The method is simple but elegant. The subject is instructed to imagine playing tennis when he/she wanted to answer “yes” and walking about their house when he/she wanted to respond “no”. Investigators studied 54 patients with disorders of consciousness.
Five of these patients, of whom 4 were vegetative, demonstrated flares of their condition, as reported in the 2006 Science paper. All of these patients had traumatic brain injury. Most remarkably, in one “vegetative” patient, the investigators were able to open a narrow-band communication channel through which the subject could respond yes/no by willful modulation.
There is much to be said about this provocative study. Ethically, it provides a feeble communication channel with individuals who are conscious but without a vector out of themselves, although the bandwidth is narrow and the reception poor, much like a bad cell phone connection (42). It also seems to provide a way to improve diagnostics, as without this intervention the patient would still be considered vegetative. But even here that nagging discordance between the physical examination and neuroimaging becomes apparent.
Thirty of 31 patients in the study who were minimally conscious were identified by careful bedside examination through behavioral assessment. Only one additional patient was identified as being conscious by neuroimaging, although for that patient this made a profound difference. Here we have one assessment that is utilitarian and most sensitive, while the other is really deontologic and most specific: having identified the one conscious patient who was behaviorally diagnosed as vegetative and who could not communicate, albeit having done this at a rudimentary level.
There is also a scientific paradox: that patient was had a lower functional level than other patients who could not make use of this communication channel. This variation points to important questions about mechanisms of injury and recovery (43), that my colleagues and I are studying at Weill Cornell and which we hope to clarify in years to come at the Consortium for the Advanced Study of Brain Injury (CASBI).
MIND'S EYE
In the meantime, we need a prudential ethic about the limits of the technology and the on going value of the physical examination and the broader narrative elements of a patient's life that will help to contextualize tentative efforts to communicate, especially about “big” questions in terms of whether a patient might want to live in the patient's current state (44). Responses are wholly dependent upon the questions that are asked, as patients cannot initiate queries. A non-response is not dispositive, because it could be due to distraction, the latency of response, the weighing of choices, or an outright failure of the method itself.
So we need to appreciate the paradox that even as we give voice to some patients, we need to be careful not to undermine their prior articulations because of doubts that might be engendered by a non-response or a response that is incomplete or inconsistent. To do that would create the worrisome paradox that a prosthetic for communication could undermine the patient's voice, potentially eroding the patient's right to determine how to live and even die.
ACKNOWLEDGEMENTS
The author is grateful to his ACCA nominator and sponsors for the privilege of this wonderful association, and dedicates this paper to the memory of the late Fred Plum, M.D.
Footnotes
Potential Conflicts of Interest: Dr Fins reports receiving grants from The Robert Wood Johnson Foundation, Buster Foundation, Jerrold B. Katz Foundation, Richard Lounsbery Foundation, Charles A. Dana Foundation, and the NIH Clinical and Translational Science Center UL1-RR024966 (Weill Cornell Medical Center).
This point about the more dire prognosis associated with anoxic brain injury is now an open question for patients who have sustained cardiac arrest and received hypothermia therapy with chilled saline concurrent with their injury. Induced hypothermia is neuroprotective and will probably dramatically alter outcomes in anoxic brain injury.
REFERENCES
- 1.Reynolds HY. R.T.H. Laennec, M.D. - Clinicopathologic observations, using the stethoscope, made medicine scientific. President's Address, Trans Am Clinical and Climatol Associ. 2004;115:1–29. [PMC free article] [PubMed] [Google Scholar]
- 2.Verghese A. 123rd Annual Meeting of the American Clinical and Climatological Association; October 24, 2010. [Google Scholar]
- 3.Fins JJ. Neuroethics and Neuroimaging: Moving towards Transparency. Am J Bioethics. 2008;8(9):46–52. doi: 10.1080/15265160802334490. [DOI] [PubMed] [Google Scholar]
- 4.Fins JJ. Minds, monuments and moments: musings on time and disorders of consciousness. Front Neurosci. Submitted. [Google Scholar]
- 5.Ad Hoc Committee of the Harvard Medical School to examine the definition of brain death. A definition of irreversible coma. JAMA. 1968;205:337–40. [PubMed] [Google Scholar]
- 6.Fins JJ. Caring for the Dying, Identification and Promotion of Physician Competency: New Additions to Personal Narratives. Philadelphia: American Board of Internal Medicine; 1999. When brain death pulls at the heart strings. [Google Scholar]
- 7.Laureys, Owen, & Schiff, Brain function in brain death, coma, vegetative state, minimally conscious state and the locked-in syndrome. Lancet Neurol. 2004;3(9):537–46. doi: 10.1016/S1474-4422(04)00852-X. [DOI] [PubMed] [Google Scholar]
- 8.Fins JJ. Affirming the right to care, preserving the right to die: disorders of consciousness and neuroethics after Schiavo. Palliat Support Care. 2006;4(2):169–78. doi: 10.1017/s1478951506060238. [DOI] [PubMed] [Google Scholar]
- 9.Hauser SL, Johnston SC. Fred Plum (1924–2010) and the founding of the Annals of Neurology. Ann of Neurol. 2010;68(3):A5–7. doi: 10.1002/ana.22225. [DOI] [PubMed] [Google Scholar]
- 10.Jennett B, Plum F. Persistent vegetative state after brain damage. A syndrome in search of a name. Lancet. 1972;(1):734–37. doi: 10.1016/s0140-6736(72)90242-5. [DOI] [PubMed] [Google Scholar]
- 11.Cantor NL. Twenty-five years after Quinlan: a review of the jurisprudence of death and dying. J Law Med Ethics. 2001;29(2):182–96. doi: 10.1111/j.1748-720x.2001.tb00339.x. [DOI] [PubMed] [Google Scholar]
- 12.Fins JJ. Lessons from the injured brain: a bioethicist in the vineyards of neuroscience. Camb Q Health Ethics. 2009;18(1):7–13. doi: 10.1017/S0963180108090038. [DOI] [PubMed] [Google Scholar]
- 13.Fins JJ. Rethinking Disorders of Consciousness: New Research and Its Implications. Hastings Cent Rep. 2005;35(2):22–4. [PubMed] [Google Scholar]
- 14.Kinney HC, Korein J, Panigrahy A, Dikkes P, Goode R. Neuropathological findings in the brain of Karen Ann Quinlan. The role of the thalamus in the persistent vegetative state. N Engl J Med. 1994;330(21):1469–75. doi: 10.1056/NEJM199405263302101. [DOI] [PubMed] [Google Scholar]
- 15. Matter of Karen Quinlan, 70 N.J. 10, 355 A.2d 677 (1976)
- 16.Fins JJ. Constructing an ethical stereotaxy for severe brain injury: balancing risks, benefits and access. Nat Rev Neurosci. 2003;4:323–27. doi: 10.1038/nrn1079. [DOI] [PubMed] [Google Scholar]
- 17.Fins JJ. A Palliative Ethic of Care: Clinical Wisdom at Life's End. Sudbury, MA: Jones and Bartlett Publishers; 2006. [Google Scholar]
- 18.Medical aspects of the persistent vegetative state (1 and 2). The Multi-Society Task Force on PVS. N Engl J Med. 1994;330(21)(22):1499–508. 1572–9. doi: 10.1056/NEJM199405263302107. [DOI] [PubMed] [Google Scholar]
- 19.Schiff ND, Ribary U, Moreno DR. Residual cerebral activity and behavioural fragments can remain in the persistently vegetative brain. Brain. 2002;125:1210–1234. doi: 10.1093/brain/awf131. [DOI] [PubMed] [Google Scholar]
- 20.Posner J, Saper C, Schiff ND, Plum F. 4th ed. New York: Oxford University Press; 2007. Plum and Posner's Diagnosis of Stupor and Coma. [Google Scholar]
- 21.Fins JJ. Neuroethics and neuroimaging: moving towards transparency. Am J Bioethics. 2008;8(9):46–52. doi: 10.1080/15265160802334490. [DOI] [PubMed] [Google Scholar]
- 22.Giacino JT, Ashwal S, Childs N, et al. The minimally conscious state: definition and diagnostic criteria. Neurology. 2002;58(3):349–53. doi: 10.1212/wnl.58.3.349. [DOI] [PubMed] [Google Scholar]
- 23.Andrews K, Murphy L, Munday R, et al. Misdiagnosis of the vegetative state: retrospective study in a rehabilitation unit. B M J. 1996;313:13–16. doi: 10.1136/bmj.313.7048.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Childs NL, Mercer WN, Childs HW. Accuracy of diagnosis of persistent vegetative state. Neurology. 1993;43:1465–67. doi: 10.1212/wnl.43.8.1465. [DOI] [PubMed] [Google Scholar]
- 25.Wilson FC, Harpur J, Watson T, et al. Vegetative state and minimally responsive patients: regional survey, Long-term case outcomes and service recommendations. Neuro Rehabilitation. 2002;17:231–236. [PubMed] [Google Scholar]
- 26.Schnakers C, Vanhaudenhuyse A, Giacino J. Diagnostic accuracy of the vegetative and minimally conscious state: clinical consensus versus standardized neurobehavioral assessment. BMC Neurol. 2009;9:35. doi: 10.1186/1471-2377-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fins JJ, Master MG, Gerber LM, Giacino JT. The minimally conscious state: a diagnosis in search of an epidemiology. Arch Neurol. 2007;64(10):1400–5. doi: 10.1001/archneur.64.10.1400. [DOI] [PubMed] [Google Scholar]
- 28.Schiff ND, Fins JJ. Hope for “Comatose” Patients. Cerebrum. 2003;5(4):7–24. [Google Scholar]
- 29.Voss HU, Uluc AM, Dyke JP, et al. Possible axonal regrowth in late recovery from minimally conscious state. J Clin Invest. 2006;116:2005–11. doi: 10.1172/JCI27021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fins JJ, Schiff ND, Foley KM. Late recovery from the minimally conscious state: ethical and policy implications. Neurology. 2007;68:304–7. doi: 10.1212/01.wnl.0000252376.43779.96. [DOI] [PubMed] [Google Scholar]
- 31.Fins JJ, Plum F. Neurological diagnosis is more than a state of mind: diagnostic clarity and impaired consciousness. Arch Neurol. 2004;61(9):1354–5. doi: 10.1001/archneur.61.9.1354. [DOI] [PubMed] [Google Scholar]
- 32.Laureys S, Faymonville ME, Peigneux P, et al. Cortical processing of noxious somatosensory stimuli in the persistent vegetative state. Neuroimage. 2002;17(2):732–41. [PubMed] [Google Scholar]
- 33.Schiff ND, Rodriguez-Moreno D, Kamal A, et al. fMRI reveals large-scale network activation in the minimally conscious state. Neurology. 2005;64(3):514–23. doi: 10.1212/01.WNL.0000150883.10285.44. [DOI] [PubMed] [Google Scholar]
- 34.Fins JJ. Clinical pragmatism and the care of brain injured patients: towards a palliative neuroethics for disorders of consciousness. Prog Brain Res. 2005;150:565–82. doi: 10.1016/S0079-6123(05)50040-2. [DOI] [PubMed] [Google Scholar]
- 35.Owen AM, Coleman MR, Boly M, et al. Willful modulation of brain activity in disorders of consciousness. Detecting awareness in the vegetative state. Science. 2006;313(5792):1402. doi: 10.1126/science.1130197. [DOI] [PubMed] [Google Scholar]
- 36.Fins JJ, Schiff ND. Shades of gray: new insights from the vegetative state. Hastings Cent Rep. 2006;36(6):8. doi: 10.1353/hcr.2006.0094. [DOI] [PubMed] [Google Scholar]
- 37.Owen AM, Coleman MR, Boly M, et al. Willful modulation of brain activity in disorders of consciousness. Detecting awareness in the vegetative state. Science. 2006;313(5792):1402. doi: 10.1126/science.1130197. Supplemental material to: [DOI] [PubMed] [Google Scholar]
- 38.Fins JJ. Border zones of consciousness: another immigration debate? Am J Bioethics. 2007;7(1):51–4. doi: 10.1080/15265160601064231. [DOI] [PubMed] [Google Scholar]
- 39.Fins JJ, Shapiro Z. Neuroimaging and Neuroethics: Clinical and Policy Considerations. Curr Opin Neurol. 2007;20(6):650–54. doi: 10.1097/WCO.0b013e3282f11f6d. [DOI] [PubMed] [Google Scholar]
- 40.Fins JJ, Illes J, Bernat JL, Hirsch J, Laureys S, Murphy E Participants of the Working Meeting on Ethics, Neuroimaging and limited states of consciousness. Neuroimaging and disorders of consciousness: envisioning an ethical research agenda. Am J Bioethics. 2008;8(9):3–12. doi: 10.1080/15265160802318113. [DOI] [PubMed] [Google Scholar]
- 41.Monti MM, Vanhaudenhuyse A, Coleman MR, et al. N Engl J Med. 2010;362(7):579–89. doi: 10.1056/NEJMoa0905370. [DOI] [PubMed] [Google Scholar]
- 42.Fins JJ. Quote of the Day. The New York Times. 2010 Feb 5;:A-2. [Google Scholar]
- 43.Schiff ND. Recovery of consciousness after brain injury: a mesocircuit hypothesis. Trends Neurosci. 2010;33(1):1–9. doi: 10.1016/j.tins.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fins JJ, Schiff ND. In the Blink of the Mind's Eye. Hastings Cent Rep. 2010;40(3):21–3. doi: 10.1353/hcr.0.0257. [DOI] [PubMed] [Google Scholar]