Abstract
Although body temperature is tightly regulated in humans, elevated temperatures are frequently encountered during febrile illnesses and exertional and environmental hyperthermia. Such temperature increases exert profound effects on cell signaling and gene expression patterns, which have important consequences for innate immune function and cell injury, apoptosis, and recovery. The lung offers a framework for understanding how these effects can either benefit or harm the host. We present data demonstrating that exposure to febrile-range hyperthermia (∼39.5°C) exerts multiple biologic effects that converge on enhanced neutrophil recruitment to the lung, and describe the consequences of these effects for pathogen clearance and collateral tissue injury. We also discuss the influence of temperature on apoptosis in lung epithelium. Collectively, the data presented identify body temperature as a modifiable factor that exerts profound influence on the outcome of infection and injury.
Fever is a complex physiologic and behavioral response to infection or injury, the key feature of which is a temporary resetting of the body's thermostatic setpoint that causes an increase in core temperature. Although fever is recognized as a component of the acute-phase response to infection, the risk:benefit relationship of fever in the infected host has been an ongoing source of controversy. In this paper we review the limited information available from retrospective clinical studies and data from animal models that describe the effects of direct manipulation of core body temperature on outcome in infection and injury, and the possible mechanisms and consequences of fever. We propose a conceptual model to explain how fever and febrile-range hyperthermia (FRH) may confer either benefit or harm, depending on the underlying pathologic process, and provide data from animal models of lung infection and injury to support this concept.
AN EVOLUTIONARY PERSPECTIVE OF FEVER
Although fever is generally perceived to be a response limited to mammals and birds, many ectothermic animals, including lower vertebrates, arthropods, and annelids, also increase their core temperature in response to infection or injury (1). The prevalence of fever in such diverse modern animals suggests that it first appeared over 600 million years ago. The evolutionary persistence of fever is even more remarkable when one considers its substantial metabolic cost. In humans, generating fever through thermogenic shivering requires an approximately 6-fold increase in metabolic rate (2), and maintaining a physiologic core temperature at febrile levels requires an approximately 12% increase in basal metabolic rate per 1°C increase in core temperature (3, 4). In ectothermic animals with infections, moving to warmer environs not only obligates increased energy expenditure, but may also expose vulnerable individuals to attack by predators. Therefore, fever must confer benefit that more often than not outweighs these costs in the infected or injured host. Furthermore, given the phylogenetic age of fever, the immunologic processes that are active during febrile illnesses have had ample opportunity to evolve for optimal function at febrile temperatures. We therefore reasoned that changes in core temperature within the febrile range act as a biologic response modifier and, like most biologic response modifiers, may be harmful or beneficial depending on the clinical context, in which they occur.
THE EFFECT OF FEVER ON OUTCOME IN INFECTIONS
Observational Studies in Humans
Clinical studies suggest that the effects of fever depend in part on the severity of the underlying illness (5). Studies in non-life-threatening illnesses have shown evidence of both beneficial effects of fever and harmful effects of antipyretic therapy on the outcome of non-life-threatening infections. In children with chickenpox, those treated with acetaminophen experience a longer period before total crusting than do those given placebo (6). Graham et al. (7) reported a trend toward a longer duration of rhinovirus shedding in association with antipyretic therapy, and showed that the use of aspirin or acetaminophen is associated with suppression of the serologic neutralizing-antibody response and with increased nasal symptoms and signs. Stanley et al. (8) reported that adults infected with rhinovirus have more nasal viral shedding when they receive aspirin than when given placebo. In an analysis of experimental human shigellosis, Mackowiak and colleagues reported that an elevated oral temperature during clinical illness was associated with more severe symptoms but a shorter disease duration (9).
The influence of fever in severe sepsis is less clear. Approximately 90% of patients with sepsis are febrile (10–12), while many of the remainder are hypothermic. Studies of patients with invasive bacterial infections generally show fever to be associated with improved survival, but less consistently so than in patients with milder infections. In a retrospective analysis of 218 patients with Gram-negative bacteremia, Bryant and colleagues (13) reported that survival was 2.4-fold greater (71% vs. 29%) in patients who were febrile on the day of bacteremia (maximum daily temperature > 38.3°C) than in those who remained afebrile. A similar relationship was observed in an analysis of 184 cases of polymicrobial sepsis (14), and Weinstein et al. identified an association between a core temperature > 38°C and increased survival in their series of patients with spontaneous bacterial peritonitis (15). In a similar retrospective analysis of patients with spontaneous bacterial peritonitis, Hoefs and colleagues showed that the mean core temperature was greater in survivors than in nonsurvivors (100.9°C vs. 99.7°C, respectively) (16). In contrast, Dupont et al. failed to detect a difference in survival in febrile and afebrile patients in a series of 655 adults with bacteremia (17). In a re-analysis of these data in which we ranked the studies on the basis of acuity of illness as reflected by the incidence of sepsis and overall shock, we showed that the fever-associated improvement in survival was lost in more severe disease (5). Additional studies (14, 18) show that survival decreases when core temperature exceeds 39.4°C, suggesting there is an upper limit to the optimal febrile range.
Animal and Cell Culture Models of FRH
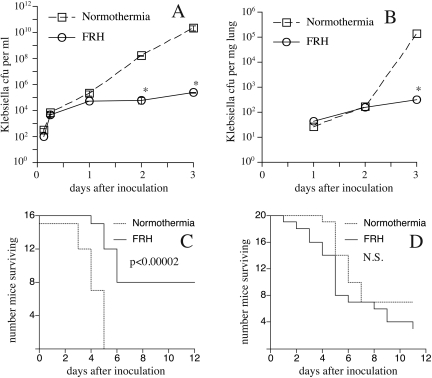
Animal models have provided more definitive information about the mechanisms and consequences of FRH during infections and injuries. We developed a mouse model of FRH in which mice exposed to an ambient temperature of 35°C to 37°C change their core temperature by ∼2°C but maintain normal circadian patterns and appear otherwise healthy and active (19). Using this model, we showed that FRH increased survival in experimental Klebsiella pneumoniae peritonitis from 0% to 50% and reduced the intraperitoneal bacterial load by 100,000-fold compared with K. pneumoniae-infected mice that remained normothermic (20) (Fig. 1A, C). K. pneumoniae had comparable in vitro proliferation rates at 37° and 39.5°C (20), suggesting that the reduced pathogen burden was achieved through effects on host bacterial clearance mechanisms. In a second study, we used the same model to analyze the consequences of FRH in pneumonia using the same K. pneumoniae pathogen (21). As occurred in the peritonitis model, co-exposure to FRH greatly reduced pathogen burden over the first three days of infection, but unlike the peritonitis model, FRH conferred no survival advantage (Fig. 1B, D). We reasoned that the difference in survival in the two models reflected differences in the balance between accelerated pathogen clearance and enhanced collateral tissue injury stimulated by FRH. Simply put, the lung is more susceptible to immune-mediated injury than the peritoneal cavity and the consequences for survival are greater in diffuse lung inflammation than peritoneal inflammation.
Fig. 1.
Comparison of FRH effects on experimental Klebsiella pneumoniae peritonitis and pneumonia. Bacterial load experiments: Mice were inoculated with 100 CFU of K. pneumoniae via intraperitoneal injection in 1 mL PBS (Panel A) or 250 CFU K. pneumoniae via intratracheal instillation in 50 μL PBS (Panel B), and then housed at 25°C (normothermia) or 35–37°C to maintain a core temperature of ∼39.5°C (FRH). Groups of 6 normothermic and FRH-exposed mice were sequentially euthanized and the bacterial load in the animals' peritoneal fluid (Panel A) and in homogenized lung tissue (Panel B) was quantified by culturing and counting colonies. Values are given as mean ± SE;* P < 0.05 versus normothermic mice. Survival experiments: Groups of 8 to 10 mice were inoculated with K. pneumoniae via intraperitoneal injection (Panel C) or via intratracheal instillation (Panel D) and were, then housed under conditions of normothermia or FRH and their survival monitored. Duplicate experiments were performed. Febrile ranger hyperthermia improved survival in the peritonitis model (Panel C) but not the pneumonia model (Panel D) by a log rank test.
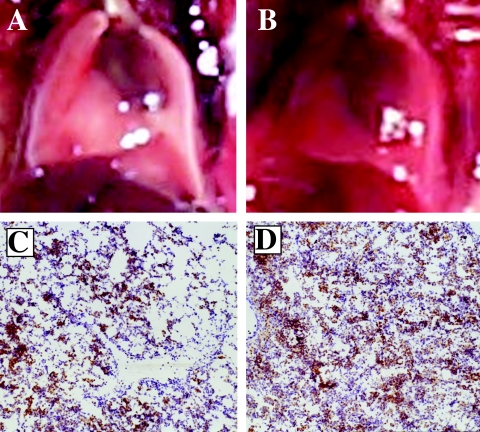
To analyze the contribution of collateral lung injury vs. bacterial infection to excess mortality in the FRH-exposed mouse pneumonia model, we treated mice with ceftriaxone 2 days after intratracheal inoculation with K. pneumoniae, which eliminated viable pathogen and imporved survival to 100% in the normothermic mice. However, mortality in the FRH-exposed mice with ceftriaxone-treated pneumonia was 50% despite complete pathogen clearance (21). Similarly, in a model of lung inflammation and injury induced by intratracheal instillation of bacterial endotoxin, FRH exposure reduced survival from 100% to 50% (21) and enhanced lung injury as can be seen by the gross appearance of the inflation-fixed lungs (Fig. 2A, B) and reflected in increased protein leak into lung lavage (21). Collectively, these studies suggest that excess lung injury in FRH-exposed mice with pneumonia was caused by an immune response rather than by proliferating pathogens. In another model of non-infectious lung injury, consisting of pulmonary oxygen toxicity, lethal lung injury caused by exposure to >95% oxygen was greatly accelerated in mice exposed to FRH. Thus, the occurrence of fever during lung injury or infection appears to worsen lung injury and survival.
Fig. 2.
Effect of exposure to FRH on LPS-induced acute lung injury. Mice received instillations of 50 μg LPS in 50 μL PBS and were then housed at 25°C (Panels A and C) or at 35–37°C to maintain a core temperature of ∼39.5°C (Panels B and D). Mice were euthanized after 24 hours and their lungs fixed in Prefer™ fixative at 20 cm H2O pressure. Representative pictures of the inflated lungs (Panels A and B) and micrographs of lung tissue stained for neutrophils with anti-GR-1 antibody (Panels C and D) are shown. Neutrophils appear as brown-staining cells.
We found that FRH exposure was associated with greatly increased neutrophil infiltration in models of both lipopolysaccharide (Figure 2C,D) and hyperoxia-induced acute lung injury (21, 22). Interestingly, Bernheim et al. (23) showed in the ectothermic desert lizard, Diposaurus dorsalis, that increasing core temperature from 38°C to 40°C after intradermal inoculation with the Gram-negative pathogen Aeromonas hydrophila increased survival, reduced bacterial burden, and increased the accumulation of granulocytic leukocytes at the inoculation site. This last-named study suggests that the mechanisms of FRH-augmented neutrophil recruitment, pathogen clearance, and injury observed in mammals are phylogenetically ancient and well conserved.
We have shown that exposure to FRH augments multiple steps required for neutrophil delivery to sites of infection and injury, including expanded neutrophil production, increased generation of endogenous chemotaxins, and modifications in endothelial and neutrophil function that facilitate transendothelial neutrophil migration. We found that exposure to FRH activates G-CSF expression and expands the circulating pool of polymorphonuclear neutrophils (PMN) (24), enhances the expression of GM-CSF (21), and augments the expression of CXC chemokines (21, 22, 25), thereby increasing both the pool of recruitable PMNs and the gradient for cellular chemotaxis. We showed that the increased expression of chemokines is mediated through a newly appreciated function for the heat/stress-activated transcription factor heat shock factor-1 (HSF-1) (25). We had previously shown that exposing human pulmonary artery endothelial cells to a temperature of 39.5°C in vitro increases their capacity for allowing neutrophil transmigration (26). More recently, we utilized a mouse assay of in vivo transalveolar neutrophil migration to analyze the effects of FRH on the capacity for chemokine-directed neutrophil migration (27). In this model, a fixed chemokine gradient is established in the lung by the intratracheal instillation of recombinant human IL-8, and the neutrophil content in lung tissue and lung lavage subsequently determined. We found that pre-exposure to FRH markedly increased the IL-8-directed transalveolar migration of neutrophils (27). Adoptive neutrophil transfer between normothermic and FRH-exposed neutrophil donors and recipients demonstrated that enhanced neutrophil migration capacity required the FRH exposure of both the donors and recipients (27), suggesting that FRH exerts interdependent effects on neutrophils and endothelium. Additional in vivo, studies in mice, and in vitro studies of human lung endothelial cells showed that FRH may exert its effects on neutrophil migration by activating extracellular signal-related kinases (ERK) and p38 mitogen-activated protein (MAP) kinases (27). Additional ongoing studies in our laboratory also demonstrate that mice exposed to FRH shift β2-integrin expression on circulating neutrophils from Mac-1 to LFA-1; however the consequences of this effect are not yet known.
In the models of both intratracheal LPS-induced lung injury (21) and pulmonary oxygen toxicity (22), co-exposure to FRH greatly increased the extravasation of serum protein into the bronchoalveolar compartment, indicating a loss of both endothelial and epithelial barrier function. Exposing human lung endothelial cells to a temperature of 39.5°C augments tumor necrosis factor (TNF)-α induced opening of the paracellular pathway to macromolecules without causing cell injury (26, 28). By contrast, FRH exposure caused marked airway epithelial injury in intratracheal LPS-challenged mice (22, 29). Lipke et al. (29) recently showed that exposing the mouse lung epithelial-cell line MLE15 and primary cultured mouse lung epithelial cells to FRH enhanced TNFα-induced apoptosis.
Taken together, these animal and cell-culture studies demonstrate that exposure to FRH in the setting of acute lung injury or infection augments development of the central pathophysologic features of human adult respiratory distress syndrome (ARDS), including neutrophil accumulation, loss of endothelial and epithelial barrier function, and epithelial injury (30). Adult respiratory distress syndrome occurs in one-fourth of humans with heat stroke (31), demonstrating that exposure to hyperthermia is sufficient to activate some of the pathways leading to neutrophil accumulation and lung injury. Such effects of FRH may also contribute to pathogenesis of the acute lung injury that complicates influenza and other respiratory viral infections.
CHALLENGES TO FEVER MANAGEMENT IN CRITICALLY ILL PATIENTS
Although the retrospective clinical studies and animal and cell-culture studies discussed above clearly establish the importance of prospective studies of fever management in critically ill patients, there is a surprising lack of effective therapies for fever management in this patient population. Acetaminophen is largely ineffective in critically ill patients (32, 33). Nonsteroidal anti-inflammatory agents such as ibuprofen are more effective in reducing fever (11), but the associated toxicity profile (e.g., renal toxicity and platelet dysfunction) raises concerns about their use in many critically ill patients. Physical cooling methods can reduce core temperature with variable efficiency, but all methods for accomplishing this cause shivering (34–36) and increase the metabolic rate (37–39).
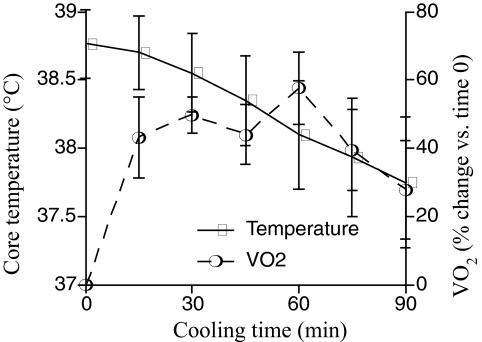
To quantify the metabolic and cardiopulmonary stress produced by external cooling in critically ill patients, we analyzed the cooling rate, rate of oxygen consumption (VO2), heart and respiratory rate, and blood pressure in 6 patients with systemic inflammatory response syndrome during external cooling for fever that persisted despite acetaminophen treatment. Two Cincinnati Sub-Zero Blanketrol II cooling blankets (Cincinnati: Sub-Zero Products, Inc., Cincinnati, OH) set to 4°C were placed above and below the patient, respectively, and axillary and inguinal icepacks were applied. Vo2 was measured with a Viasys Vmax 229 metabolic cart (CareFusion, Inc., San Diego, CA). Baseline values were established over a 15-minute period and Vo2 was measured every 15 minutes during the 90 min cooling period (Figure 3). The change in Vo2 was analyzed by calculating the maximal increase in Vo2 during cooling as compared with its baseline value, as well as by calculating the area under the curve of Vo2 versus time, using the trapezoidal rule. The core temperature before cooling (mean ± SD) was 38.3 ± 0.25°C, and decreased by 0.67°C per hour during cooling. During cooling, Vo2 reached maximal levels (mean ± SEM) of 57.6 ± 10.5% above basal levels. Analysis of the area under the curve revealed a 34.8 ± 7.5% increase in oxygen consumption for the 90-minute, cooling period as compared with its pre-cooling level. Mean arterial pressure increased by 15.1 ± 4.6%. These data reveal the limited effectiveness and metabolic and cardiovascular consequences of physical cooling that limit its success in febrile, critically ill patients, and demonstrate the feasibility of analyzing new cooling protocols in the ICU. The dangers of such an approach in critically ill patients are demonstrated by the results of a study of fever management in patients with non-neurologic trauma (40). The authors reported a 7-fold higher mortality in patients who received aggressive fever management (acetaminophen for a core temperature >38.5°C and a cooling blanket for a temperature > 39.5°C) than in those receiving permissive treatment (acetaminophen and a cooling blanket for a core temperature >40°C). Given the poor antipyretic activity of acetaminophen in this population, the metabolic and cardiovascular stress during external cooling may have contributed to the excess mortality in the patients who had aggressive fever management. Furthermore, 4 of the 7 deaths in the group having aggressive fever management occurred in patient >73 years old, identifying the elderly as possibly more vulnerable to the adverse consequences of external cooling. Several pharmacologic agents have been reported to reduce the shivering component of the compensatory thermogenic response to cooling; however, most of the studies in which this was found were done in the setting of forced hypothermia or post-anesthesia shivering. Paralytic agents have been most effective for reducing shivering (41), but have a high complication rate in ICU patients (42). Other agents, including meperidine, dexmedetomidine, buspirone, and clonidine, are of limited use because of sedation and hypotension (39, 43, 44).
Fig. 3.
Metabolic consequences of external cooling in febrile, critically ill patients. Six patients with SIRS and persistent fever >38.3°C despite receiving acetaminophen were subjected to physical cooling with two Cincinnati Sub-zero Blanketrol II cooling blankets set to 4°C, with one blanket above and one below the patient, and with axillary and inguinal icepacks. Vo2 was measured with a Viasys Vmax 229 metabolic cart for 15 minutes before cooling, and the measurement was repeated every 15 minutes during a 90-minute cooling period. Values are given as mean ± SE.
SUMMARY
We have provided information suggesting that the increase in core temperature that occurs during fever or other hyperthermic states is a potent biologic response modifier with consequences that are profound but difficult to predict, especially in the critically ill. Optimal fever management will require better understanding of the underlying mechanisms through which FRH exerts its effects, and empiric studies of fever management in well-characterized disease states. However, such clinical studies require the development of safer and more effective tools for fever management.
ACKNOWLEDGEMENTS
Dr. Hasday thanks his colleague Matthew Weir for the latter's support. This work was supported by grants GM066855, HL69057, and HL085256 (from the NIH to Dr. Hasday), and by a VA Merit Review grant to Dr. Hasday.
Footnotes
Potential Conflicts of Interest: None disclosed.
DISCUSSION
Sacher, Cincinnati: This brings to mind malarial therapy for cèrtain diseases. Certainly syphilis was one of the infections treated in this way, but also apparently in China they are using malarial therapy for the treatment of AIDS. Do you have any comment on that?
Hasday, Baltimore: First, using malarial therapy is not quite the same as applying febrile-range hyperthermia through increased ambient temperature. With malarial therapy the increased temperature is induced and accompanied by the generation of high levels of pyretic and pro-inflammatory cytokines. I heard the results of a study of extreme hyperthermia in patients with AIDS presented at a hyperthermia meeting. The study was performed in Siberia, and hyperthermia was achieved by hot water immersion of patients who had to be sedated and mechanically ventilated to withstand the procedure. The data that were presented showed that this therapy resulted in long-lasting remission with an undetectable viral load. However, I have not yet seen the data published.
Henrich, San Antonio: Thank you, Jeff, for that very nice presentation. So, on the one hand, hyperthermia stimulates the migration of white cells to kill the bacteria that might be present in infection, but on the other hand it seems that those cells promote the leakage that would produce the untoward effects of adult respiratory distress syndrome and the downward spiral that occurs in patients in ICUs. So it would seem like the logical next step would be combination therapy, whereby you might use hyperthermia, but then if you had something that could retard The leakage, you could use that in combination with it, and you might get the benefit of both arms of a therapeutic strategy. Is that along the right track?
Hasday, Baltimore: That's correct. Obviously the idea is to apply the beneficial actions of febrile-range hyperthermia and avoid the harmful effects by either blocking the harmful effects during fever or by pharmacologically reproducing the beneficial effects. We are currently trying to develop agents that selectively block phosphorylation of certain p38 MAP kinase substrates that are phosphorylated in response to febrile-range hyperthermia and which may exert harmful effects. Combining hyperthermia (or fever) with agents such as these might preserve some of the benefit of hyperthermia or fever while limiting the harmful effects.
Suthanthiran, New York: I really enjoyed your presentation. Regarding the switch in adhesion molecule expression from Mac-1 to LFA-1, and its contribution to neutrophil migration, is it possible to think about using an anti-LFA-1 antibody to block this interaction so as to preserve the beneficial effects of neutrophil trafficking at sites other than the lung. An approach like this may preserve the benefit of fever without paying the price.
Hasday, Baltimore: I agree. This is similar to the concept suggested by Dr. Henrich. The important first step is to recognize that temperature matters during inflammation, infection, and injury. I consider myself an apostle for getting out the message that temperature does exert profound biologic effects. Until we understand all of its effects and consequences, it is going to be very difficult to develop therapeutic management strategies that can maximize the beneficial effects and minimize the harmful effects of fever and hyperthermia.
Southwick, Gainsville: I want to tie your experiments to the talk by Jerry Mandell on bioterrorism. That lethal factor released by anthrax actually blocks, through an MAP kinase kinase, the p38 pathway, and it turns out that when you block that p38 pathway, you block the phosphorylation of a heat-shock protein called HSP27. As its cycles, HSP27 probably releases actin monomers, allows them to polymerize, and thus allows neutrophils to move. Therefore, I think that may be part of the mechanism you are seeing.
Hasday, Baltimore: I agree completely. Although there was not adequate time to address it in my presentation, one of the actions of p38 that may have consequences for leukocyte migration and endothelial barrier function is phosphorylation of HSP27. It has been shown that p38, via the HSP27 pathway, contributes to leakage of lung fluid, and just recently Paul Hassoun's group at Johns Hopkins published a study showing in a mouse model of ventilator-induced lung injury that blocking the phosphorylation of HSP27 mitigated lung injury.
Kenney, Madison: Hyperthermia has also been shown to induce massive changes in a post-translational modification called sumoylation. Have you looked at that?
Hasday, Baltimore: You make an excellent point. Hyperthermia has been shown to cause several types of post-translational protein modifications, including sumoylation of heat shock factor-1, which is the central regulatory molecule controlling the heat-shock response, as well as influencing several genes involved in the inflammatory response. The total level of sumoylated proteins increases in cells exposed to febrile-range hyperthermia, including some of the molecules we believe are related to lung injury, but we have not yet surveyed all the molecules that are modified.
Stevenson, Palo Alto: I'm a neonatologist and the question I have relates to the newborn. Are you or anybody else in your field looking at the newborn as a model, because newborns really don't regulate their temperature well for a variety of reasons, and they are also more vulnerable to infections. The question is whether there is a relationship there that we should be looking at?
Hasday, Baltimore: The situation with temperature regulation in neonates is very different than in adults, as neonates do not usually develop fever in the neonatal ICU, but more often are hypothermic. Temperature regulation in human newborns resembles temperature regulation in our model, in which the mouse hypothalamus attempts to increase core temperature in the face of infection, but fails because of insufficient effector mechanisms. In the case of the mouse, the failure is caused by the animal's large surface-to-mass ratio. In this regard, neonates physiologically resemble mice, but are less furry and more expensive to maintain. However, when neonates are sick, they cry, which activates a response in the parents to pick them up and cuddle them. This may help the neonate increase its core temperature, much as increasing ambient temperature in our mouse model allowed an increase in core temperature. This parent-neonate interaction is less likely to occur in the neonatal ICU, and to my knowledge a study to measure the effects of this parental behavior on core temperature in a sick neonate has not yet been done.
REFERENCES
- 1.Kluger MJ, Kozac W, Conn CA, Leon LR, Soszynski D. The adaptive value of fever. In: Mackowiak PA, editor. Fever: Basic Mechanisms and Management. ed. 2. New York: Raven Press; 1996. pp. 255–66. [Google Scholar]
- 2.Horvath S, Spurr G, Hutt B, Hamilton L. Metabolic cost of shivering. J Appl Physiol. 1956;8:595–602. doi: 10.1152/jappl.1956.8.6.595. [DOI] [PubMed] [Google Scholar]
- 3.Manthous C, Hall J, Olson D, et al. Effect of cooling on oxygen consumption in febrile critically ill patients. Am J Respir Crit Care Med. 1995;151:10–4. doi: 10.1164/ajrccm.151.1.7812538. [DOI] [PubMed] [Google Scholar]
- 4.Schumacker P, Rowland J, Saltz S, Nelson D, Wood L. Effects of hyperthermia and hypothermia on oxygen extraction by tissues during hypovolemia. J Appl Physiol. 1987;63:1246–52. doi: 10.1152/jappl.1987.63.3.1246. [DOI] [PubMed] [Google Scholar]
- 5.Hasday JD, Fairchild KD, Shanholtz C. The role of fever in the infected host. Microbes Infect. 2000;2:1–14. doi: 10.1016/s1286-4579(00)01337-x. [DOI] [PubMed] [Google Scholar]
- 6.Doran TF, DeAngelis C, Baumgardner RA, Mellits ED. Acetaminophen: more harm than good for chicken pox? J Pediatr. 1989;114:1045–8. doi: 10.1016/s0022-3476(89)80461-5. [DOI] [PubMed] [Google Scholar]
- 7.Graham NMH, Burrell CJ, Douglas RM, Debelle P, Davies L. Adverse effects of aspirin, acetaminophen, and ibuprofen on immune function, viral shedding, and clinical status in rhinovirus-infected volunteers. J Infect Dis. 1990;162:1277–82. doi: 10.1093/infdis/162.6.1277. [DOI] [PubMed] [Google Scholar]
- 8.Stanley ED, Jackson GG, Panusarn C, Rubenis M, Dirda V. Increased viral shedding with aspirin treatment of rhinovirus infection. JAMA. 1975;231:1248–51. [PubMed] [Google Scholar]
- 9.Mackowiak PA, Wasserman SS, Levine MM. An analysis of the quantitative relationship between oral temperature and severity of illness in experimental shigellosis. J Infect Dis. 1992;166:1181–4. doi: 10.1093/infdis/166.5.1181. [DOI] [PubMed] [Google Scholar]
- 10.The Veterans Administration Systemic Sepsis Cooperative Study Group. Effect of high-dose glucocorticoid therapy on mortality in patients with clinical signs of systemic sepsis. N Engl J Med. 1987;317:659–65. doi: 10.1056/NEJM198709103171102. [DOI] [PubMed] [Google Scholar]
- 11.Bernard GR, Wheeler AP, Russell JA, et al. The effects of ibuprofen on the physiology and survival of patients with sepsis. The Ibuprofen in Sepsis Study Group [see comments] N Engl J Med. 1997;336:912–8. doi: 10.1056/NEJM199703273361303. [DOI] [PubMed] [Google Scholar]
- 12.Clemmer TP, Fisher CJ, Jr, Bone RC, Slotman GJ, Metz CA, Thomas FO. Hypothermia in the sepsis syndrome and clinical outcome. The Methylprednisolone Severe Sepsis Study Group [see comments] Crit Care Med. 1992;20:1395–401. doi: 10.1097/00003246-199210000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Bryant RE, Hood AF, Hood CE, Koenig MG. Factors affecting mortality of gram-negative rod bacteremia. Arch Intern Med. 1971;127:120–8. [PubMed] [Google Scholar]
- 14.Mackowiak PA, Browne RH, Southern PM, Jr., Smith JW. Polymicrobial sepsis: an analysis of 184 cases using log linear models. Am J Med Sci. 1980;280:73–80. doi: 10.1097/00000441-198009000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Weinstein MP, Iannini PB, Stratton CW, Eickhoff TC. Spontaneous bacterial peritonitis. A review of 28 cases with emphasis on improved survival and factors influencing prognosis. Am J Med. 1978;64:592–8. doi: 10.1016/0002-9343(78)90578-8. [DOI] [PubMed] [Google Scholar]
- 16.Hoefs JC, Canawati HN, Sapico FL, Hopkins RR, Weiner J, Montgomerie JZ. Spontaneous bacterial peritonitis. Hepatology. 1982;2:399–407. doi: 10.1002/hep.1840020402. [DOI] [PubMed] [Google Scholar]
- 17.DuPont HL, Spink WW. Infections due to gram-negative organisms: an analysis of 860 patients with bacteremia at the University of Minnesota Medical Center, 1958–1966. Medicine (Baltimore) 1969;48:307–32. doi: 10.1097/00005792-196907000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Hodgin UG, Sanford JP. Gram-negative rod bacteremia. An analysis of 100 patients. Am J Med. 1965;39:952–60. doi: 10.1016/0002-9343(65)90118-x. [DOI] [PubMed] [Google Scholar]
- 19.Sareh H, Tulapurkar ME, Shah NG, Singh IS, Hasday JD. Response of mice to continuous 5-day passive hyperthermia resembles human heat acclimation. Cell Stress Chaperon. 2010 doi: 10.1007/s12192-010-0240-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang Q, Cross AS, Singh IS, Chem TT, Viscardi RM, Hasday JD. Febrile core temperature is essential for optimal host defense in bacterial peritonitis. Infect Immun. 2000;68:1265–70. doi: 10.1128/iai.68.3.1265-1270.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rice P, Martin E, He J-R, et al. Febrile-range hyperthermia augments neutrophil accumulation and enhances lung injury in experimental gram-negative bacterial pneumonia. J Immunol. 2005;174:3676–85. doi: 10.4049/jimmunol.174.6.3676. [DOI] [PubMed] [Google Scholar]
- 22.Hasday J, Garrison A, Singh I, et al. Febrile-range hyperthermia augments pulmonary neutrophil recruitment and amplifies pulmonary oxygen toxicity. Am J Pathol. 2003;162:2005–17. doi: 10.1016/S0002-9440(10)64333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernheim HA, Bodel PT, Askenase PW, Atkins E. Effects of fever on host defence mechanisms after infection in the lizard Dipsosaurus dorsalis. Br J Exp Pathol. 1978;59:76–84. [PMC free article] [PubMed] [Google Scholar]
- 24.Ellis G, Carlson D, Hester L, Bagby G, Singh IS, Hasday J. G-CSF, but not corticosterone mediates circulating neutrophilia induced by febrile-range hyperthermia. J Appl Physiol. 2005;98:1799–804. doi: 10.1152/japplphysiol.01376.2004. [DOI] [PubMed] [Google Scholar]
- 25.Singh IS, Gupta A, Nagarsekar A, et al. Heat Shock Co-activates interleukin-8 transcription. Am J Respir Cell Mol Biol. 2008;39:235–42. doi: 10.1165/rcmb.2007-0294OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasday JD, Bannerman D, Sakarya S, et al. Exposure to febrile temperature modifies endothelial cell response to tumor necrosis factor-α. J Appl Physiol. 2001;90:90–8. doi: 10.1152/jappl.2001.90.1.90. [DOI] [PubMed] [Google Scholar]
- 27.Almutairy EA, Shah NG, Tulapurkar ME, Hasday JD. Febrile range hypethermia (FRH) augments neutrophil recruitment to lung via modulation of Lung endothelium and neutrophils. Am J Respir Crit Care Med. 179:A4012. 209. [Google Scholar]
- 28.Shah NG, Tulapurkar ME, Almutairy EA, Hasday JD. Febrile range hyperthermia augments TNF-α induced permeability in human microvascular endothelial cells in the lung (hMVEC-L) Am J Respir Crit Care Med. 2009;179:A4014. [Google Scholar]
- 29.Lipke AB, Matute-Bello G, Herrero R, et al. Febrile-range hyperthermia augments lipopolysaccharide-induced lung injury by a mechanism of enhanced alveolar epithelial apoptosis. J Immunol. 2010;184:3801–13. doi: 10.4049/jimmunol.0903191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomashefski JF., Jr Pulmonary pathology of acute respiratory distress syndrome. Clin Chest Med. 2000;21:435–66. doi: 10.1016/s0272-5231(05)70158-1. [DOI] [PubMed] [Google Scholar]
- 31.el-Kassimi FA, Al-Mashhadani S, Abdullah AK, Akhtar J. Adult respiratory distress syndrome and disseminated intravascular coagulation complicating heat stroke. Chest. 1986;90:571–4. doi: 10.1378/chest.90.4.571. [DOI] [PubMed] [Google Scholar]
- 32.Greenberg RS, Chen H, Hasday JD. Acetaminophen has limited antipyretic activity in critically ill patients. J Crit Care. 2010;25:363 el–7. doi: 10.1016/j.jcrc.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mackenzie I, Forrest K, Thompson F, Marsh R. Effects of acetaminophen administration to patients in intensive care. Intens Care Med. 2000;26:1408. doi: 10.1007/s001340000614. [DOI] [PubMed] [Google Scholar]
- 34.Badjatia N, Strongilis E, Gordon E, et al. Metabolic impact of shivering during therapeutic temperature modulation: the Bedside Shivering Assessment Scale. Stroke. 2008;39:3242–7. doi: 10.1161/STROKEAHA.108.523654. [DOI] [PubMed] [Google Scholar]
- 35.Carhuapoma JR, Gupta K, Coplin WM, Muddassir SM, Meratee MM. Treatment of refractory fever in the neurosciences critical care unit using a novel, water-circulating cooling device. A single-center pilot experience. J Neurosurg Anesthesiol. 2003;15:313–8. doi: 10.1097/00008506-200310000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Mayer SA, Kowalski RG, Presciutti M, et al. Clinical trial of a novel surface cooling system for fever control in neurocritical care patients. Crit Care Med. 2004;32:2508–15. doi: 10.1097/01.ccm.0000147441.39670.37. [DOI] [PubMed] [Google Scholar]
- 37.Eyolfson DA, Tikuisis P, Xu X, Weseen G, Giesbrecht GG. Measurement and prediction of peak shivering intensity in humans. Eur J Appl Physiol. 2001;84:100–6. doi: 10.1007/s004210000329. [DOI] [PubMed] [Google Scholar]
- 38.Kimberger O, Ali SZ, Markstaller M, et al. Meperidine and skin surface warming additively reduce the shivering threshold: a volunteer study. Crit Care. 2007;11:R29. doi: 10.1186/cc5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mokhtarani M, Mahgoub AN, Morioka N, et al. Buspirone and meperidine synergistically reduce the shivering threshold. Anesth Analg. 2001;93:1233–9. doi: 10.1097/00000539-200111000-00038. [DOI] [PubMed] [Google Scholar]
- 40.Schulman CI, Namias N, Doherty J, et al. The effect of antipyretic therapy upon outcomes in critically ill patients: a randomized, prospective study. Surg Infect (Larchmt) 2005;6:369–75. doi: 10.1089/sur.2005.6.369. [DOI] [PubMed] [Google Scholar]
- 41.Dupuis JY, Nathan HJ, DeLima L, Wynands JE, Russell GN, Bourke M. Pancuronium or vecuronium for treatment of shivering after cardiac surgery. Anesth Analg. 1994;79:472–81. doi: 10.1213/00000539-199409000-00012. [DOI] [PubMed] [Google Scholar]
- 42.Gehr LC, Sessler CN. Neuromuscular blockade in the intensive care unit. Semin Respir Crit Care Med. 2001;22:175–88. doi: 10.1055/s-2001-13831. [DOI] [PubMed] [Google Scholar]
- 43.Doufas AG, Lin CM, Suleman MI, et al. Dexmedetomidine and meperidine additively reduce the shivering threshold in humans. Stroke. 2003;34:1218–23. doi: 10.1161/01.STR.0000068787.76670.A4. [DOI] [PubMed] [Google Scholar]
- 44.Schwarzkopf KR, Hoff H, Hartmann M, Fritz HG. A comparison between meperidine, clonidine and urapidil in the treatment of postanesthetic shivering. Anesth Analg. 2001;92:257–66. doi: 10.1097/00000539-200101000-00051. [DOI] [PubMed] [Google Scholar]