Abstract
Adenosine plays an important role in the pathophysiology of heart failure and in myocardial protection during ischemia and reperfusion. The action of adenosine in the heart is mediated by four G-protein-coupled receptors: A1-AR and A3-AR, which act via Gα1, and A2A-AR and A2B-AR, which act via Gαs. Understanding of cellular signaling pathways triggered by adenosine has been complicated by the availability of only partially specific adenosine agonists/antagonists. Adenosine signaling appears to be at times redundant in receptor function, and cellular signaling pathways for adenosine are multiple, parallel, and interrelated. Data obtained about the specific role of individual adenosine receptors, through the genetic modulation of receptors in murine hearts have provided important information about the role of adenosine receptors in the heart. Here we review existing data and present new results that clarify the function of individual adenosine receptors in the heart and their role in the development of left ventricular dysfunction, and about the downstream signaling systems that are modified by adenosine receptor activation.
In 1929, Drury and Szent-Gyorgyi published their landmark article in the Journal of Physiology in which they demonstrated that simple extracts from heart muscle, brain, kidney, and spleen had a “definite and transient effect upon the mammalian heart,” and reported their finding that the substance responsible for this effect was adenosine (1). They recognized that adenosine would “slow the rate of beating, impair conduction from auricle to ventricle, and arrest experimentally produced auricular fibrillation.” Dr. Robert M. Berne found in 1963 that adenosine was a critical signaling molecule in the heart, and hypothesized that adenosine, released by cardiac myocytes, exerts its effects on the vasomotor tone of the coronary arteries and thus couples metabolic demands of the myocardium to coronary blood flow (2).
More recently, interest in the role of adenosine in the heart has been due to a series of studies demonstrating that myocardial adenosine levels increase in response to metabolic stress and cell damage, such as inflammation, hypoxia, ischemia, and trauma (3, 4), and that this protects the heart during ischemia and reperfusion (5). Furthermore, adenosine mediates the effects of pre-ischemic conditioning—the phenomenon whereby repetitive and brief episodes of ischemia “pre-condition” the heart so that the subsequent total occlusion of a coronary artery results in a myocardial infarction that is small in relation to that seen in animals that have not had such pre-conditioning (6). Nonetheless, the exact mechanisms responsible for the salutary effects of adenosine remain undefined (7, 8).
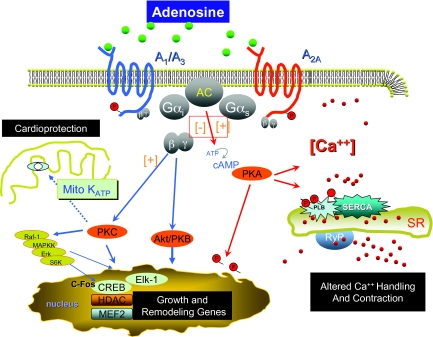
Adenosine is a ubiquitous purine nucleoside generated by the dephosphorylation of the 51 adenosine monophosphate (AMP) and by the hydrolysis of S-adenosylhomocysteine (9). Adenosine action in the heart is mediated by four G-protein-coupled receptors: A1-AR and A3-AR, which act via Gαi, and A2A-AR and A2B-AR which act via Gα (9). When a receptor is stimulated, the α subunit releases guanosine diphosphate (GDP), allowing guanosine triphosphate (GTP) to bind in its place, and the G-protein complex dissociates into two activated components: an α subunit and a βγ subunit. Gαi and Gαs have been classically associated with inhibition and stimulation (respectively) of adenyl cyclase, and activation of the Gαs pathway is important in the modulation of calcium handling (Figure 1) (9). Gβγ mediates adenosine action in the cell nucleus by modifying the expression of genes involved in cell growth and remodeling (10, 11).
Fig. 1.
Signaling pathway for adenosine receptors.
Although the role of adenosine in mediating the effects of ischemic pre-conditioning and protection from reperfusion injury is well known, we hypothesized that adenosine and adenosine receptor subtypes could play a critical role in the development of left ventricular (LV) dysfunction, hypertrophy, and failure. This hypothesis was due in large part to the fact that the family of cardiac adenosine receptors activated a cascade of downstream signaling molecules, many of which were implicated in cardiac hypertrophy, apoptosis, or the regulation of intracellular calcium fluxes (Figure 1). These critical signaling molecules, which act downstream of the adenosine receptors, include Akt, protein kinase C (PKC), the nuclear regulatory proteins MEF2, CERB, HDAC, and c-Fos, the mitochondrial KATP channel, and the protein kinase A (PKA) signaling system that regulates the function of the calcium regulatory proteins phospholamban and Ca2+ ATP-ase.
Our speculation that adenosine and adenosine receptors might behave quite differently in the failing heart than in the presence of cardiac ischemia was also supported by our studies in mice with heart failure secondary to over-expression of the pro–inflammatory cytokine TNFα or to trans-aortic banding. While hearts exposed to cardiac ischemia demonstrate marked increases in tissue levels of adenosine, adenosine levels were decreased by 70% in hearts with LV dysfunction secondary to over–expression of TNFα. In addition, in the mice with heart failure, A1-AR levels were increased by 4-fold, and a 50% reduction in the levels of A2A-AR was observed (12).
Early attempts to tease apart the regulatory properties of the adenosine receptors have been limited by the highly homologous nature of the three cardiac adenosine receptors and by the fact that adenosine receptors are ubiquitous and can be found in the vasculature as well as in the central nervous system and the kidney. Therefore, we and others have utilized transgenic models and gene-transfer technology to perturb the expression of selected adenosine receptor subtypes in the myocardium.
GENETIC MANIPULATION OF CARDIOVASCULAR A1-ADENOSINE RECEPTORS
Matherne and colleagues created one of the first transgenic murine models with altered adenosine signaling (13). They designed a construct containing a rat A1-adenosine receptor, human growth hormone polyadenylylation signal, and mutated α-Major histocompatibility complex (MHC) promoter. Over-expression of up to 100-fold of this functional A1-adenosine receptor in C57/black mice was associated with bradycardia, delayed conduction through the sinoatrial (SA)and atrioventricular(AV) nodes, atrial arrhythmias, and moderate ventricular hypertrophy (13–16). Altered response to β-adrenoceptor signaling (13) was associated with augmented A1-AR signaling. Furthermore, A1-AR over-expression mediated enhanced ischemic tolerance during ischemia-reperfusion injury (14) and hypertrophic remodeling of the myocardium (13, 16). A limitation of these studies was that the transcript was expressed during late fetal development as well as during the early post-natal period. Furthermore, the studies were performed in C57/black mice, a strain that may obviate the ability to discern the development of heart failure.
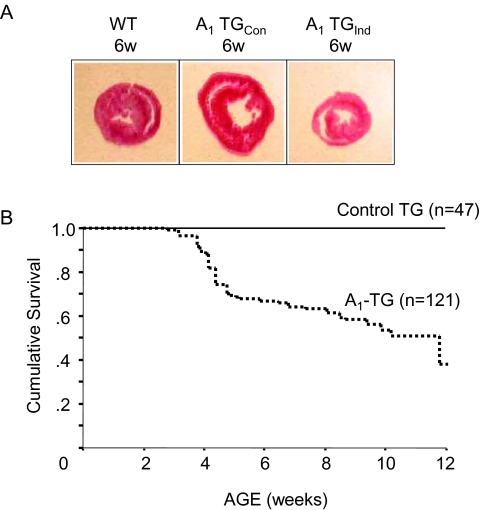
To mitigate some of the limitations of constitutive over-expression of a G-protein-coupled receptor transgene, we created transgenic mice in a FVB background (12), using a construct of human A1-AR cDNA cloned into a cardiac-specific vector composed of a modified mouse a-myosin heavy-chain promoter fused with nucleotide binding sites for tetracycline transactivating factor (tTA). This experimental design allowed on/off switching of A1-AR over-expression. In the presence of doxycycline in the diet of pregnant or nursing mouse mothers or in the food of young mice that had been weaned, the transgene was turned-off, whereas in the absence of doxycycline the transgene was turned-on. Constitutive A1-AR overexpression induced a dilated cardiomyopathy and death at 6 to 12 weeks (Figure 2). Mice in which A1-AR over-expression was induced in adulthood achieved a normal phenotype after birth and a had more robust survival than did mice with constitutive over-expression of the transgene; however, these mice still demonstrated a decreased survival and LV dysfunction later in life as compared with non-transgenic littermate controls.
Fig. 2.
Constitutive induction (Con) and adult induction (Ind) of A1-AR expression in transgenic mice. For adult A1-AR induction doxycycline, (DOX) was removed from transgenic mice at 3 weeks of age (Panel A): HE staining of 6-Week-old mouse myocardium cross-section. (Panel B): Kaplan-Meier survival curves for mice constitutively expressing A1-AR (A1-transgenic construct [TGCon]) and for mice with A1-AR induced at 3 weeks of age by removal of DOX (A1-TGInd).
Both constitutive and controlled overexpression of the A1-AR was associated with ventricular hypertrophy, an increase in the size and length of individual myocytes, a lower heart rate, increased ventricular fibrosis, diminished calcium cycling, and recapitulation of the heart-failure “genotype,” including increased production of atrial natriuretic peptide (ANP), and decreased sarco(endo) plasmic reticulum calcium-ATPase (SERCA) and phospholamban expression. Activity of the cardoprotective kinase. Akt was also decreased (17). The change in ventricular function could not be attributed to a decrease in heart rate, because diminished ventricular function was also seen when hearts from transgenic mice were isolated, perfused, and paced at a defined heart rate.
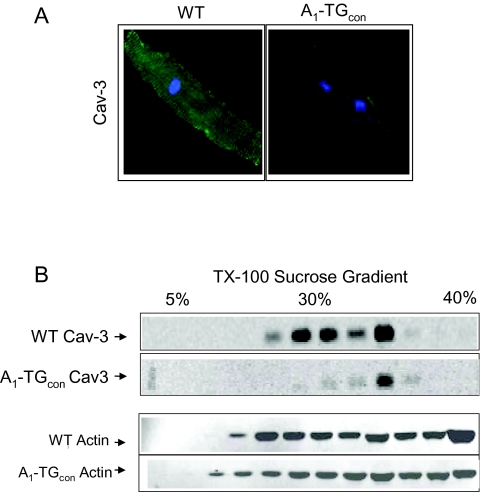
A1-AR-induced cardiomyopathy was also associated with a marked decrease in the expression of caveolin-3: levels of both caveolin-3 mRNA and caveolin-3 protein were decreased (Figure 3) (18). Caveolin-3 anchors cardiac receptors and calcium-signaling proteins in the caveolae of the cardiac T-tubule system, and is also crucial in A1-AR internalization, recycling, and signal transduction. The nuclear regulatory protein myogenin is both necessary and sufficient for expression of the caveolin-3 gene, while the protein ID2 can limit the ability of myogenin to interact with its binding sites in the promoter region of the caveolin-3 gene. Over-expression of the A1-AR had no effect on cardiac levels of myogenin or ID2. Therefore, the mechanism whereby over-expression of A1-AR decreases the expression of caveolin-3 remains to be defined.
Fig. 3.
(Panel A): Immunofluorescence staining of caveolin-3 isolated myocytes. Confocal images represent at least 3 mice/genotype group and a minimum of 20 myocytes/mice examined. (Panel B): Caveolin-3 (Cav3) protein expression profile in sucrose gradient. Ventricular extracts from 10-week-old WT and A1-TGcon mice were separated in a 5%-30%–40% step-sucrose gradient and probed with indicated antibodies.
GENETIC MANIPULATION OF CARDIOVASCULAR A2A-ADENOSINE RECEPTOR
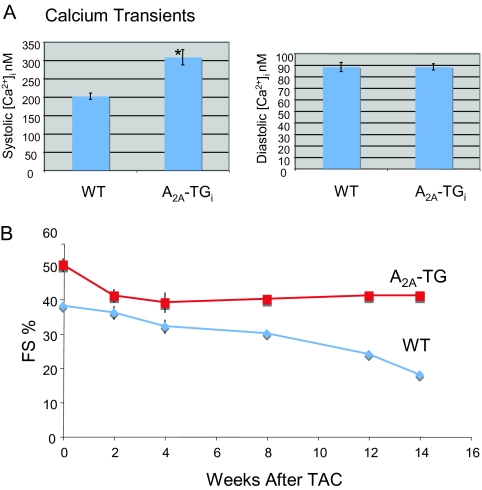
To better understand the role of the A2A-AR, we generated transgenic mouse lines with high and low expression of the A2A-AR by putting the cDNA for the human receptor under control of the cardiac-specific promoter, with the on/off switch for gene expression that we used in generating mice with A1-AR over-expression. In the absence of doxycycline, there was expression of the A2A-AR at levels four-times those seen in the normal non-transgenic mice in the line with high levels of A2A-AR expression and approximately a two-fold greater expression than in the mouse line with low levels of A2A-AR expression. Constitutive overexpression of the A2A-AR in young mice was associated with increased cardiac contractility, higher heart rates, and a small increase in LV mass (Figure 4A). Increased SERCA2 expression and Ca2+ uptake by the sarcoplasmic reticulum were found in association with augmented signaling through the A2A-AR, and correlated with physiologic changes (19). The role of A2A-AR-mediated signaling in this mouse model during ischemia-reperfusion injury suggested salutary benefits of the A2A-AR on cardiac function and cardiac hemodynamics, but the effects of long-term, controlled over-expression of the A2A-AR remain to be determined.
Fig. 4.
(Panel A): Calcium transient response in WT and A2A-TG mice. Adult myocytes from WT and A2A-TG mice were used to measure systolic and diastolic [Ca2+]i* *P <0.01. (Panel B): Echocardiography of A2A-TG and WT mice. Wild-type (n = 17) and A2A-TG (n = 10) mice were evaluated at 0, 2, 4, 8, and 14 weeks after TAC—transaortic constriction Graph shows percent fractional shortening (% FS).
Recently, we have shown that adult over-expression of the cardic A2A-AR modifies the heart's response to pressure overload. Trans-thoracic banding in wild-type (WT) mice leads to decreased cardiac function, an increase in end-systolic and end-diastolic dimensions, a greater ratio of heart weight to body weight, and marked fibrosis as compared with these measures in sham-operated controls. These changes were markedly attenuated by over expression of the A2A-AR (P < 0.001 for each measure) (Figure 4B).
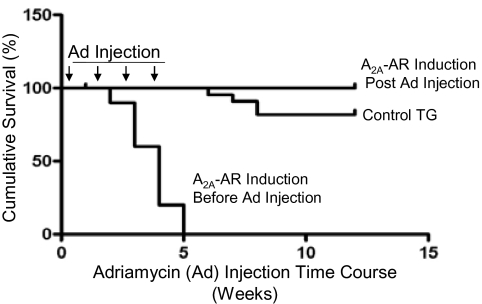
Interestingly, over-expression of A2A-AR is not cardioprotective in all experimental situations. When WT mice were injected intra-perioneally with adriamycin (5 mg/kg/wk for 4 weeks), all of the mice survived. However, when mice over-expressing the A2A-AR were given this same treatment, all of the mice died by 5 weeks (Figure 5). Telemetry showed progressive prolonation of the QT interval, bradyarrhythmias, heart block, and sudden death in the A2A-AR over-expressing mice as compared with WT mice. The duration of the action potential also increased dramatically in the A2A-AR-expressing myocyte of adriamycin-treated mice (20).
Fig. 5.
Timing of A2A-AR expression affected survival after adriamycin treatment. Eight-week-old control and A2A-TG mice were injected with adriamycin (Ad) and monitored over 15 weeks. Expression of the A2A-AR transgene was induced either before (starting at 3 weeks of age) or after cessation of adriamycin administration.
Perhaps the most important finding was that over-expression of the A2A-AR could rescue the heart-failure phenotype in mice with constitutive over-expression of the A1-AR. Concurrent over-expression of A2A-AR and A1-AR resulted in mice with a normal or near-normal cardiac phenotype in terms of ventricular function, survival, and Ca2+ handling (19). In addition, mice concurrently over-expressing the A1-AR and A2A-AR had levels of brain natriuretic peptide (BNP), phosphlamban, and Ca2+-ATPase that mirrored those in non-transgenic controls, and their expression of caveolin-3 was normal. Importantly, low levels of over-expression of the A2A-AR were unable to overcome LV dysfunction in A1-AR over-expressing mice. These results suggested that the normal homeostatic function of the heart requires balanced activation of the A1-AR and A2A-AR—a stoichoimetric relationship that occurs under physiologic conditions because of similarities in the inherent affinity of the A1-AR and A2A-AR for both endogenous and exogenous adenosine. Our results also suggest that pharmacologic manipulation of a single adenosine receptor subtype or a genetic mutation that alters the function of a single adenosine receptor subtype might have adverse affects on the heart.
ADENOSINE RECEPTOR “KNOCKOUT” STUDIES
Studies in which adenosine receptors have been genetically kocked-out have not significantly improved our understanding of their role in heart failure, owing largely to the fact that in most of these models, adenosine receptors have been deleted from all organs. For example, mice in which the A1-AR has been genetically deleted appear to have a normal cardiac phenotype in the absence of stress (21, 22). The effect of the A1-AR deletion on coronary blood flow, vascular sensitivity (23–25), and ischemic preconditioning (26, 27) remain to be defined (26–28).
Studies done with A2A-AR-knockout transgenic mice have highlighted the role of the A2A-AR in blood pressure control, local vasodilatation, and coronary vasoregulation, as well as in regulating the inflammatory response following myocardial ischemia-reperfusion injury and in regulating angiogenesis. Deletion of the A2A-AR was associated with a significant increase in blood pressure and associated bradycardia (29, 30), and with reduced aortic relaxation and endothelial function (31–33). Although A2A-AR-independent arterial dilation in A2A-AR-knockout hearts is probably mediated by the A2B-AR, the receptor was found to be up-regulated in the coronary arteries of A2A-AR knockout mice (34).
Deletion of A2A-AR-mediated signaling augments inflammation in a variety of cells and model systems (35–37), through effects including inhibition of the bone-marrow-derived cell response to injury, specifically by T-lymphocytes (38). Studies of A2A-AR knockout have also implicated a role for the A2A-AR in angiogenesis (39–41). However, the effects of A2A-AR ablation on the development of cardiac hypertrophy and failiure are unknown.
GENETIC MANIPULATION OF CARDIOVASCULAR A3-AR TRANSGENIC A3-AR OVER-EXPRESSION.
Far less is known about the role of the A3-AR in development of the heart failure phenotype than is known about the role of the A2A-AR. Enhanced expression of the A3-AR during early embryogenesis in some strains of mice is lethal (42). In other strains with high levels of receptor expression, a phenotype with prominent bradycardia and arrhythmogenesis emerges (42, 43), while experimental approaches inducing lower levels of A3-AR overexpression have observed its effects only on the cardiac response to myocardial ischemia (42–44). The basis of cardiomyopathy triggered by A3-AR overexpression is thought to be secondary to enhanced Gαi signaling (42).
Mice with an A3-AR-knockout transgene (45) were found to exhibit modulation of inflammatory processes (45), elevations in cAMP in cardiac and vascular tissue, and an exaggerated systemic hypotensive response to exogenous adenosine (although the animals baseline blood pressure was unaltered) (46). Talukder and colleagues provide evidence that A3-AR agonism modestly counters vasodilatation mediated by A2A-ARs (47), and a group of studies demonstrated a cardioprotective effect of the A3-AR (48, 49). Thus, for example, deletion of the A3-AR reduced infarct size (48) without significantly modifying the intracellular energy state (49). However, both the genetic background of the mouse model and the design of ischemic preconditioning studies may be important in understanding the role of the A3-AR (7, 50, 51). The only information regarding the role of the A3-AR in heart failure comes from studies in which this receptor has been knocked out. Lu and colleagues found that genetic ablation of the A3-AR blunted pathologic cardiac remodeling, including hypertrophic growth and fibrotic change (52).
TRANSLATING MOUSE BIOLOGY TO HUMANS: ADENOSINE IN HEART FAILURE
Rolofylline KW-3902, Merck Research Laboratories, West Point, PA was the first receptor-subtype-specific adenosine antagonist to be evalutated for the treatment of decompensated heart failure. This specific A1-AR antagonist, with 890-fold binding selectivity for the A1-AR over the A2A-AR, and no effect on the A3-AR, exerts its effect by inhibiting sodium reabsorption in the proximal tubule of the kidney and by blocking adenosine-mediated constriction of the afferent glomerular arterioles. This results in diuresis and natriuresis. Thus, rolofylline was developed because of its presumed effect on renal function, independently of whatever effects it might have on cardiac muscle.
In early phase I clinical trials diuresis was seen at 3 hours, after a dose of 30 mg of rolofylline, and the effect persisted for up to 8 hours after active drug administration (88). In a phase II trial, 146 patients with decompensated class II-III congestive heart failure (CHF) and an estimated creatinine clearance of 20 to 80 mL/min were randomized to receive placebo or from 1 to 4 doses of rolofylline infused for up to 2 hours daily for up to 3 days. The drug increased urine output, enhanced diuresis, and reduced the need for concomitant intravenous furosemide. One seizure was reported in a patient receiving the highest dose of rolofylline (53). With these encouraging results in preliminary studies, 2,033 patients hospitalized for CHF were randomized in a 2:1 ratio to receive either rolofylline 30 mg/day or placebo, administered as a 4-hour daily infusion that was repeated for 3 days. The primary end-point of the PROTECT trial was a three-category ordered outcome of treatment success, unchanged, patient condition, or treatment failure (54). Secondary end-points were time to death or rehospitalization for cardiovascular or renal causes, and persistent renal impairment.
No significant difference was found among rolofylline-and placebo-treated patients with respect to the primary end-point or the secondary endpoints. A higher rate of persistent renal impairment was noted in the rolofylline group. In addition, more patients in the rolofylline group experienced seizures (11 [0.8%] vs. 0 in the placebo group) as well as strokes (16[1.2%] vs. 3 [0.5%]). Thus, despite promising preliminary findings, the PROTECT trial did not yield a positive results in heart failure, owing in large part to the increased incidence of neurologic events in patients receiving rolofylline. Although the mechanisms responsible for these adverse effects were not identified by the study, one could only conclude that the use of receptor-specific antagonists (or agonists) is not a rational approach to treating heart failure in light of the basic science data showing an adverse effect of receptor subtype-specific interventions.
To further evaluate this hypothesis, we sought to identify whether mutations in individual adenosine-receptor subtypes could predict outcomes in patients with heart failure. We sequenced the genes encoding the human A1-, A2A-, and A3-AR receptors in a cohort of patients with normal cardiac function and in a matched group of patients with idiopathic dilated cardiomyopathy or ischemic heart failure (55). These studies identified a group of genetic variants that occurred in a relevant percentage of the general population. Several of these variants were of particular interest. These consisted of three variants in the 3′-untranslated region of the A1-AR gene (nt 1689 C/T; nt 2206 Tdel, nt 2683 del 36) that predicted a change in the three-dimensional structure of the mRNA tail and thus a change in stability of the mRNA for the receptor; a non-informative single nucleiotide polymorphism (SNP) in the coding region of the A1-AR gene (nt 717 T/G); and an informative SNP in the coding region of the A2A adenosine gene (nt 1509 A/C). The four genetic variants in the A1-AR gene were associated with either an increase or a decrease in infarct size in patients with heart failure secondary to ischemic heart disease and myocardial infarction. In addition, the non-informative SNP at nt 717(T/G) in the coding region of the A1-AR gene was associated with an increase in infarct size as well as with an increase in the combined end-point of death or cardiovascular hospitalization in the same population (Feldman, AM, unpublished data). Thus, the finding that alterations in the genetic structure—and presumably function—of a sub-type-specific AR gene could change the outcome of disease in a population of patients with heart failure supports the findings in mice with controlled or constitutive over-expression of the A1-AR.
CONCLUSIONS AND FUTURE DIRECTIONS
Since 1963, when adenosine was initially identified as an important signaling molecule in the heart by Robert M. Berne, its role in the heart has been extensively studied; however, the vast majority of these studies have focused on the role of adenosine in hearts with ischemic injury. Our recent studies demonstrate that adenosine receptors can play an important role in the development of heart failure. In particular, we have found that normal cardiac homeostasis requires a stoichiometric balance between signaling through the A1/A3 Gαi protein-coupled receptors and the A2A Gαs protein-coupled receptor. The need for “balanced” signaling provides a teleologic explanation for how nature could develop a signaling system in which a single ligand binds to multiple receptor subtypes—some of which activate down-stream signaling pathways having diametrically opposite cellular effects Additional studies will be required to identify the role of novel down-stream signaling proteins and pathways that transform the signals for activation of a specific-receptor subtype into physiologic effects, as these downstream signaling pathways may provide more appropriate targets for future therapeutic interventions for altering the heart failure phenotype and modifying disease progression.
Footnotes
Potential Conflicts of Interest: None disclosed.
DISCUSSION
Hasday, Baltimore: It's been 25 years since I've done anything with adenosine receptors. One of the few things that I remember about them is that they have different βmax values and different affinities for adenosine, and I wonder how the concentration of adenosine may affect the balance between the two signaling pathways.
Feldman, Philadelphia: We've looked at that, and actually the affinity of adenosine for the A1 and the A2A receptor is somewhat similar. Nonetheless, what was interesting is that we have three different lines of the A2A adenosine receptor. Two of the lines that had very low levels of expression would not rescue the mouse cardiac phenotype with A1 receptor over-expression, but the higher expression line would dido this. So we were actually able to tinker with those lines and find the one that actually gave the correct stoichiometric relationship.
Blantz, San Diego: I have two sorts of unrelated questions: In your over-expressed models, did you ever look at the feedback regulatory loop in terms of adenosine generation? Does endonucleotidase activity change or do tissue adenosine levels change as a result of an over-expressed model, because that might spill into the other receptor and have secondary effects that would be confounding?
Feldman, Philadelphia: That's a great question. We actually did look at adenosine levels and they were not different in the two models.
Blantz, San Diego: One second, smaller question. In terms of the human studies, or I guess the ones you described at the end, the kidney has adenosine receptors that act in somewhat opposite directions. Is that part of the complexity?
Feldman, Philadelphia: The interesting part of that story is that there were two large trials done over the past 3 or 4 years, and both looked at an A1 adenosine receptor antagonist and it's role in treating patients with congestive heart failure. The hypothesis was, as you alluded to, that you could antagonize the renal A1 adenosine receptors and in so doing improve diuresis. In fact, those molecules did what we wanted them to do in terms of increasing diuresis. But in the large morbidity-mortality trials, there was actually a signal toward an increase in mortality with an A1 adenosine receptor antagonist, which we think happens because it changes the critical balance in the myocardium between A1- and A2A-mediated receptor signaling. In addition, those patients receiving active drug showed a substantially higher incidence of seizures, because they were also experienced perturbed adenosine receptor balance in the central nervous system. As a result of these studies, further study of both drugs was discontinued.
Shannon, Philadelphia: Elegant stuff as always. Does the A2A receptor undergo down-regulation or desensitization like other G-coupled protein receptors, or do we know the answer to that?
Feldman, Philadelphia: That's a great question. It turns out that if you look at models of failing hearts—and we've looked at three models—you see very interesting changes in receptor levels. We've looked at a banding model; we've looked at a TNFα overexpression model; and we've looked at a model of cardiac-specific overexpression of calsequestrin (CSQ). In all cases, when heart failure occurs, the levels of adenosine actually go down. On the basis of data from models of cardiac ischemia, you would expect that they would go up, but they don't. In these same models, the decrease in adenosine levels is accompanied by up-regulation of the A1 adenosine receptor but down-regulation of the A2A adenosine receptor. Thus, this system is far more complex than we might imagine.
Abboud, Iowa City: Is the expression of A2A and A1 in a protective function related in any way to cellular effects on potassium channels versus calcium channels?
Feldman, Philadelphia: That is an excellent question, but at this point in time we don't know the answer to it. We actually embarked on this project on the basis of work by fellow who was working in my laboratory at the time, Dr. Daniel Wagner. He found that adenosine was the most potent inhibitor of TNF2. Recognizing that TNFα could adversely influence the cardiac phenotype, we thought that adenosine molecules would be excellent molecules with which to modulate the expression of the pro–inflammatory cytokines that were over-expressed in congestive heart failure. Then we were faced with the confounding problems that I discussed today, with over-expression models demonstrating heart failure. We need to get back to that underlying hypothesis eventually, but as yet we haven't.
Oates, Nashville: This is certainly beautiful work in selecting out the different adenosine receptors. I'm curious about some of the effects other than contractility as we go forward in thinking about therapeutic interventions focused on a specific receptor subtype.
Feldman, Philadelphia: I think we have to understand what these receptors are doing to a greater degree before we start putting molecules into humans, and I think that we often make assumptions about what the biology of the receptor is without really knowing what it's going to do in a clinical situation.
REFERENCES
- 1.Drury AN, Szent-Gyorgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol. 1929;68(3):213–37. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berne RM. Cardiac nucleotides in hypoxia: possible role in regulation of coronary blood flow. Am J Physiol. 1963;204:317–22. doi: 10.1152/ajplegacy.1963.204.2.317. [DOI] [PubMed] [Google Scholar]
- 3.Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol. 2001;41:775–87. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]
- 4.Fredholm BB, AP IJ, Jacobson KA, et al. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53(4):527–52. [PMC free article] [PubMed] [Google Scholar]
- 5.Olafsson B, Forman MB, Puett DW, et al. Reduction of reperfusion injury in the canine preparation by intracoronary adenosine: importance of the endothelium and the no-reflow phendmenon. Circulation. 1987;76(5):1135–45. doi: 10.1161/01.cir.76.5.1135. [DOI] [PubMed] [Google Scholar]
- 6.Woolfson RG, Patel VC, Yellon DM. Pre-conditioning with adenosine leads to concentration-dependent infarct size reduction in the isolated rabbit heart. Cardiovasc Res. 1996;31(1):148–51. [PubMed] [Google Scholar]
- 7.Ashton KJ, Peart JN, Morrison RR, et al. Genetic modulation of adenosine receptor function and adenosine handling in murine hearts: insights and issues. J Mol Cell Cardiol. 2007;42(4):693–705. doi: 10.1016/j.yjmcc.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Peart JN, Headrick JP. Adenosinergic cardioprotection: multiple receptors, multiple pathways. Pharmacol Ther. 2007;114(2):208–21. doi: 10.1016/j.pharmthera.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Schroder J. Boston: Martinus Nijhoff; 1983. Metabolism of Adenosine and Site of Production in the Heart. [Google Scholar]
- 10.Feldman AM, Koch WJ, Force TL. Developing strategies to link basic cardiovascular sciences with clinical drug development: another opportunity for translational sciences. Clin Pharmacol Ther. 2007;81(6):887–92. doi: 10.1038/sj.clpt.6100160. [DOI] [PubMed] [Google Scholar]
- 11.Wettschureck N, Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol Rev. 2005;85(4):1159–204. doi: 10.1152/physrev.00003.2005. [DOI] [PubMed] [Google Scholar]
- 12.Funakoshi H, Zacharia LC, Tang Z, et al. A1 adenosine receptor upregulation accompanies decreasing myocardial adenosine levels in mice with left ventricular dysfunction. Circulation. 2007;115(17):2307–15. doi: 10.1161/CIRCULATIONAHA.107.694596. [DOI] [PubMed] [Google Scholar]
- 13.Gauthier NS, Morrison RR, Byford AM, Jones R, Headrick JP, Matherne GP. Functional genomics of transgenic overexpression of A1 adenosine preceptors in the Heart. Drug Dev Res. 1998;45:402–409. [Google Scholar]
- 14.Matherne GP, Linden J, Byford AM, et al. Transgenic A1 adenosine receptor overexpression increases myocardial resistance to ischemia. Proc Natl Acad Sci USA 10. 1997;94(12):6541–6. doi: 10.1073/pnas.94.12.6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neumann J, Vahlensieck U, Boknik P, et al. Functional studies in atrium overexpressing Al-adenosine receptors. Br J Pharmacol. 1999;128(7):1623–9. doi: 10.1038/sj.bjp.0702963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirchhof P, Fabritz L, Fortmuller L, et al. Altered sinus nodal and atrioventricular nodal function in freely moving mice overexpressing the A1 adenosine receptor. Am J Physiol Heart Circ Physiol. 2003;285(1):H145–53. doi: 10.1152/ajpheart.01036.2002. [DOI] [PubMed] [Google Scholar]
- 17.Funakoshi H, Chan TO, Good JC, et al. Regulated overexpression of the A1-adenosine receptor in mice results in adverse but reversible changes in cardiac morphology and function. Circulation. 2006;114(21):2240–50. doi: 10.1161/CIRCULATIONAHA.106.620211. [DOI] [PubMed] [Google Scholar]
- 18.Cheskis Feiner ECP, Francois Jasmin J, Zhang J, et al. Left ventricular dysfunction in murine models of heart failure and in failing human heart is associated with a selective decrease in the expression of caveolin-3. J Card Fail. 2010 doi: 10.1016/j.cardfail.2010.10.008. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan TO, Funakoshi H, Song J, et al. Cardiac-restricted overexpression of the A(2A)-adenosine receptor in FVB mice transiently increases contractile performance and rescues the heart failure phenotype in mice overexpressing the A(1)-adenosine receptor. Clin Transl Sci. 2008;1(2):126–33. doi: 10.1111/j.1752-8062.2008.00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamad EA, Li X, Song J, et al. Effects of cardiac-restricted overexpression of the A(2A) adenosine receptor on adriamycin-induced cardiotoxicity. Am J Physiol Heart Circ Physiol. 2010;298(6):H1738–47. doi: 10.1152/ajpheart.00688.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johansson B, Halldner L, Dunwiddie TV, et al. Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc Natl Acad Sci USA. 2001;98(16):9407–12. doi: 10.1073/pnas.161292398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun D, Samuelson LC, Yang T, et al. Mediation of tubuloglomerular feedback by adenosine: evidence from mice lacking adenosine 1 receptors. Proc Natl Acad Sci USA. 2001 Aug 14;98(17):9983–8. doi: 10.1073/pnas.171317998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tawfik HE, Schnermann J, Oldenburg PJ, et al. Role of A1 adenosine receptors in regulation of vascular tone. Am J Physiol Heart Circ Physiol. 2005;288(3):H1411–6. doi: 10.1152/ajpheart.00684.2004. [DOI] [PubMed] [Google Scholar]
- 24.Tawfik HE, Teng B, Morrison RR, et al. Role of A1 adenosine receptor in the regulation of coronary flow. Am J Physiol Heart Circ Physiol. 2006;291(1):H467–72. doi: 10.1152/ajpheart.01319.2005. [DOI] [PubMed] [Google Scholar]
- 25.Zucchi R, Cerniway RJ, Ronca-Testoni S, et al. Effect of cardiac A(1) adenosine receptor overexpression on sarcoplasmic reticulum function. Cardiovasc Res. 2002;53(2):326–33. doi: 10.1016/s0008-6363(01)00471-0. [DOI] [PubMed] [Google Scholar]
- 26.Schulte G, Sommerschild H, Yang J, et al. Adenosine A receptors are necessary for protection of the murine heart by remote, delayed adaptation to ischaemia. Acta Physiol Scand. 2004;182(2):133–43. doi: 10.1111/j.1365-201X.2004.01350.x. [DOI] [PubMed] [Google Scholar]
- 27.Morrison RR, Teng B, Oldenburg PJ, et al. Effects of targeted deletion of A1 adenosine receptors on postischemic cardiac function and expression of adenosine receptor subtypes. Am J Physiol Heart Circ Physiol. 2006;291(4):H1875–82. doi: 10.1152/ajpheart.00158.2005. [DOI] [PubMed] [Google Scholar]
- 28.Reichelt ME, Willems L, Molina JG, et al. Genetic deletion of the A1 adenosine receptor limits myocardial ischemic tolerance. Circ Res. 2005;96(3):363–7. doi: 10.1161/01.RES.0000156075.00127.C3. [DOI] [PubMed] [Google Scholar]
- 29.Ledent C, Vaugeois JM, Schiffmann SN, et al. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature. 1997;388(6643):674–8. doi: 10.1038/41771. [DOI] [PubMed] [Google Scholar]
- 30.Scislo TJ, O'Leary DS. Purinergic mechanisms of the nucleus of the solitary tract and neural cardiovascular control. Neurol Res. 2005;27(2):182–94. doi: 10.1179/016164105X21959. [DOI] [PubMed] [Google Scholar]
- 31.Ponnoth DS, Sanjani MS, Ledent C, et al. Absence of adenosine-mediated aortic relaxation in A(2A) adenosine receptor knockout mice. Am J Physiol Heart Circ Physiol. 2009;297(5):H1655–60. doi: 10.1152/ajpheart.00192.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrison RR, Talukder MA, Ledent C, et al. Cardiac effects of adenosine in A(2A) receptor knockout hearts: uncovering A(2B) receptors. Am J Physiol Heart Circ Physiol. 2002;282(2):H437–44. doi: 10.1152/ajpheart.00723.2001. [DOI] [PubMed] [Google Scholar]
- 33.Talukder MA, Morrison RR, Ledent C, et al. Endogenous adenosine increases coronary flow by activation of both A2A and A2B receptors in mice. J Cardiovasc Pharmacol. 2003;41(4):562–70. doi: 10.1097/00005344-200304000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Teng B, Ledent C, Mustafa SJ. Up-regulation of A 2B adenosine receptor in A 2A adenosine receptor knockout mouse coronary artery. J Mol Cell Cardiol. 2008;44(5):905–14. doi: 10.1016/j.yjmcc.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Day YJ, Marshall MA, Huang L, et al. Protection from ischemic liver injury by activation of A2A adenosine receptors during reperfusion: inhibition of chemokine induction. Am J Physiol Gastrointest Liver Physiol. 2004;286(2):G285–293. doi: 10.1152/ajpgi.00348.2003. [DOI] [PubMed] [Google Scholar]
- 36.Yu L, Huang Z, Mariani J, et al. Selective inactivation or reconstitution of adenosine A2A receptors in bone marrow cells reveals their significant contribution to the development of ischemic brain injury. Nat Med. 2004;10(10):1081–7. doi: 10.1038/nm1103. [DOI] [PubMed] [Google Scholar]
- 37.McColl SR, St-Onge M, Dussault AA, et al. Immunomodulatory impact of the A2A adenosine receptor on the profile of chemokines produced by neutrophils. FASEB J. 2006;20(1):187–9. doi: 10.1096/fj.05-4804fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Z, Day YJ, Toufektsian MC, et al. Myocardial infarct-sparing effect of adenosine A2A receptor activation is due to its action on CD4+ T lymphocytes. Circulation. 2006;114(19):2056–64. doi: 10.1161/CIRCULATIONAHA.106.649244. [DOI] [PubMed] [Google Scholar]
- 39.Montesinos MC, Desai A, Chen JF, et al. Adenosine promotes wound healing and mediates angiogenesis in response to tissue injury via occupancy of A(2A) receptors. Am J Pathol. 2002;160(6):2009–18. doi: 10.1016/S0002-9440(10)61151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leibovich SJ, Chen JF, Pinhal-Enfield G, et al. Synergistic up-regulation of vascular endothelial growth factor expression in murine macrophages by adenosine A(2A) receptor agonists and endotoxin. Am J Pathol. 2002;160(6):2231–44. doi: 10.1016/S0002-9440(10)61170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Desai A, Victor-Vega C, Gadangi S, et al. Adenosine A2A receptor stimulation increases angiogenesis by down-regulating production of the antiangiogenic matrix protein thrombospondin 1. Mol Pharmacol. 2005;67(5):1406–13. doi: 10.1124/mol.104.007807. [DOI] [PubMed] [Google Scholar]
- 42.Black RG, Jr., Guo Y, Ge ZD, et al. Gene dosage-dependent effects of cardiac-specific overexpression of the A3 adenosine receptor. Circ Res. 2002;91(2):165–72. doi: 10.1161/01.res.0000028007.91385.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fabritz L, Kirchhof P, Fortmuller L, et al. Gene dose-dependent atrial arrhythmias, heart block, and brady-cardiomyopathy in mice overexpressing A(3) adenosine receptors. Cardiovasc Res. 2004;62(3):500–8. doi: 10.1016/j.cardiores.2004.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cross HR, Murphy E, Black RG, et al. Overexpression of A(3) adenosine receptors decreases heart rate, preserves energetics, and protects ischemic hearts. Am J Physiol Heart Circ Physiol. 2002;283(4):H1562–8. doi: 10.1152/ajpheart.00335.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salvatore CA, Tilley SL, Latour AM, et al. Disruption of the A(3) adenosine receptor .gene in mice and its effect on stimulated inflammatory cells. J Biol Chem. 2000;275(6):4429–34. doi: 10.1074/jbc.275.6.4429. [DOI] [PubMed] [Google Scholar]
- 46.Zhao Z, Makaritsis K, Francis CE, et al. A role for the A3 adenosine receptor in determining tissue levels of cAMP and blood pressure: studies in knock-out mice. Biochim Biophys Acta. 2000;1500(3):280–90. doi: 10.1016/s0925-4439(99)00111-8. [DOI] [PubMed] [Google Scholar]
- 47.Talukder MA, Morrison RR, Jacobson MA, et al. Targeted deletion of adenosine A(3) receptors augments adenosine-induced coronary flow in isolated mouse heart. Am J Physiol Heart Circ Physiol. 2002;282(6):H2183–9. doi: 10.1152/ajpheart.00964.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo Y, Bolli R, Bao W, et al. Targeted deletion of the A3 adenosine receptor confers resistance to myocardial ischemic injury and does not prevent early preconditioning. J Mol Cell Cardiol. 2001;33(4):825–30. doi: 10.1006/jmcc.2001.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harrison GJ, Cerniway RJ, Peart J, et al. Effects of A(3) adenosine receptor activation and gene knock-out in ischemic-reperfused mouse heart. Cardiovasc Res. 2002;53(1):147–55. doi: 10.1016/s0008-6363(01)00424-2. [DOI] [PubMed] [Google Scholar]
- 50.Jacobson KA. Adenosine A3 receptors: novel ligands and paradoxical effects. Trends Pharmacol Sci. 1998;19(5):184–91. doi: 10.1016/s0165-6147(98)01203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ge ZD, Peart JN, Kreckler LM, et al. Cl-IB-MECA [2-chloro-N6-(3-iodobenzyl)adenosine-5′-N-methylcarboxamide]; reduces ischemia/reperfusion injury in mice by activating the A3 adenosine receptor. J Pharmacol Exp Ther. 2006;319(3):1200–10. doi: 10.1124/jpet.106.111351. [DOI] [PubMed] [Google Scholar]
- 52.Lu Z, Fassett J, Xu X, et al. Adenosine A3 receptor deficiency exerts unanticipated protective effects on the pressure-overloaded left ventricle. Circulation. 2008;118(17):1713–21. doi: 10.1161/CIRCULATIONAHA.108.788307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang Z, Diamond MA, Chen JM, et al. Polymorphisms in adenosine receptor genes are associated with infarct size in patients with ischemic cardiomyopathy. Clin Pharmacol Ther. 2007;82(4):435–40. doi: 10.1038/sj.clpt.6100331. [DOI] [PubMed] [Google Scholar]