Abstract
Interrupting human-to-human transmission of the agents (Neisseria meningitidis, Haemophilus influenzae, and Streptococcus pneumoniae) of bacterial meningitis by new capsular polysaccharide-protein conjugate vaccines (PPCVs) has proven to be a remarkable (and unanticipated) contributor to vaccine effectiveness. Herd immunity accounts for ∼50% of the protection by meningococcal serogroup C PPCVs, pneumococcal PPCV7, and H. influenzae b PPCVs. Nasopharyngeal carriage can be reduced ≥75% for vaccine serotypes; the decrease in carriage is correlated with disease reduction in unvaccinated individuals, and the impact of herd immunity lasts for years. Based on these data, models for using herd immunity in vaccine-based prevention strategies are underway for control of meningitis in sub-Saharan Africa. Although the immunologic basis of herd immunity and impact on microbial biology need more study, protecting the unvaccinated by altering pathogen transmission dynamics is a powerful effect of PPCVs and increasingly important in vaccine introduction, implementation, and evaluation strategies.
The polysaccharide-protein conjugate vaccines (PPCVs) for the pathogens responsible for bacterial meningitis have since their introduction demonstrated remarkable effectiveness, far beyond the predicted individual efficacy. Three examples are the Haemophilus influenzae b conjugate vaccines introduced in the US in the early 1900s (1), the Neisseria meningitidis serogroup C conjugate vaccines introduced in the UK in 1999 (2), and the heptavalent pneumococcal conjugate vaccine (PCV7) introduced in the US in 2000 (3). The message is that approximately half of the effectiveness of these vaccines in preventing disease is due to altering the human-to-human transmission dynamics of these pathogens at upper respiratory mucosal surfaces, leading to herd immunity. I will review how these unanticipated successes are influencing vaccine implementation strategies and the duration of protection, and comment on the immune basis and the effects of these vaccines on pathogen biology.
Conjugate vaccine technology is the covalent linkage of a saccharide to an immunogenic carrier protein to create a glycoconjugate. This concept dates back to the work in the 1930s at the Rockefeller Institute by Oswald Avery and Walther Goebel (4). Hamilton Smith, Porter Anderson, John Robbins, and Rachael Schneerson were also major contributors to conjugate vaccine development in the 1960s, 1970s, 1980s, and 1990s, for which they received the Lasker Award in 1996. Conjugation results in replacement of a T-cell-independent process, seen with the polysaccharide alone, with the T-cell-dependent antigen recruitment of T-cell help by the carrier protein.
PPCVs, in contrast to polysaccharide vaccines (Table 1), induce predominantly an IgG immune response as well as immunological memory and recall, have a booster effect, lack the effect of hypo-responsiveness to repeated vaccinations, have expanded efficacy in infants and young children, and importantly for this discussion, can reduce human-to-human pathogen transmission and induce herd immunity.
TABLE 1.
Comparison of Properties of Polysaccharide-Protein Conjugate versus Polysaccharide Vaccines
| Property | Polysaccharide | Conjugate |
|---|---|---|
| T-cell-dependent immune response | No (IgM) | Yes (IgG) |
| Immune memory/recall | No | Yes |
| Booster effect | No | Yes |
| Lack of hypo-responsiveness | No | Yes |
| Efficacy in infants/young children | No | Yes |
| Reduction of transmission/herd immunity | No | Yes |
PPCVS FOR HAEMOPHILUS INFLUENZAE SEROTYPE B
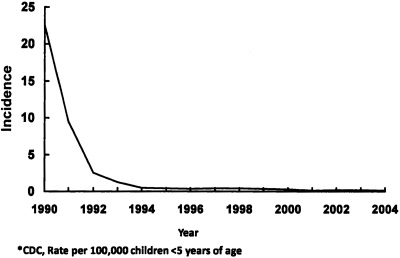
Haemophilus influenzae type b (hib) disease, prior to the introduction of the conjugate Hib vaccines, caused over 20 cases of infection per 100,000 children <5 years of age (Figure 1). Type b H. influenzae expresses a polyribitol phosphate capsule and is transmitted via respiratory droplets from human nasopharyngeal carriers, with the highest carriage rates in young children. Before introduction of the conjugate Hib vaccines, ∼20,000 cases per year occurred in children <5 years, with meningitis, epiglottitis, and pneumonia as major clinical syndromes. Also significant was Hib disease in adults, including pregnant women and those with human immunodeficiency virus (HIV).
Fig. 1.
Decline in the US incidence of invasive Haemophilus Influenzae serotype b disease following introduction of Hib PPCVs, 1990–2004 (5 and National Immunization Program, CDC).
Beginning in 1990, three Hib conjugate vaccines were licensed and introduced; all utilized different carrier proteins: meningococcal group B OMP; CRM197, a nontoxic mutant of diphtheria toxin; and tetanus toxoid, and required a primary series in infancy of two or three doses and a booster at 12–15 months.
The introduction of Hib PPCVs in 1990, first in older children and then in infants, as part of a routine immunization series, very rapidly reduced the incidence of Hib disease (5). Since 1990, over 400,000 cases have been prevented in the U.S., including 20,000 deaths and 80,000 cases with neurologic sequelae. The very rapid decline in disease with these vaccines, and one-third to one-half of their overall effectiveness, have been shown to be due to herd immunity by decreasing the carriage and transmission of Hib among young children (6). Cases of Hib disease in non-vaccinated adults have also disappeared due to loss of the Hib reservoir.
Hib vaccination strategies are now successfully applied in many parts of the world; for example, the virtual elimination of Hib disease in The Gambia followed Hib conjugate vaccination (7).
No major effect on the biology of H. influenzae (such as significant replacement disease with other serotypes) has been seen. Although there are differences in the immunologic properties of the Hib conjugate vaccines, and differences in transmission dynamics in different populations, the impact of these vaccines on Hib disease has been remarkable. The future elimination of this disease as a human threat is now being discussed as a real possibility.
PPCVS FOR NEISSERIA MENINGITIDIS
The global epidemiology of meningococcal disease continues to include epidemic outbreaks of N. meningitidis of capsular serogroups A, B, C, Y, W-135 and, most recently, X (8). Although meningococcal polysaccharide vaccines for A, C, Y, W-135 have been in use since the 1970s and 1980s, and have efficacy in older children and adults, they are poorly immunogenic in young children (<2 years), have a short duration of protection, produce no significant herd immunity, and demonstrate immunological hypo-responsiveness on repeated administration. Over the past 20 years, major vaccine manufacturers and recently a unique public/private partnership have developed new meningococcal conjugate vaccines for serogroups C, A, and A, C, Y, and W-135 (9).
In 1999, meningococcal serogroup C conjugate vaccines were introduced in the UK via a broad catch-up campaign for those <19 years of age (2). Disease due to serogroup C, but not serogroup B, rapidly declined (10). The vaccine efficacy on nasopharyngeal carriage of serogroup C in adolescents (the main reservoir) was reduced >75% (11). Reduction in carriage has persisted for 10 years, and vaccine effectiveness in young children who were not protected on a long-term basis by the vaccine schedule initially used has been due to the prolonged efficacy against carriage in older age groups (12). There has been no evidence of serogroup replacement, but an increase in carriage of non-encapsulated genotypes identical to the clonal strain was found.
Data from studies by Ramsey et al. (13) and Balmer et al. (14), using active surveillance and case-control methodology, demonstrated significant reductions in meningococcal serogroup C disease in the UK owing to herd immunity. Although the impact varied by age, ∼50% or more of all disease prevented was because of herd immunity.
Meningococcal A, C, Y, and W-135 conjugates were introduced for adolescents in the US beginning in 2005, but because of a vaccine shortage and other factors, uptake and coverage has been slow to develop in the adolescent population, and the impact of herd immunity has not been clearly demonstrated. Ortez-Sanchez et al. have proposed a model for meningococcal conjugate vaccine effectiveness and impact with and without herd immunity (15).
PNEUMOCOCCAL PPCVS
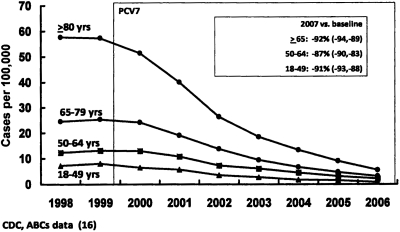
Rates (in cases per 100,000) of PCV7-type invasive pneumococcal disease among US adults >18 years old (comparing the 1998/1999 baseline with the incidence of disease following introduction of PCV7 in 2000) are shown in Figure 2. Remarkably, disease caused by the PCV7 serotypes of S. pneumoniae has declined ∼ 90% in all adult populations (16). Yet these individuals were not vaccinated. In a number of studies of this phenomenon, the decline was found to be due to interference with the transmission of vaccine serotypes between children (the largest reservoir for pneumococci) and adults (3). Recent data also indicate that non-invasive pneumonia in adults has been reduced 15–20% following the introduction of PCV7.
Fig. 2.
Rates of PCV-7 serotype invasive pneumococcal disease among unvaccinated US Adults, 1998/99–2006 (16 and National Center for Immunization and Respiratory Diseases, CDC).
The Centers for Disease Control (CDC) estimated that the prevention of PCV7-type pneumococcal disease is by direct (one-third of cases) and by indirect (two-thirds of cases) effects based on prospective population based surveillance (17). Although there has been serotype replacement following introduction of the vaccine (such as the emergence of serotype 19A), the overall impact of this vaccine, both in unvaccinated young children and unvaccinated adults, has been quite remarkable. The introduction in 2010 of a new conjugate PCV13 for children, which includes serotype 19A, is predicted to further advance the quest to reduce pneumococcal disease in all age groups.
Importantly, declines in the incidence of pneumococcal disease in adults, through herd immunity, were seen when vaccine coverage in children was less than 40% and with schedules not achieving 3–4 doses (18).
BASIS OF HERD IMMUNITY
Herd immunity is not a new concept, and has been identified for other vaccine-preventable diseases and used in vaccination programs such as the smallpox eradication campaign. However, the estimated vaccine coverage threshold for inducing herd immunity is considerably higher (>75%) for these other vaccines than for the bacterial conjugate vaccines. This is related to the higher basic reproduction numbers (Ro), consisting of the number of secondary cases of infection produced by a single index case in a susceptible population (19). For N. meningitidis serogroup C, this number is estimated to be much lower (1.36) and the coverage necessary to achieve herd immunity (17–26%) considerably lower than for other vaccines (20).
The immunological basis for herd immunity with the PPCVs is not well understood. Mucosal immunoglobulins and/or transudation of high-avidity serum IgG to interfere with organism acquisition have been proposed as mechanisms. However, recent interest has focused on introduction of Th17 immunity by CD4+ cells expressing IL-17 and recruiting macrophages or neutrophils to eliminate or control colonization of pathogens at mucosal sites. For example, Zhang et al. (21) have recently shown macrophages mediating CD4+ Th17-dependent clearance of pneumococcal colonization in a mouse model (22). Protecting against the bacterial meningitis pathogens through herd immunity is a remarkable, powerful, and unanticipated effect of PPCVs and accounts for one half of their effectiveness.
Herd immunity is an increasingly important consideration in strategies for PPCV introduction, cost-effectiveness, implementation of use, and evaluation. This fall a mass vaccination campaign was begun in countries of the African meningitis belt, with a serogroup A meningococcal conjugate (produced at $0.40 a dose), with a goal of individual protection and to maximize the impact of herd immunity (21). The remarkable indirect effects of PPCVs are having a global impact on reducing the burden of disease caused by the meningitis pathogens. Continued surveillance is needed to assess the long-term impact of the PPCVs on natural human immune responses and pathogen biology.
ACKNOWLEDGEMENTS
I thank Mary Whitley for manuscript preparation and the Georgia Emerging Infections Program and the Active Bacterial Core Surveillance (ABCs)/Emerging Infections Program Network at CDC for their support.
Footnotes
Potential Conflicts of Interest: None disclosed
DISCUSSION
Blaser, New York: David, there has been remarkable progress in the development and use of the bacterial meningitis conjugate vaccines, and congratulations to you and your colleagues for all of the lives that have been saved. I want to go to the issue that you raised about serogroup replacement. Certainly, we shouldn't let fear of replacement slow us down, but we must know where we're going, and as you know with pneumococcal vaccine, there has been replacement by non-vaccine-susceptible serotypes of Streptococcus pneumoniae. Now there is some evidence for replacement by unanticipated organisms, such as Staphylococcus aureus. So the question is: with the meningococcal vaccines, what kinds of replacements are being seen so far?
Stephens, Atlanta: As you mentioned, replacement disease with pneumococcal serotype 19A, for example, has been a problem with PCV7. Serotype 19A is included in the new PCV13 vaccine, and hopefully that will deal with replacement disease associated with this serotype. For the meningococcal conjugates—and the experience in the UK is really the best data that we have—there has been no evidence of meningococcal serogroup replacement disease. There has been some increase, based on carriage studies, of non-typeable meningococci of the same genetic type that caused the serogroup C problem, but essentially no replacement disease has been seen in the 10-plus years following serogroup C vaccine introduction. I think the issue of replacement is very important; these vaccine introductions are great experiments in essence in human populations. We constantly need to have active surveillance and to monitor for replacement disease. However, to emphasize the pneumococcal story, although we saw 19A replacement following the introduction of PCV7, the overall impact of these vaccines on the prevention of infection by PCV7 serotypes has been immense.
Denson, Iowa City: David, I enjoyed your talk as usual. Following up on Marty's question, it seems to me that the techniques that are used to look at replacement, as I understand them, are largely gross techniques, like culture, but DNA techniques, like those being employed to study the human microbiome, may be more sensitive. Are you aware that those studies are going on, because it seems to me that it's not so much to look at the disease, but to look at what's happening at the larger microlevel of the environment right there on the mucosa. I also have a second question: given that these vaccines are so effective and basically use the same conjugates, do you think that it is necessary to demonstrate for each one of the conjugates their safety before they're employed, particularly given the magnitude of disease, say, in Africa?
Stephens, Atlanta: In answer to your second question first, I didn't talk about safety. These vaccines have been found to be incredibly safe. There was some concern of a Guillain-Barré signal after the ACYW-135 meningococcal conjugate was introduced in the US for adolescents, but subsequent work has shown that there is not such a signal for this vaccine. So remarkably, these vaccines are very, very safe. You do have to evaluate each vaccine separately; the immunologic carrier protein and the specific polysaccharides do create differences. There is strong interest now, for example, in the area of multiple vaccinations and immune interference, if you will, when multiple conjugates are given at the same time.
In terms of your colonization question, most of the data we have on replacement is with cross-sectional, point-prevalence carriage studies based on culture. PCR has been used in some of these studies and does enhance the sensitivity and specificity for meningococci, but surprisingly, not to a great extent. So I think that the current methodology remains a reasonable sample of what changes have occurred at the mucosal level, and that PCR hasn't really helped us to better define different questions. However, whole-genome microbiome sequencing is an interesting idea. Your question and Marty's earlier question concerning replacement with other organisms such as S. aureus are important ones. We do see other organisms that may occupy these ecologic niches and were unanticipated. However, the association with S. aureus is, at least in my view, a modest one, and certainly should not limit the use of these vaccines.
REFERENCES
- 1.Robbins JB, Schneerson R, Anderson P, Smith DH. Prevention of systemic infections, especially meningitis, caused by Haemophilus influenzae type b. Impact on public health and implications for other polysaccharide-based vaccines. JAMA. 1996;276(14):1181–5. doi: 10.1001/jama.276.14.1181. [DOI] [PubMed] [Google Scholar]
- 2.Miller E, Salisbury D, Ramsay M. Planning, registration, and implementation of an immunisation campaign against meningococcal serogroup C disease in the UK: a success story. Vaccine. 2001;20(suppl 1):S58–S67. doi: 10.1016/s0264-410x(01)00299-7. [DOI] [PubMed] [Google Scholar]
- 3.Whitney CG, Farley MM, Hadler J, et al. Active Core Surveillance of the Emerging Infections Program Network. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348(18):1737–46. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 4.Avery OT, Goebel WF. Chemo-immunological studies on conjugated carbohydrate-proteins: V. The immunological specificity of an antigen prepared by combining the capsular polysaccharide of type III pneumococcus with foreign protein. J Exp Med. 1931;54(3):437–47. doi: 10.1084/jem.54.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CDC. Progress toward elimination of Haemophilus influenzae type b invasive disease among infants and children—United States, 1998–2000. MMWR Morbid Mortal Wkly Rep. 2002;51(11):234–7. [PubMed] [Google Scholar]
- 6.Steinhoff M, Goldblatt D. Conjugate Hib vaccines. Lancet. 2003;361(9355):360–1. doi: 10.1016/S0140-6736(03)12441-5. [DOI] [PubMed] [Google Scholar]
- 7.Adegbola RA, Secka O, Lahai G, et al. Elimination of Haemophilus influenzae type b (Hib) disease from The Gambia after the introduction of routine immunization with a Hib conjugate vaccine: a prospective study. Lancet. 2005;366(9480):144–50. doi: 10.1016/S0140-6736(05)66788-8. [DOI] [PubMed] [Google Scholar]
- 8.Stephens DS, Greenwood B, Brandizaeg P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet. 2007;369(9580):2196–210. doi: 10.1016/S0140-6736(07)61016-2. [DOI] [PubMed] [Google Scholar]
- 9.Zimmer SM, Stephens DS. Meningococcal conjugate vaccines. Expert Opin Pharmacother. 2004;5(4):855–63. doi: 10.1517/14656566.5.4.855. [DOI] [PubMed] [Google Scholar]
- 10.Gray SJ, Trotter CL, Ramsay ME, et al. Epidemiology of meningococcal disease in England and Wales 1993/94 to 2003/04: contribution and experiences of the Meningococcal Reference Unit. J Med Microbiol. 2006;55(Pt 7):887–96. doi: 10.1099/jmm.0.46288-0. [DOI] [PubMed] [Google Scholar]
- 11.Maiden MC, Ibarz-Pavon AB, Urwin R, et al. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J Infect Dis. 2008;197(5):737–43. doi: 10.1086/527401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trotter CL, Borrow R, Findlow J, et al. Seroprevalence of antibodies against serogroup C meningococci in England in the Postvaccination Era. Clin Vaccine Immunol. 2008;15(11):1694–1698. doi: 10.1128/CVI.00279-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramsay ME, Andrews NJ, Trotter CL, et al. Herd immunity from meningococcal serogroup C conjugate vaccination in England: database analysis. BMJ. 2003;326(7385):365–6. doi: 10.1136/bmj.326.7385.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balmer P, Borrow R, Miller E. Impact of meningococcal C conjugate vaccine in the UK. J Med Microbiol. 2002;51(9):717–22. doi: 10.1099/0022-1317-51-9-717. [DOI] [PubMed] [Google Scholar]
- 15.Ortega-Sanchez IR, Meltzer MI, Shepard C, et al. Active Bacterial Core Surveillance Team. Economics of an adolescent meningococcal conjugate vaccination catch-up campaign in the United States. Clin Infect Dis. 2008;46(1):1–13. doi: 10.1086/524041. [DOI] [PubMed] [Google Scholar]
- 16.Pilishvili T, Lexau C, Farley M, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32–41. doi: 10.1086/648593. [DOI] [PubMed] [Google Scholar]
- 17.CDC. Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease - United States, 1998–2003. MMWR Morbid Mortal Wkly Rep. 2005;54(36):893–897. [PubMed] [Google Scholar]
- 18.Haber M, Barskey A, Baughman W, et al. Herd immunity and pneumococcal conjugate vaccine: a quantitative model. Vaccine. 2007;25(29):5390–8. doi: 10.1016/j.vaccine.2007.04.088. [DOI] [PubMed] [Google Scholar]
- 19.Fine Paul EM. Herd immunity: history, theory, practice. Epidemiol Rev. 1993;15:265–302. doi: 10.1093/oxfordjournals.epirev.a036121. [DOI] [PubMed] [Google Scholar]
- 20.Trotter CL, Gay NJ, Edmunds WJ. Dynamic models of meningococcal carriage, disease, and the impact of serogroup C conjugate vaccination. Am J Epidemiol. 2005;162:89–100. doi: 10.1093/aje/kwi160. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z, Clarke Thomas B, Weiser Jeffrey N. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J Clin Invest. 2009;119(7):1899–1909. doi: 10.1172/JCI36731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LaForce FM, Konde K, Viviani S, et al. The Meningitis Vaccine Project. Vaccine. 2007;25S:A97–A100. doi: 10.1016/j.vaccine.2007.04.049. [DOI] [PubMed] [Google Scholar]