Abstract
Normal function of the glomerular filtration barrier requires wild-type differentiation of the highly specialized glomerular epithelial cell, the podocyte. Podocytes express three distinct domains, consisting of a cell body, primary processes, and secondary foot processes (FP). These FP express slit diaphragms, which are highly specialized cell-cell contacts critical for filtration-barrier function. Foot processes are dynamic structures that reorganize within minutes through actin cytoskeletal rearrangement. Glomerular diseases are characterized by a persistent simplification in podocyte domain structure with loss of FP, a phenotype described as FP effacement. The generation of such phenotypic plasticity requires that signaling pathways in subcellular compartments be integrated dynamically for a cell to respond appropriately to information flow from its microenvironment. We have identified a LIM-domain-containing protein, Wilm's tumor interacting protein (WTIP), that regulates podocyte actin dynamics to maintain stable cell contacts. After glomerular injury, the WTIP molecule shuttles to the podocyte nucleus in response to changes in slit-diaphragm assembly, and changes gene transcription to permit podocyte remodeling. Defining regulatory pathways of podocyte differentiation identifies novel, druggable targets for chronic kidney diseases characterized by glomerular scarring.
CHRONIC KIDNEY DISEASE (CKD): A COMMON DISEASE WITH A HEAVY PUBLIC HEALTH BURDEN AND A COMPLEX PATHOGENESIS
A subtle but persistent change, described as an epidemic of chronic, non-communicable diseases, has occurred in the profile of human diseases around the world (1). Health-care delivery now focuses less on saving patients from acute, catastrophic illness, and more on palliating chronic and debilitating diseases. Although the change in life expectancy for patients with AIDS most clearly documents this shift in illness acuity, a walk through the medical floors of any hospital illustrates that most patients suffer from chronic, debilitating but preventable, conditions. Data from the US Centers for Disease Control and Prevention (CDC) document that the burden of chronic disease is onerous for both society generally and patients individually (2). Chronic diseases account for 70% of all deaths in the US; more than 1.7 million people succumb to a chronic disease each year, and nearly 133 million Americans live with at least one chronic illness. The medical care costs of diagnosing and treating chronic diseases account for more than 75% of the US health care budget.
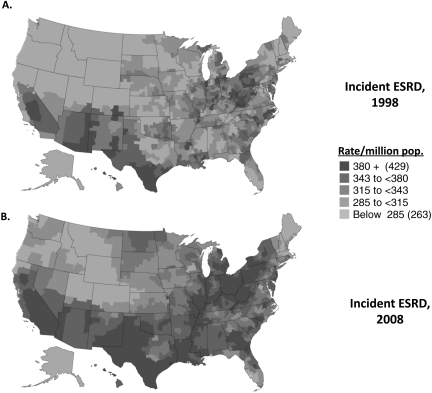
Kidney disease is the ninth leading cause of death in the US according to CDC data. The prevalence of treated end-stage renal disease (ESRD) in the US is approximately 1,700 per million population (3), and the 2010 US Renal Data System Report documents the growth in ESRD incidence rates across the US from 1998 through 2008 (Figure 1). The prevalence of patients with chronic kidney disease (CKD) is even greater; approximately 6%–7% of the US population has abnormal kidney filtration function (4). An analysis of NHANES data by Coresh and colleagues shows that the prevalence of CKD in the United States in 1999–2004 was higher than it was in 1988–1994 (5). Patients with a diagnosis of kidney disease account for 31% of Medicare expenditures (8). The risk for progressive kidney disease has risen disproportionately to the increasing incidence of systemic diseases associated with kidney injury, such as diabetes, hypertension, and obesity (5, 7). Furthermore, growth in incident ESRD has outpaced growth in prevalent chronic renal insufficiency (8), suggesting that the progressive kidney disease epidemic in the US is not merely a function of more cases of diabetes or hypertension or of an increased prevalence of CKD. Rather, these data suggest that undefined genetic, biochemical, and environmental mechanisms collaborate to promote progressive kidney injury on a background of an epidemic of diseases that contributes to CKD.
Fig. 1.
Adjusted incidence rates of ESRD per million population in 1998 (panel A) and 2008 (panel B). Incidence rates are adjusted for age, gender, and race. Shading shows geographic variation. Data and figure adapted from US Renal Data System, USRDS 2010 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2009.
CKD PHENOTYPES: PROTEINURIC KIDNEY DISEASES AND PODOCYTE PLASTICITY
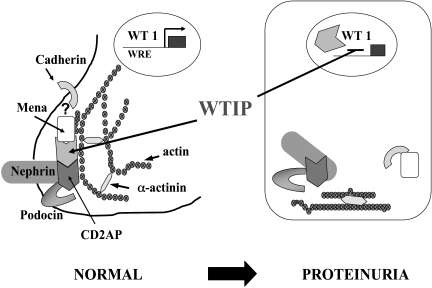
Chronic kidney disease is a complex trait characterized by progressive loss of function of the glomerular filtration barrier, manifested both by a decline in the glomerular filtration rate (GFR) and the onset of abnormal albuminuria. The glomerular filtration barrier comprises three distinct components: the fenestrated glomerular endothelial cell, the glomerular basement membrane (GBM), and the glomerular epithelial cell or podocyte (9, 10). The glomerular mesangial cell structures the filtration barrier into capillary loops (11, 12). The work in our laboratory has concentrated on characterization of the mechanisms regulating the remarkable podocyte phenotype plasticity that is a feature of proteinuric glomerular diseases. With proteinuria in the nephrotic range, regardless of the etiology, podocytes undergo marked morphologic changes. The actin cytoskeleton rearranges into a cytoskeletal mat below the plasma membrane opposed to the GBM, slit-diaphragm structures are lost, and the podocyte assumes a cuboidal shape. This change in phenotype is reversible; the podocyte can re-develop its wild-type morphology with successful treatment of nephrotic syndrome. A number of gene mutations that result in a podocyte phenotype switch have been mapped in families with nephrotic syndrome. In the absence of mutations, molecular mechanisms that regulate podocyte phenotype remain unclear. We postulated that podocytes express intracellular molecules that can reversibly translate changes in extracellular physical forces or soluble signals into appropriate changes in cellular structure. This paper describes our work on a molecule, Wilm's tumor interacting protein (WTIP), that appears to reversibly regulate a change in podocyte phenotype in response to acquired disease. Our model for WTIP function, which is detailed in the rest of this paper, is shown in Fig 2. In normal glomeruli, WTIP is part of a multi-protein complex in the foot process of the podocyte that may link molecules that mediate podocyte cell-cell contacts to the actin cytoskeleton. After injury, WTIP translocates into the nucleus, where it represses Wilms' tumor protein (WT1)-dependent gene expression to permit simplification of the podocyte phenotype in response to injury. Loss of WTIP from its location of cell-cell contact promotes a redistribution of slit-diaphragm proteins and rearrangement of actin characteristic of foot-process effacement.
Fig. 2.
Podocyte plasticity and WTIP function. The podocyte undergoes a remarkable change in morphology in proteinuric kidney diseases. The transcription factor WT1 regulates the podocyte differentiation state. The WT1-interacting protein WTIP can shuttle reversibly from podocyte cell-cell contacts into the nucleus to inhibit WT1 activity and permit podocyte adaption to a disease state. Used with permission (32).
Podocytes and the Architecture of the Filtration Barrier
Podocytes arise from the metanephric mesenchyme after a tightly orchestrated differentiation program to produce their highly specialized phenotype. The podocyte contains three different domains: the cell body, primary processes, and foot processes. Genetic and biochemical studies have in part defined the molecular architecture of the filtration barrier, which requires elaboration of podocyte foot processes and assembly of cell-matrix and specialized cell-cell junctions, known slit diaphragms, for normal function (13). The slit diaphragm is a modified adherens junction that contains specialized podocyte proteins, which were discovered by positional cloning in families with nephrotic syndrome (10, 14, 15). Nephrin, an immunoglobulin superfamily member, is thought to be a major transmembrane component of the slit diaphragm. Podocin, which is similar to scaffold molecules of the stomatin family, has a single hairpin-like structure with both the N-terminal and C-terminal domains in the cytosol. CD2AP was originally identified as an adapter protein that interacts with the cytoplasmic domain of CD2, a T-cell adhesion protein, but whose absence in mice was subsequently found to cause progressive glomerulosclerosis. Mutations in human CD2AP have been found in sporadic and familial glomerulosclerosis. F-actin which is restricted to podocyte foot processes (16), appears to be a central link in the appropriate assembly of these molecules (17). Foot-process retraction is the stereotypical podocyte response to injury, the hallmark of both primary and secondary glomerulosclerosis, and is characterized both in vivo and in vitro by marked actin cytoskeletal rearrangement (18, 19), an observation further supporting a significant role for actin as a central organizer of podocyte foot-process architecture. ACTN4, which encodes the actin cross-linking protein α-actinin-4, has been linked to familial focal segemental gloemrulosclerosis (20). A mutation in INF2, which encodes a member of the formin family of actin-regulating proteins, has also been linked to familial proteinuric kidney disease (21).
Although the identification of genes linked to familial proteinuric kidney diseases has been highly instructive about the molecular function of the glomerular filtration barrier, the mechanisms for regulated changes in podocyte phenotype in acquired disease remain unclear. Given its unique microenvironment with exposure to hemodynamic forces and a high flow of ultrafiltrate, the podocyte must be able to rapidly respond to changes in physical forces or soluble signals, a process that undoubtedly involves dynamic regulation of actin cytoskeletal organization. The dynamic nature of foot-process architecture is highlighted by the observation that appropriate treatment can restore normal podocyte structure.
The Dysregulated Podocyte Phenotype and Glomerulosclerosis
At a molecular level in both human biopsies and experimental models, the stereotypical morphologic response of podocytes described above is associated with changes in the cytoplasmic, plasma membrane, and nuclear expression of podocyte differentiation markers that varies somewhat with disease etiology (22–25). In experimental models, both nephrin and podocin redistribute and may decrease in abundance. In addition to actin rearrangements and loss of junctional complexes, podocytes in glomerular disease can re-develop a less differentiated phenotype, more characteristic of the developing than of the fully differentiated glomerulus (26). The change in podocyte morphology is contemporaneous with the onset of heavy proteinuria and is associated with genome-wide changes in transcript abundance (27), suggesting that the molecular mechanisms that regulate the cell fate of podocytes involve changes in gene expression. On this assumption, we focused our work on the regulation of podocyte transcriptional activity. Data derived from genetically modified mice and human families with syndromes including glomerular disease have identified several transcription factors required for podocyte specification and differentiation, including WT1.
THE TRANSCRIPTION FACTOR WT1 IS A KEY REGULATOR OF PODOCYTE PHENOTYPE
The correct temporal and spatial expression of WT1 is critical for the induction and maintenance of a differentiated podocyte phenotype (28). WT1 protein is widely expressed developmentally in mesothelium, adrenal glands, retina, lung, kidney and gonads, but its expression is limited to the glomerular podocytes in the adult kidney. Zinc finger mutations in WT1 are responsible for two rare causes of familial glomerulosclerosis, Denys-Drash syndrome (DDS) and Frasier syndrome (29). Variants of WT1 have also been identified in both familial and sporadic focal segmental glomerulosclerosis (30). Knock-in mice that express human WT1 mutations, recapitulate the glomerulosclerosis and gonadal dysgenesis that characterize DDS and Frasier phenotypes, documenting that WT1 function is required for the normal podocyte phenotype (31). Transgenic models that permit mice to survive show that reduced levels of wild-type WT1 are responsible for crescentic nephritis and glomerulosclerosis (31).
THE WT1 REGULATOR, WTIP, AND THE PODOCYTE
Given the importance of WT1 in the regulation of podocyte phenotype, we used a yeast two-hybrid assay to identify proteins that interacted with and regulated WT1, and identified WTIP as a novel LIM-domain-containing protein (32). We initially expressed the WTIP fragment isolated in the two-hybrid trap (which lacks the amino-terminal 159 amino acids [NΔWTIP]) in COS cells and found that it co-localized with WT1 in the nucleus, as expected from the yeast two-hybrid assay. When a full-length WTIP was cloned, we identified a consensus, Crm1-dependent nuclear export signal, and postulated that full-length WTIP shuttles between the cytosol and nucleus in a manner similar to that of other members of the zyxin family of cytosolic LIM-domain-containing proteins. In contrast to NΔWTIP, full-length WTIP localizes in COS-cell cytoplasm. However, treatment with leptomycin B, an inhibitor of Crm1-dependent nuclear export, caused full-length WTIP to accumulate in the nucleus. These data suggest that WTIP normally localizes in the cytosol and functions as a communication signal between cytosolic structures and the nucleus. Because false-positive interactions could arise in the yeast two-hybrid screen, we next used immunoprecipitation and GST pull-down assays to confirm that WT1 and WTIP associate in COS cells. Myc-NΔWTIP is co-precipitated by both anti-WT1 and anti-Flag antibodies. Although tagged, full-length WTIP is excluded from the nucleus, full-length WTIP does co-precipitate with WT1 when its nuclear export is blocked by leptomycin B. Neither full-length zyxin (a paralogous LIM-domain- containing protein) nor a truncated zyxin protein containing only the three LIM domains (NΔZyxin) that localized to the nucleus, co-precipitated with WT1. Critically, when retained in the nucleus, WTP but not zyxin inhibited WT1 transcriptional activity.
To define WTIP localization in vivo, we generated a Wtip-deficient mouse line using β-galactosidase-neomycin (β-geo) gene-trap technology. The β-geo expression vector integrated into the second intron of Wtip, and sequencing after RT-PCR confirmed that Wtip exon 2 spliced in-frame to β-geo. Immunoblotting with an anti-β-gal antibody revealed an Mr 170 protein, the size predicted for the Wtip-β-gal fusion protein. No Wtipβ-geo/β-geo animals were identified among live-born mice or embryos as young as E8.5 (n = 300 mice). The morphology and viability of Wtipβ-geo/+ and wild-type mice was similar. Injection of mice with lipopolysaccharide (LPS) causes transient nephrotic syndrome (33). After LPS injection, Wtipβ-geo/+ mice developed statistically greater and more prolonged proteinuria compared to the wild-type littermates. To map endogenous WTIP expression patterns, we assayed X-gal staining in Wtipβ-geo/+ mouse embryo and adult tissues. No X-gal staining was observed in the renal vesicle, Comma-shaped, or S-shaped stages of glomerular development. However, X-gal was seen in the capillary-loop stage and in mature glomeruli, which co-localized with the podocyte marker synaptopodin but not with the endothelial marker PECAM or α-vascular smooth-muscle actin. WTIP is also expressed in developing myocardium as early as E9.5 and persists through E14.5, but is not seen in the adult heart. Taken together, these data suggest that WTIP is essential for mouse embryogenesis and is expressed in podocytes from the capillary loop stage into adulthood. Resolution of abnormal proteinuria after injection of LPS is delayed in Wtipβ-geo/+ mice, suggesting that WTIP regulates the function of the glomerular filtration barrier. Given embryonic lethality, mice with targeted Wtip deletion will further characterize the role of WTIP in podocyte phenotype regulation and filtration-barrier function.
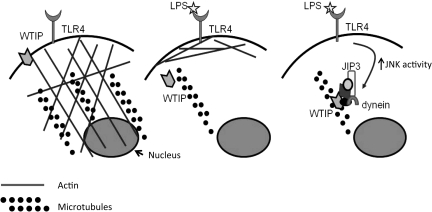
To test the hypothesis that WTIP is a reporter of environmental cues and contributes to regulation of the podocyte phenotype by translocating to the nucleus, we next developed a cell-culture model of nephrotic syndrome. Puromycin aminonucleoside (PAN), which causes proteinuria in animal models (34), disrupted cultured podocyte cell-cell junctions and increased albumin flux across differentiated podocytes cultured on collagen-coated Transwell filters (35). WTIP moved to the nuclei of PAN-treated podocytes and down-regulated the expression of a WT1-target gene, Rbbp7 (35). To further investigate mechanisms of nucleocytoplasmic translocation of WTIP in vitro, we treated podocytes with LPS, which as noted earlier is another molecule that induces nephrotic syndrome in mice. Podocytes that stably expressed an inducible, epitope-tagged WTIP were treated with LPS. After LPS treatment, WTIP rapidly disassembled from cell contacts, transited along the cytoskeleton, and localized in podocyte nuclei by 6 hours, shifting back to the plasma membrane at 24 hours (36). Cell fractionation and immunoblotting confirmed shifts in WTIP localization within intracellular compartments. LPS stimulated podocyte Janus Kinase (JNK) activity in a time-dependent manner, and a JNK, but not p38 inhibitor, prevented translocation of WTIP to nuclei. Intact actin cytoskeleton and microtubular networks, as well as activity of the motor protein dynein, are required for LPS-stimulated WTIP translocation (Figure 3).
Fig. 3.
LPS-stimulated WTIP nuclear translocation requires JNK activity, which assembles a multiprotein complex of the scaffolding protein JIP3 and the molecular motor dynein. In uninjured glomeruli, WTIP is part of a multi-protein signalsome that links podocyte adherens junction to the actin cytoskeleton (left panel). After stimulation with LPS, an inducer of transient nephrotic syndrome, the actin cytoskeleton rearranges. WTIP tranlocation requires intact microtubules (middle panel). Concomitant assembly of a dynein motor-containing protein complex ferries WTIP to the nucleus (right panel).
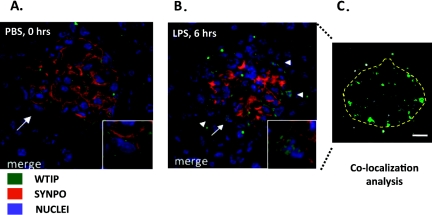
To determine whether WTIP translocates to podocyte nuclei in vivo after glomerular injury, we injected LPS into wild-type C57BL/6 mice and analyzed them for albuminuria and podocyte localization of WTIP (36). Injection of LPS resulted in albuminuria within 24 hours, whereas albumin excretion did not change significantly from baseline in mice injected with PBS. As previously shown, LPS-induced albuminuria was transient and returned to baseline levels within 72 hours after LPS injection. Immunohistochemistry of kidney sections demonstrated that the WTIP immunostaining pattern changed after LPS treatment as compared to that in untreated and PBS-injected controls (Figure 4). The linear staining pattern of WTIP distribution observed in the PBS-injected control transitioned to a more diffuse cytosolic and nuclear pattern by 6 hours after LPS injection, or approximately the same time at which we observed an increase in urinary albumin excretion in vivo.
Fig. 4.
WTIP shifts into podocyte nuclei in a mouse model of nephrotic syndrome. (Panel A:) Glomerular co-staining of kidney cross sections of WTIP (green) and the podocyte-foot-process actin-binding protein synaptopodin (red). Nuclei are identified by TOPRO-3 (blue). Inset shows a zoom image of the podocyte marked by the arrow. WTIP and synaptopodin co-localize within podocyte foot processes, but no WTIP staining is seen within the podocyte nuclei. (Panel B:) Six hours after administration of LPS, WTIP has shifted into podocyte nuclei (inset), a finding confirmed with Image J analysis (shown in panel C). Image J identifies the spatial distribution of WTIP and TOPRO-3 pixels, and correlations above random overlap are pseudocolored green. The anatomic locations of the correlated pixels are consistent with podocyte nuclei. Used with permission (32).
IMPLICATIONS FOR UNDERSTANDING HUMAN PROTEINURIC KIDNEY DISEASE
Chronic kidney disease is prevalent, mortal, a significant burden on health-care delivery, and a driver of health-care costs. The glomerular filtration barrier is a critical determinant of kidney function, and abundant evidence suggests that it is morphologically and functionally dynamic in response to changes in the glomerular microenvironment. The podocyte is one of three distinct components of the capillary wall that can undergo remarkable, reversible changes in phenotype with disease onset. We have identified a molecule, WTIP, that appears to regulate this process in podocytes. It is to be hoped that better understanding of pathways and molecular processes that regulate the function of the glomerular filtration barrier will lead to targeted therapies for a group of diseases with few treatment options.
ACKNOWLEDGEMENTS
Support for this project was provided by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-07470, P50 DK-054178, DK-064719, and F30 DK083897.
Footnotes
Potential Conflicts of Interest: None disclosed.
DISCUSSION
Mackowiak, Baltimore: I noticed in one of the maps you showed of the distribution incidence of chronic renal disease that Alaska has almost none as compared to the rest of the US. I was just wondering if that was one more reason to vote for Sarah Palin. I know this might not be your particular area of interest, but are those data real? If so, why might there be less chronic renal failure in Alaska than elsewhere, because these data reported chronic renal failure per million population. So it couldn't just be due to the small population.
Sedor, Cleveland: That's a good question. I don't have a clear answer. I assume the CDC incidence data on end-stage renal disease are correct. I'm not sure about those data. I know that more recently, as people have reported the epidemiology of chronic kidney disease and albuminuria in surveys done on American Indian/Alaska Native populations, the burden of disease overall is approximately 3 times greater in these people than in whites, although regional variation exists between tribes. Regardless of incidence, kidney disease is a problem in Alaska that disproportionately affects Alaska natives, although the overall incidence appears less than in other regions of the United States. I can't explain the CDC maps.
Mitch, Houston: Thank you. Very elegant. Two quick questions. One is: What is the turnover time of podocytes? And the second one is: What does diabetes do to your elegant working out of those proteins?
Sedor, Cleveland: The podocyte turns over very slowly if at all, at least in the normal cell state, and in this regard it is similar to the neuron. The podocyte is terminally differentiated, and in fact, one of the hallmarks of podocyte disease is the rearrangement of the actin cytoskeleton. The podocyte actually detaches into the urinary space. Podocyte renewal through repopulation by progenitor cells is an active area of investigation. So the answer to the first question, about the rate of turnover, is: extremely slow. It's not even clear that it turns over at all. We are just looking at diabetes models now. I've been fortunate enough to be part of an animal-models study that the NIH has done, and I think we are now finally getting some animal models that seem to better replicate human diabetic kidney disease.
Stokes, Iowa City: John, very nice work. I had a general question that might be relevant for people that are listening to this and thinking about glomerular disease. So, we know that there are many protein defects in the podocyte that can produce proteinuria and predispose to chronic kidney disease and progressive renal failure. If one takes a list of these defects—I think the list may be more than 20; I haven't kept track right now—but there are a lot of proteins that can be damaged or deranged in the podocyte that will produce these kinds of effects. So one question for you: To what extent might your interacting protein, which is essentially a transcription factor, alter the transcription and production of a variety of proteins that might be deranged in a whole variety of illnesses such as diabetes, or something like that, and to what extent do these things all come together to produce illness?
Sedor, Cleveland: I'll answer your question in two parts. We're interested in knowing what genes WTIP regulates, and in fact we wanted to use laser-capture microdissection of the glomerulus and compare gene expression between wild-type and the knockout Wtip null mouse to that in the normal mouse. WT-1, which interacts with WTIP in the nucleus, for example, does regulate a number of the genes that you are referring to. So I think it is a likely possibility that WTIP can regulate the expression of genes known to cause glomerular diseases. I think the other thing we need to do is to start to synthesize the data. When we have looked at the data published on other proteins in the WTIP family, we've found that all of these molecules regulate assembly of the actin cytoskeleton, in part though changes in activity of Rho family G-proteins. These G proteins may be interesting targets for the pharmacologic treatment of glomerular diseases. Some drugs affect pathways regulated by Rho family G-protein activity, and others are under development. Drug companies aren't particularly interested in developing new therapies for chronic kidney diseases, but as the basic mechanisms in the pathogenesis of glomerular disease are defined, we can take advantage of drugs developed to treat other diseases. The use of ACE inhibitors to treat kidney disease provides a successful example of co-opting a drug developed for one use, in the case of ACE inhibitors as anti-hypertensives agents, for use in kidney disease. I think the big problem, for our discipline, has been that we don't have a durable infrastructure where you can begin to design some of these trials for glomerular diseases. I hope that in the next few years we nephrologists will be able to address this issue by using the approaches successfully implemented by oncologists, who rapidly test new drugs in humans.
Rosenwasser, Kansas City: Congratulations on a very elegant study. When I was chief resident at UCSF, Holly Smith told me that if you are a hammer, everything looks like a nail. So when I look at a podocyte, I am wondering if it's related, at all, to dendritic cells or macrophages or cells of the innate immune response. For example, is WTIP expressed in a host response to an immune inflammatory trigger?
Sedor, Cleveland: We haven't looked at that. I'm not sure the podocyte is related to dendritic cells. In terms of differentiated podocytes and podocyte cytoskeletal architecture it seems to be most related to neurons. There's a lot of similarity between them in some of their structural proteins.
Barondess, New York: I want to ask a reciprocal of Phil Mackowiak's question. I, too, was struck particularly with the earlier display of the geographic distribution of chronic kidney disease and renal failure. The particularly heavy incidence in the Southeast overlies a similar map that was produced some years ago describing a sarcoid belt in this country. That earlier map also overlaps with the distribution of low-income populations in the US, and the difference in distribution of the African-American population at that time. A related comment is that if you're looking at end-stage kidney disease, you're looking at the results of insults that occurred some time before. So I guess my question is, what about the possibility of socio-cultural influences on this and racial and ethnic influences on the risk of the initial insult that leads to this end stage?
Sedor, Cleveland: Well that's a complex question, and I think that there's no question that socio-cultural influences contribute to chonic kidney disease prevalence. In fact, chronic kidney disease is one of the “poster children” for investigators working on health disparities. My colleague Ashwini Sehgal has an NIH P60 grant to look at the effect of socio-cultural disparities and their role in the prevalences of a variety of kidney diseases. I might add that I have been fortunate enough to be a part of a big consortium that's looking at genes for nephropathy. We've identified a locus associated with non-diabetic kidney disease in African-American patients. The specific genetic variants underlying the association were discovered by Martin Pollack at Harvard. So the answer to your question is that chronic kidney disease likely reflects both underlying pathobiology and the results of socio-economic disparities. That actually puts some genetic basis to the fact that African-Americans may have an excess burden of kidney disease. So I think the answer is that it's both things. And clearly, socioeconomic disparities are important in the prevalence of chronic kidney disease.
Alexander, Atlanta: I also had a question about the map. The possible epidemiology in that that map also looks very similar to the maps for body mass index as it has expanded from the stroke belt or kidney-failure belt or whatever you like to call it to virtually extend nationally, and there is something in the environment causing that. I guess the lesson is that there are environmental elements that are contributing powerfully to this.
Sedor, Cleveland: I think that you are absolutely correct and have identified the inter-relatedness between a number of conditions that comprise the chronic-disease epidemic discussed in an article in Nature several years ago. CKD is just one of those conditions in this epidemic.
REFERENCES
- 1.Daar AS, Singer PA, Leah PD, et al. Grand challenges in chronic non-communicable diseases. Nature. 2007;450:494–496. doi: 10.1038/450494a. [DOI] [PubMed] [Google Scholar]
- 2.Chronic Disease Prevention and Health Promotion. [Accessed February 1, 2011]. http://www.cdc.gov/chronicdisease/overview/index.htm.
- 3.Collins AJ, Foley RN, Herzog C, et al. Precis: an introduction to end-stage renal disease in the United States. Am J Kidney Dis. 2011;57:e209–e222. [Google Scholar]
- 4.Collins AJ, Foley RN, Herzog C, et al. Chronic kidney disease in the general population. Am J Kidney Dis. 2011;57:e39–e52. [Google Scholar]
- 5.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 6.Collins AJ, Foley RN, Herzog C, et al. US Renal Data System 2010 Annual Data Report. Am J Kidney Dis. 2011;57:e1–e526. doi: 10.1053/j.ajkd.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Jones CA, Krolewski AS, Rogus J, Xue JL, Collins A, Warram JH. Epidemic of end-stage renal disease in people with diabetes in the United States population: Do we know the cause? Kidney Int. 2005;67:1684–1691. doi: 10.1111/j.1523-1755.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- 8.Hsu CY, Vittinghoff E, Lin F, Shlipak MG. The incidence of end-stage renal disease is increasing faster than the prevalence of chronic renal insufficiency. Ann Intern Med. 2004;141:95–101. doi: 10.7326/0003-4819-141-2-200407200-00007. [DOI] [PubMed] [Google Scholar]
- 9.Jarad G, Miner JH. Update on the glomerular filtration barrier. Curr Opin Nephrol Hypertens. 2009;18:226–232. doi: 10.1097/mnh.0b013e3283296044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patrakka J, Tryggvason K. Molecular make-up of the glomerular filtration barrier. Biochem Biophys Res Commun. 2010;396:164–169. doi: 10.1016/j.bbrc.2010.04.069. [DOI] [PubMed] [Google Scholar]
- 11.Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 1994;8:1875–1887. doi: 10.1101/gad.8.16.1875. [DOI] [PubMed] [Google Scholar]
- 12.Kikkawa Y, Virtanen I, Miner JH. Mesangial cells organize the glomerular capillaries by adhering to the G domain of laminin α5 in the glomerular basement membrane. J Cell Biol. 2003;161:187–196. doi: 10.1083/jcb.200211121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patrakka J, Tryggvason K. New insights into the role of podocytes in proteinuria. Nat Rev Nephrol. 2009;5:463–468. doi: 10.1038/nrneph.2009.108. [DOI] [PubMed] [Google Scholar]
- 14.Mathieson PW. Update on the podocyte. Curr Opin Nephrol Hypertens. 2009;18:206–211. doi: 10.1097/mnh.0b013e328326f3ca. [DOI] [PubMed] [Google Scholar]
- 15.Tryggvason K, Patrakka J, Wartiovaara J. Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med. 2006;354:1387–1401. doi: 10.1056/NEJMra052131. [DOI] [PubMed] [Google Scholar]
- 16.Drenckhahn D, Franke RP. Ultrastructural organization of contractile and cytoskeletal proteins in glomerular podocytes of chicken, rat, and man. Lab Invest. 1988;59:673–682. [PubMed] [Google Scholar]
- 17.Kerjaschki D. Caught flat-footed: podocyte damage and the molecular bases of focal glomerulosclerosis. J Clin Invest. 2001;108:1583–1587. doi: 10.1172/JCI14629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shirato I, Sakai T, Kimura K, Tomino Y, Kriz W. Cytoskeletal changes in podocytes associated with foot process effacement in Masugi nephritis. Am J Pathol. 1996;148:1283–1296. [PMC free article] [PubMed] [Google Scholar]
- 19.Oh J, Reiser J, Mundel P. Dynamic (re)organization of the podocyte actin cytoskeleton in the nephrotic syndrome. Pediatr Nephrol. 2004;19:130–137. doi: 10.1007/s00467-003-1367-y. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan JM, Kim H, North KN, et al. Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet. 2000;24:251–256. doi: 10.1038/73456. [DOI] [PubMed] [Google Scholar]
- 21.Brown EJ, Schlondorff JS, Becker DJ, et al. Mutations in the formin gene INF2 cause focal segmental glomerulosclerosis. Nat Genet. 2009;42:72–76. doi: 10.1038/ng.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barisoni L, Kriz W, Mundel P, D'Agati V. The dysregulated podocyte phenotype: A novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 1999;10:51–61. doi: 10.1681/ASN.V10151. [DOI] [PubMed] [Google Scholar]
- 23.Sharif K, Goyal M, Kershaw D, Kunkel R, Wiggins R. Podocyte phenotypes as defined by expression and distribution of GLEPP1 in the developing glomerulus and in nephrotic glomeruli from MCD, CNF, and FSGS. A dedifferentiation hypothesis for the nephrotic syndrome. Exp Nephrol. 1998;6:234–244. doi: 10.1159/000020528. [DOI] [PubMed] [Google Scholar]
- 24.Bariety J, Bruneval P, Meyrier A, Mandet C, Hill G, Jacquot C. Podocyte involvement in human immune crescentic glomerulonephritis. Kidney Int. 2005;68:1109–1119. doi: 10.1111/j.1523-1755.2005.00503.x. [DOI] [PubMed] [Google Scholar]
- 25.Yang D-H, Goyal M, Sharif K, et al. Glomerular epithelial protein 1 and podocalyxin-like protein 1 in inflammatory glomerular disease (Crescentic nephritis) in rabbit and man. Lab Invest. 1996;74:571–584. [PubMed] [Google Scholar]
- 26.Shono A, Tsukaguchi H, Yaoita E, et al. Podocytes participate in the assembly of tight junctions between foot processes in nephrotic podocytes. J Am Soc Nephrol. 2007;18:2525–2533. doi: 10.1681/ASN.2006101084. [DOI] [PubMed] [Google Scholar]
- 27.Hauser PV, Perco P, Muhlberger I, et al. Microarray and bioinformatics analysis of gene expression in experimental membranous nephropathy: Nephron Exp Nephrol. 2009;112:e43–e58. doi: 10.1159/000213505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SB, Haber DA. Wilms tumor and the WT1 gene. Exp Cell Res. 2001;264:74–99. doi: 10.1006/excr.2000.5131. [DOI] [PubMed] [Google Scholar]
- 29.Niaudet P, Gubler MC. WT1 and glomerular diseases. Pediatr Nephrol. 2006;21:1653–1660. doi: 10.1007/s00467-006-0208-1. [DOI] [PubMed] [Google Scholar]
- 30.Ruf RG, Schultheiss M, Lichtenberger A, et al. Prevalence of WT1 mutations in a large cohort of patients with steroid-resistant and steroid-sensitive nephrotic syndrome. Kidney Int. 2004;66:564–570. doi: 10.1111/j.1523-1755.2004.00775.x. [DOI] [PubMed] [Google Scholar]
- 31.Wagner KD, Wagner N, Schedl A. The complex life of WT1. J Cell Sci. 2003;116:1653–1658. doi: 10.1242/jcs.00405. [DOI] [PubMed] [Google Scholar]
- 32.Srichai MB, Konieczkowski M, Padiyar A, et al. A WT1 co-regulator controls podocyte phenotype by shuttling between adhesion structures and nucleus. J Biol Chem. 2004;279:14398–14408. doi: 10.1074/jbc.M314155200. [DOI] [PubMed] [Google Scholar]
- 33.Reiser J, Von Gersdorff G, Loos M, et al. Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clin Invest. 2004;113:1390–1397. doi: 10.1172/JCI20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim YH, Goyal M, Kurnit D, et al. Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int. 2001;60:957–968. doi: 10.1046/j.1523-1755.2001.060003957.x. [DOI] [PubMed] [Google Scholar]
- 35.Rico M, Mukherjee A, Konieczkowski M, et al. WT1-interacting protein and ZO-1 translocate into podocyte nuclei after puromycin aminonucleoside treatment. Am J Physiol Renal Physiol. 2005;289:F431–F441. doi: 10.1152/ajprenal.00389.2004. [DOI] [PubMed] [Google Scholar]
- 36.Kim JH, Konieczkowski M, Mukherjee A, et al. Podocyte injury induces nuclear translocation of WT1 interacting protein (WTIP) via microtubule dependent transport. J Biol Chem. 2010;285:9995–10004. doi: 10.1074/jbc.M109.061671. [DOI] [PMC free article] [PubMed] [Google Scholar]