Abstract
Scientific investigation of the relationship between atherosclerosis, inflammation, and lipoprotein metabolism originated in the mid-19th century and has increased exponentially over the past 50 years. Basic research that characterized the lipoproteins and their metabolism was followed by clinical and epidemiologic studies that began to link elevated levels of cholesterol in the blood to the development of atherosclerosis and increased risk for cardiovascular disease. The link between elevated serum cholesterol levels and cardiovascular disease, known as the “lipid hypothesis,” was confirmed with the discoveries of the low-density lipoprotein (LDL) receptor and of the statins. Subsequent results of multiple clinical trials, particularly with statins, have established that reductions in LDL cholesterol are associated with reduced risk for coronary heart disease (CHD). A growing body of evidence suggests that measures of inflammation, such as C-reactive protein (CRP), may enhance cardiovascular risk assessment and help guide clinical decision-making. Reductions in CRP in individuals with low serum levels of LDL cholesterol have been shown to reduce cardiovascular events. A variety of agents designed to further reduce LDL cholesterol, increase high-density lipoprotein (HDL) cholesterol, and target inflammation are currently in development. Future research can help clarify the roles of emerging biomarkers and lipid fractions other than LDL cholesterol in the prevention, diagnosis, and treatment of cardiovascular disease.
EVOLUTION OF THE LIPID HYPOTHESIS
The lipid hypothesis proposes that elevated levels of cholesterol in the blood lead to the development of atherosclerosis and increased risk for cardiovascular disease (CVD). Much scientific investigation currently centers on the role of inflammation in atherosclerosis, but the view that atherosclerosis is an inflammatory disease dates back to Karl von Rokitansky, an Austrian pathologist who first described inflammatory changes in the blood vessels in the 1840s (1). Von Rokitansky's observations are referred to as the “encrustation” theory, and he postulated that inflammatory changes within the arterial wall were secondary to other diseases and not primary. In 1856, Rudolf Virchow, who is often described as the father of modern pathology, also observed cellular inflammatory changes in the arterial wall, which he termed “endo-arteritis chronica deformans.” He believed that these changes originated within the arterial wall and would, therefore, be primary to atherogenesis (2). In 1904, another pathologist, Felix Marchand, first proposed the term “atherosclerosis” to describe the inflammatory and ensuing changes that take place in the vessel wall in what is now known as CVD. The term has a Greek origin: “athero” meaning gruel, and “sclerosis” meaning hardening. “Arteriosclerosis” is a more generalized description than atherosclerosis, and includes various diseases of the layers of the vessel wall, such as calcification of the media and the loss of arterial elasticity that occurs with aging. Atherosclerosis represents a subtype of arteriosclerosis that involves primarily the intima and innermost part of the media of medium-sized and large arteries (3). Stedman's Medical Dictionary defines atherosclerosis as “arteriosclerosis characterized by irregularly distributed lipid deposits in the intima of large and medium-sized arteries, causing narrowing of arterial lumens and proceeding eventually to fibrosis and calcification” (4). In 1913, Nikolai Anichkov in Russia observed a relationship between cholesterol and what we would now recognize as atherosclerosis when he produced inflammatory vascular lesions in rabbits by feeding them cholesterol that had been purified from egg yolk (5). Earlier, he had produced vascular lesions by feeding rabbits a high-protein diet, but found that it was the cholesterol component specifically that induced the lesions (6). Anichkov's discovery was a major step in the evolution of the lipid hypothesis.
Further studies helped elucidate the relationship between lipid metabolism and atherosclerosis. We now know that plasma lipids are transported in macromolecular complexes referred to as the plasma or serum lipoproteins. The body has evolved this mechanism for maintaining the very hydrophobic lipid constituents of lipoproteins—cholesteryl ester and triglyceride—in a soluble, emulsified form as they circulate in the blood. These lipids, which are insoluble in an aqueous medium, exist as complexes together with phospholipids, unesterified or free cholesterol, and proteins referred to as apolipoproteins that serve as detergents for purposes of emulsification. In terms of solubility, unesterified cholesterol is somewhere between triglyceride and cholesteryl ester, on the one hand, and apolipoprotein and phospholipid on the other. The other major lipids in plasma, the unesterified (free) fatty acids, may be associated with lipoproteins, but primarily bind to albumin and pre-albumin as they circulate in the blood (7).
The discovery of the serum lipoproteins is credited to Michel Macheboeuf, who described isolating lipoproteins from horse serum and plasma through the procedure of ammonium sulfate fractionation in 1929 (8). The fractions that Macheboeuf described most likely represent the alpha- or high-density lipoproteins (HDL), and the beta- or low-density lipoproteins (LDL). This nomenclature arises from the co-migration of the alpha-lipoproteins with the alpha globulins on electrophoresis, whereas the beta-lipoproteins migrate with the beta globulins. When separated by electrophoresis, the lipoproteins were also described as having a pre-beta fraction, which corresponds to the very-low-density lipoproteins (VLDL). In addition, what was called a “sinking pre-beta” fraction was separated and subsequently identified as lipoprotein(a), or Lp(a) (3). More recently, a pre-beta HDL fraction has been identified as lipid-poor HDL (apolipoprotein A-I [apo A-I] plus phospholipid), and been suggested as a putative mediator of reverse cholesterol transport.
John Oncley and colleagues at Harvard University studied the characteristics of the lipoproteins through a procedure called Cohn fractionation beginning in the 1940s (9). In 1949, John Gofman, at the University of California at Berkeley, studied and characterized the lipoproteins based on the basis of their rate of flotation in the analytical ultracentrifuge (10). He found that in salt solutions of varying densities, the most lipid-rich of the lipoproteins showed the most rapid rates of flotation. Thus, chylomicrons have the fastest flotation rates (measured in Svedberg flotation units), followed by VLDL, intermediate-density lipoprotein (IDL), LDL, Lp(a), and HDL. Gofman also reported that higher serum levels of LDL and VLDL, and of LDL in particular, were associated with increased risk for coronary heart disease (CHD), whereas higher levels of HDL appeared to protect against CHD. In 1951, investigators at New York Hospital-Cornell Medical Center first described higher levels of HDL in women than in men (11).
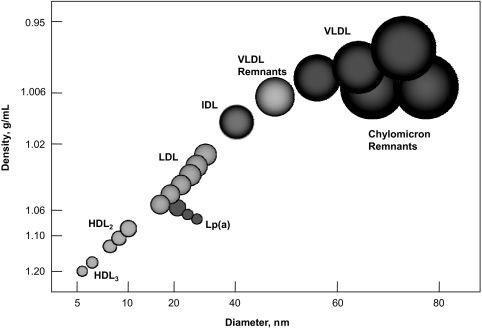
Figure 1 illustrates the multiple lipid fractions associated with CHD risk. In general, the lipoproteins appear round when examined by electron microscopy. High-density lipoprotein, VLDL, IDL, LDL, and Lp(a) are secreted primarily by the liver, while chylomicrons carry dietary lipid. Approximately two-thirds of the cholesterol in the plasma lipoproteins is esterified, and about two-thirds is transported by the LDL family of lipoproteins. All of the lipoproteins except HDL contain a very hydrophobic lipid core, with apolipoproteins and phospholipids interspersed along the surface of the lipoprotein particle. In contrast, HDL, which begins as an apolipoprotein-phospholipid bilayer, is transformed into a spherical particle by taking on cholesterol that is converted to cholesteryl ester; the spherical particle does not have a true hydrophobic core. Lipoprotein remnants represent VLDL and chylomicron particles in which the triglyceride core has been partly degraded by lipases.
Fig. 1.
Lipid fractions associated with CHD risk. (Adapted from Segrest JP, Garber DW, Brouillete CG, et al. The amphipathic α helix: a multifunctional structural motif in plasma apolipoproteins, Adv Prot Chem vol 1994;45:303–369. with permission from Elsevier.)
The lipid and apolipoprotein composition of the lipoproteins is shown in Table 1. Apo A-I and apo A-II are the major apolipoprotein constituents of HDL, although apo A-IV and apo D are also found primarily in HDL. Apo B-100 is the major protein in all of the lipoproteins other than HDL that originate in the liver (VLDL, IDL, and LDL). A truncated form of apo B-100, called apo B-48, is the major protein constituent of the chylomicrons. Apo B-48 represents a form of apo B made in the intestine, and is approximately one-half the size of apo B-100, because of the presence of a stop codon in the mRNA for apo B-100 in the intestine. Rodents, unlike humans, produce apo B-100 and apo B-48 in the liver, whereas in humans the apolipoprotein produced in the liver is almost exclusively apo B-100. Lp(a) contains apo B-100 plus an additional protein called apo(a), which has some structural homology to the kringles of plasminogen. Apo C-I, C-II, C-III, and E are additional apolipoproteins that play direct roles in lipid metabolism, although apolipoproteins going up to apo O have also been described (7, 12).
TABLE 1.
| Lipoprotein | Major Lipid Components | Major Apolipoprotein Components (Minor) |
|---|---|---|
| Chylomicrons | TG | B-48, C-I, C-II, C-III, (A-I, A-II) |
| VLDL | TG, CE | B-100, C-II, C-III, (C-I, E) |
| IDL | TG, CE | E, (B-100, C-I, C-II, C-III) |
| LDL | CE | B-100 (C-III, E) |
| HDL | CE | A-I, A-II, A-IV, C-I, D, (C-II, C-III, E) |
| Lp(a) | CE | Apo(a), B-100 |
One of the first investigators to link elevated levels of serum cholesterol, which in his patients, equated to elevated plasma levels of LDL, to increased risk for atherosclerotic CHD was Carl Müller, a Norwegian physician studying familial hypercholesterolemia (FH) in the 1930s. In a 1939 publication, Müller wrote: “I observed my first patient with xanthoma tuberosum and angina pectoris in April 1937, and by June I was able to make a preliminary report of a number of cases in which I expressed the opinion that hypercholesterolemia is a frequent and important factor in heart disease. This opinion has been strengthened beyond expectation by the study of additional patients.” (13). Others, such as Khachadurian, also studied large families with FH and determined that the disease was characterized by autosomal dominant inheritance (14). These researchers observed that persons heterozygous for FH generally have LDL cholesterol (LDL-C) levels ranging from 300 to 400 mg/dL, while the rare homozygous subject tends to have an LDL-C level over 500 mg/dL and often develops severe atherosclerotic CHD in childhood or adolescence. Thus, there could be no doubt about the association between LDL-C and risk of CHD if levels of LDL-C were sufficiently elevated.
The relationship between serum cholesterol, diet, and CHD was studied extensively by Ancel Keys at the University of Minnesota. He reported a 25-year follow-up of what was called the Seven Countries Study, which showed that the highest death rates from CHD per 1,000 men occurred in Northern Europe and the United States (15). The lowest death rates occurred in Japan and in Southern Europe, where study participants consumed a Mediterranean diet containing large quantities of vegetables and cooked primarily with olive oil, which is rich in monounsaturated fat. On the basis of additional metabolic-ward studies conducted in Minnesota, Keys developed the following formula relating the proportion of calories from dietary saturated fat to serum cholesterol levels:
where ΔTC is the change in serum cholesterol, ΔSFA and ΔPFA are changes in the percentages of total dietary calories from saturated and polyunsaturated fatty acids, respectively, and ΔCHOL is the change in dietary cholesterol in mg/1,000 kcal (16). Thus, Keys showed that the major determinant of serum cholesterol levels was the proportion of calories derived from dietary saturated fat, with dietary cholesterol contributing to these levels to a lesser extent.
In 1956 the Technical Group of the Committee on Lipoproteins and Atherosclerosis, appointed by the National Heart Institute, came to the conclusion that measuring lipoproteins provided no more diagnostic information than measuring serum cholesterol (17). This controversial view persisted for some time. Meanwhile, the Framingham Heart Study identified major risk factors for CHD, including an elevated serum plasma cholesterol level, high blood pressure, and cigarette smoking (18). Subsequent analysis of lipid and lipoprotein levels in participants in the Framingham study found that LDL-C levels were positively correlated with CHD risk, whereas HDL cholesterol (HDL-C) levels were inversely correlated (19). Years later, the Framingham Risk Score (Table 2) was incorporated into the US National Cholesterol Education Program (NCEP) guidelines to identify individuals on the basis of high risk (10-year risk >20%), intermediate risk (10-year risk 10%– 20%), and lower risk (10-year risk <10%) for CHD (20). Thus, serum plasma cholesterol, LDL-C, and HDL-C levels are used today to calculate CHD risk and are the basis for decisions about the need for dietary and/or drug therapy for CHD, as well as for the intensity of such therapy.
TABLE 2.
Predictors Used to Calculate Framingham Risk Score (10-Year Risk of Developing CHD) (19)
|
Most of the epidemiologic and clinical trial data on atherosclerotic CHD relate to LDL-C; however, some data show that apo B measurements may be superior to LDL-C, since all of the cholesterol in the plasma (excluding HDL cholesterol) is carried in atherogenic, apo B-containing particles. Another way to express risk is with the term, non-HDL-C, which encompasses LDL, Lp(a), IDL, VLDL remnants, VLDL, and chylomicron remnants. It is also believed that smaller, dense LDL particles are more atherogenic than the larger, more buoyant ones. Controversy exists in the literature about the relative weight given to LDL-C versus non-HDL-C versus apo B versus particle size distribution in terms of cardiovascular risk assessment (7).
In 1966, Fredrickson, Levy, and Lees published a seminal series of articles in The New England Journal of Medicine that characterized the plasma lipoproteins on the basis of their separation, and described phenotypic disorders associated with lipoprotein metabolism, named the hyperlipidemias (Table 3) (21). The Fredrickson classification system was based on the separation and quantification of cholesterol and the various lipid fractions in plasma by preparative plasma ultracentrifugation, using the method of Havel, Eder, and Bragdon (as opposed to analytical ultracentrifugation as used by Gofman and co-investigators at the Donner Laboratory at Berkeley), and on separation with paper electrophoresis (22). Hatch and Lees had recently discovered that adequate separation of the plasma lipoproteins by electrophoresis on paper was greatly enhanced by the use of albuminated buffer (23).
TABLE 3.
Fredrickson Classification of the Hyperlipidemias (7)
| Phenotype | Lipoprotein(s) elevated | Result | Atherogenicity |
|---|---|---|---|
| I | Chylomicrons | Very high TG | ? |
| IIa | LDL | Elevated cholesterol | +++ |
| IIb | LDL and VLDL | Elevated cholesterol and TG | +++ |
| III | IDL | Elevated cholesterol and TG | +++ |
| IV | VLDL | Elevated TG and normal to slightly elevated cholesterol | + |
| V | VLDL and chylomicrons | Very high TG and normal to slightly elevated cholesterol | + |
In the late 1960s, it was decided that a national study of diet and heart health would be excessively expensive and laborious to perform. What was needed was a clinical trial showing that reductions in plasma cholesterol and LDL-C levels would result in a decreased risk for atherosclerotic CVD. In the 1970s, the Lipid Research Clinics were established to study the prevalence and distribution of hyperlipidemia and dyslipidemia in adults and children. These 12 centers, specialized in arteriosclerosis and located in the United States and Canada, concentrated on characterizing the dyslipidemias, the plasma lipoproteins, and their relationship to the atherosclerotic process. I participated with colleagues at the Baylor College of Medicine and the Methodist Hospital in Houston at one of the 12 clinics. We recruited Dr. William Insull from the Rockefeller University to serve as the principal investigator (PI) in the Lipid Research Clinics-Coronary Primary Prevention Trial (LRC-CPPT), the second major undertaking of the clinics in addition to their studies of the prevalence and characterization of hyper- and dyslipidemia. The LRC-CPPT was a very difficult clinical trial to conduct, and the recruitment efforts for it were enormous. More than 500,000 middle-aged men were screened to find 3,806 who met the strict characteristics for the study, consisting of middle-aged men with primary hypercholesterolemia, an LDL-C ≥175 mg/dL after dieting, and no evidence of CHD at entry into the study (6, 24). The treatment arms of the study gave cholestyramine 24 g/d versus placebo. It did not prove feasible to get the participants to take this much cholestyramine because of gastrointestinal side effects, including gastric discomfort, hard stools, and constipation. The mean cholestyramine consumption was approximately 12 g/d. The men in the study were followed for 7.4 years, after which the cholestyramine group had an 8.5% greater reduction in serum cholesterol and a 12.6% greater reduction in LDL-C than did the placebo group, which translated into a 19% relative reduction in CHD-related events. I was President of the American Heart Association (AHA) at the time of the study, and the AHA strongly endorsed the significance of these findings. However, there was considerable skepticism in the cardiology community about the significance of the study findings, since the result was statistically significant only with a one-tailed t-test.
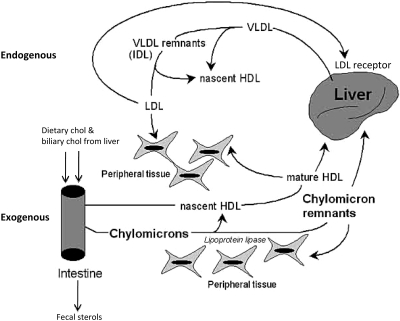
A great advance was made in 1973 with the discovery of the LDL receptor by Michael Brown and Joseph Goldstein working at the University of Texas Health Science Center at Dallas. These investigators shared the richly deserved Nobel Prize in Medicine or Physiology in 1985 for their identification of a deficiency of LDL receptors in patients with FH, thus elucidating at the cellular level the genetic defect in the patients described by Müller and others beginning in the 1930s (25). The discovery of the LDL receptor went a long way toward explaining cholesterol homeostasis in humans. Figure 2 illustrates the endogenous and exogenous pathways of plasma lipid transport by which cholesterol homeostasis is maintained (26). In the endogenous pathway, cholesterol is synthesized by the liver as VLDL and LDL, and is then secreted into the plasma or returned to the liver. When the liver senses a deficiency of cholesterol, it upregulates the levels of LDL receptor and production of hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase, the rate-limiting step in cholesterol biosynthesis. If there is an excess of cholesterol synthesis, the LDL receptor levels are reduced, as is the synthesis of HMG-CoA reductase. In the exogenous pathway, biliary cholesterol and dietary cholesterol are absorbed by the intestines and packaged as chylomicrons, which then release energy to peripheral tissues or are converted to chylomicron remnants for clearance by the liver. Brown and Goldstein also elegantly described the various transporters and transcription factors that regulate cholesterol homeostasis in the liver (27–31).
Fig. 2.
Exogenous and endogenous pathways of lipid transport. (Adapted with permission from Wu K C-W, Cooper AD. Postprandial lipoproteins and atherosclerosis, Front Biosci 2001;6:D332–354.)
DEVELOPMENT OF STATINS
A major turning point in the evolution of the lipid hypothesis occurred in 1976 when the biochemist Akira Endo, working at the Sankyo Company in Japan isolated a factor from the fungus Penicillium citrinum that he identified as a competitive inhibitor of HMG-CoA reductase (32). He called this substance compactin or mevastatin; it was the first statin to be administered to humans. Isolation of this substance was greeted with great enthusiasm by the lipid-research community, and compactin was soon being studied in clinical trials in Japan and in animals and humans in the US and elsewhere. For reasons that have never been published, Sankyo terminated the development of this drug. Merck Research Laboratories, directed at that time by Roy Vagelos, decided to pursue the development of statin drugs after being encouraged to do so by Daniel Steinberg, Jean Wilson, Roger Illingworth, and others. The first statin to be approved was lovastatin (mevinolin), which the US Food and Drug Administration (FDA) sanctioned for use on September 1, 1987. On that day I participated in the press conference announcing its approval, together with Joseph Brown, Michael Goldstein, and Drs. Edward Scolnick and Jonathan Tobert of Merck.
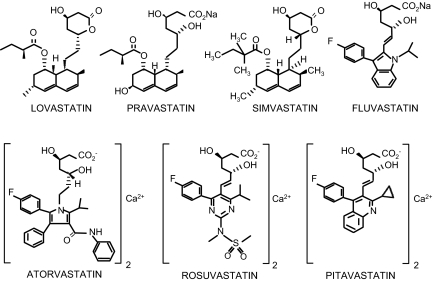
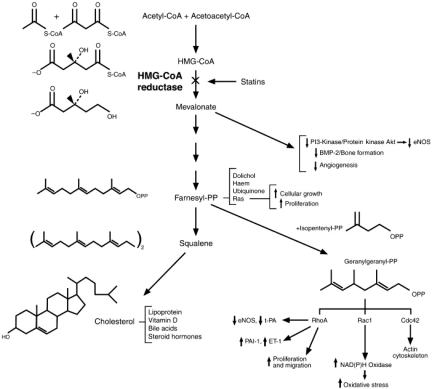
Lovastatin differs from compactin only in having a methyl group attached to the lactone ring. It was isolated in a fungal metabolite both by Dr. Endo, who by this time had left Sankyo, and by Al Alberts and colleagues at Merck Research Laboratories in the US (33). Lovastatin, pravastatin, and simvastatin were all isolated as fungal metabolites, whereas atorvastatin, fluvastatin, rosuvastatin, and most recently pitavastatin were made synthetically (Figure 3). The statin cerivastatin was approved but then withdrawn worldwide when it was found to induce significantly higher muscle toxicity than the other statins. Lovastatin and simvastatin are administered as lactones, while the other statins are administered as open-acid structures. The lactones are converted to the open-acid form in an alkaline condition, and are pro-drugs that are converted to active drugs in the body. All of the statins have side chains that serve as the chemical basis for their competitive inhibition of HMG-CoA reductase. Figure 4 illustrates the effects of inhibition of HMG-CoA reductase by statins on the cholesterol biosynthesis pathway.
Fig. 3.
Chemical Structures of the statins.
Fig. 4.
Effects of inhibition of HMG-CoA reductase by statins on the cholesterol biosynthesis pathway. (Reprinted from Liao JK. Isoprenoids as mediators of the biological effects of statins. J Clin Invest 2002;110(I3):285–88. With permission from Elsevier.)
At the time of the approval of lovastatin, there was great skepticism about the lipid hypothesis. In 1976, Michael Oliver wrote in an editorial in The British Heart Journal that “The view that raised plasma cholesterol is per se a cause of coronary heart disease is untenable” (34). Following the release of the LRC-CPPT results, Daniel Steinberg chaired a National Institutes of Health (NIH) Consensus Conference in 1984 that led to a set of national guidelines for the diagnosis and management of hypercholesterolemia. In a letter to The Lancet, Oliver opined further: that “The panel of jurists for [the Consensus Conference] was selected to include experts who would, predictably, say [ … ] that all levels of blood cholesterol in the United States are too high and should be lowered. And, of course, this is exactly what was said.” (35). Other investigators raised concerns that reducing serum cholesterol levels might increase mortality from non-vascular causes. Results in the LRC-CPPT had shown a small, statistically non-significant increase in violent deaths, which led to a subsequent meta-analysis showing a significant increase in deaths from accidents, suicide, or violence with lipid-lowering treatment as compared with placebo in 6 primary prevention trials (6, 36). Numerous trials and meta-analyses have since demonstrated that reductions in serum cholesterol and LDL-C levels in fact decrease both CHD and all-cause mortality.
Within the general public, there was a pushback against the lipid or cholesterol hypothesis, exemplified by the investigative reporter Thomas J. Moore. In 1989, Moore published a book called Heart Failure, appeared on television talk shows, and had some notable debates with Dr. John LaRosa, director of the Lipid Research Clinic at George Washington University School of Medicine. An excerpt from this book was featured in the Atlantic Monthly as an article entitled “The Cholesterol Myth,” with the cover proclaiming: that “Lowering your cholesterol is next to impossible with diet, and often dangerous with drugs—and it won't make you live any longer” (37). We now know that each of these claims is false, although we did not have the scientific evidence to disprove them in 1989. In addition, Moore postulated that there was a giant conspiracy behind the cholesterol myth involving the NIH, which wanted to get more money from Congress for its research; the AHA, which wanted to gain more money from its donors; and the pharmaceutical companies, which wanted to sell more drugs. According to Moore, this conspiracy was masterminded by a group he called the cholesterol mafia. This included Scott Grundy, John LaRosa, Robert Levy, and Daniel Steinberg, and with a name like Antonio Gotto, I wasn't surprised to be named as well.
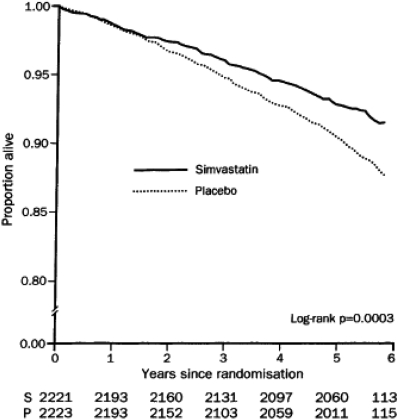
The holy grail for cardiologists came with the Scandinavian Simvastatin Survival Study headed by Terje Pedersen (38). This was a secondary prevention study in Scandinavia involving 4,444 patients who had existing CHD and a mean cholesterol level of 272 mg/dL. In this study, simvastatin 20 mg/d or 40 mg/d was compared with placebo. The LDL-C of statin-treated patients was reduced by 38% and CHD-related events in this group decreased by 34%. In addition, there was a 30% decrease in total mortality, which demonstrated for the first time that lowering cholesterol in high-risk patients could actually increase survival probability over a 5-year period (Figure 5).
Fig. 5.
Reduction in all-cause mortality in the Scandinavian Simvastatin Survival Study. (Reprinted from Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S), The Lancet, 1994;344 (8934):1383–1389. With permission from Elsevier.)
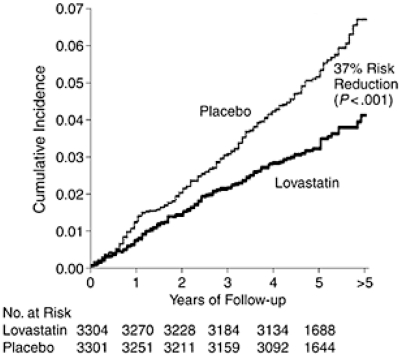
The first primary prevention study with statins was the West of Scotland Coronary Prevention Study (WOSCOPS) with pravastatin, led by Dr. James Shepherd (39). It showed a reduction in CHD events, but did not show a significant difference in total mortality, and enrolled only men. The Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS), the steering committee of which I chaired, used the originally approved statin, lovastatin, to study a group of men and women at two centers in Texas (40). Dr. John R. Downs was the PI of the study, which showed that over a 5-year period, treatment with lovastatin reduced the risk for a first acute major coronary event (measured by fatal/nonfatal myocardial infarction [MI], first occurrence of an acute coronary syndrome, and sudden cardiac death) by 37% (Figure 6). A number of other statin trials followed. The Heart Protection Study (HPS) was a large study with over 20,000 subjects in the UK. (41). Benefit was found with 40 mg/d of simvastatin regardless of baseline LDL-C level. The relative risk reduction in major vascular events was 24%.
Fig. 6.
Reduction in first acute major coronary event in the trial. AFCAPS/TexCAPS (Reprinted from Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA, 1998;279(20):1615–22. With permission from the American Medical Association. Copyright © 1998 American Medical Association. All rights reserved.)
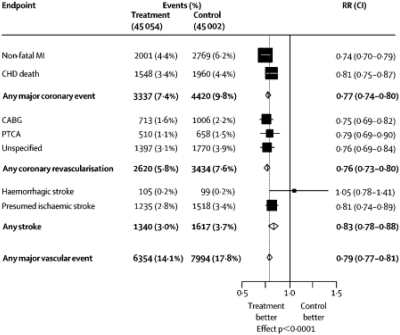
The 4 studies shown in Table 4 all supported the concept that “lower is better.” In these trials of intensive statin therapy, subjects with lower levels of attained LDL-C experienced greater benefit than those with lesser reductions (42). Further evidence of benefit with statin therapy was provided by the Cholesterol Treatment Trialists (CTT) meta-analysis in 2005, which reported the results for more than 90,000 subjects treated with statins in primary and secondary prevention trials (43). It found that for every 1 mmol/L (39 mg/dL) reduction in LDL-C, there was a 21% relative reduction in risk for major vascular events (Figure 7). All clinical endpoints were favorably affected except for hemorrhagic stroke, for which there was a neutral effect. An update of the CTT meta-analysis was published at the end of 2010 and included trials of intensive statin therapy (44). Its findings, which showed a 22% reduction in major vascular events per 1 mmol/L reduction in LDL-C, were similar to those of the earlier meta-analysis, and suggest that there is no lower threshold of benefit in terms of LDL-C reduction.
TABLE 4.
Clinical Outcome Trials Demonstrating “Lower is Better”
| Trial | Population | # | Years | LDL-C Reduction, mg/dL | Risk Reduction in Primary End Point, % | Risk Reduction in CHD Death or MI, % |
|---|---|---|---|---|---|---|
| PROVE IT-TIMI | ACS | 4,162 | 2 | 33 | 16 | 16 |
| A-to-Z (Z phase) | ACS | 4,497 | 2 | 14 | 11 | 15 |
| TNT | Stable CAD | 10,001 | 5 | 24 | 22 | 22 |
| IDEAL | Stable CAD | 8,888 | 5 | 23 | 11 | 12 |
Adapted with permission from Cannon CP (42).
Fig. 7.
Proportional effects on major vascular events per mmol/L LDL cholesterol reduction in the Cholesterol Treatment Trialists Meta-Analysis. (Reprinted from Cholesterol Treatment Trialists' Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomized trials of statins, The Lancet, 2005;366 (9493):1267–78. Copyright 2005, with permission from Elsevier.
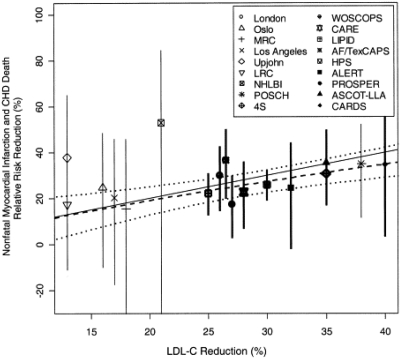
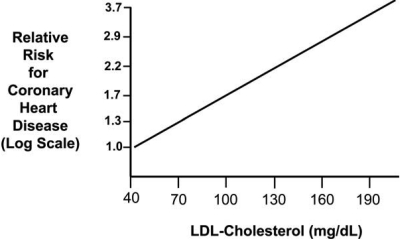
With respect to LDL-C reduction and its relation to CHD risk, a plot of various statin and non-statin trials shows roughly a 1:1 ratio of these two variables, with a 1% reduction in LDL-C resulting in a 1% relative reduction in CHD death and nonfatal MI (Figure 8) (45). This relationship was observed regardless of whether the intervention to reduce LDL-C involved diet, use of a bile-acid resin, partial ileal bypass surgery, or a statin. It occurred in subjects in primary and secondary prevention studies in different parts of the world. Another way to express the association between LDL-C and CHD risk is by plotting LDL-C levels against CHD risk on a log scale (Figure 9) (46). This log-linear relationship, which is consistent with epidemiologic and clinical-trial data, suggests that a 30 mg/dL decrease in LDL-C reduces relative risk for CHD by approximately 30%. This estimate is similar to that calculated in the 2005 CTT meta-analysis, which reported a 23% reduction in major coronary events per 39 mg/dL decrease in LDL-C (43). It should be noted that in Figure 9, the relative risk for CHD is set at 1.0 when LDL-C is equal to 40 mg/dL. This corresponds to the average LDL-C level in humans early in life, and is difficult to achieve.
Fig. 8.
1:1 Relationship between LDL-C reduction and CHD risk reduction maintained between statin and non-statin trials. Estimated change in the 5-year relative risk of non-fatal myocardial infarction or CHD death associated with mean LDL-C reduction (dashed line) along with the 95% probability interval (dotted line). The solid line has a slope = 1. The crude risk estimates from the individual studies are plotted along with their associated 95% confidence intervals. (Reprinted from Robinson JG, Smith B, Maheshwari N, Schrott H. Pleiotropic effects of statins: benefit beyond cholesterol reduction?: A meta-regression analysis, J Am Coll Cardiol 2005;46(10):1855–62. Copyright 2005, with permission from Elsevier.)
Fig. 9.
Log-linear relationship between LDL-C levels and relative risk for CHD. (Reprinted from Grundy SM, Cleeman JI, Merz CNB, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol 2004;44(3):720–32. Copyright 2004, with permission from Elsevier.)
With the exception of cerivastatin, the statins have been remarkably safe. The 2005 CTT meta-analysis showed that LDL-C reduction with statins was not associated with increased risk for cancer, and that the 5-year excess risk for rhabdomyolysis, the primary serious adverse reaction with statins, was extremely low and non-significant (absolute excess = 0.01% [SE 0.01]; P = 0.4) (44). Myopathy and rhabdomyolysis were shown to be potential complications of statin therapy very early on. They were seen in patients who were immunosuppressed or on immunosuppressant drugs, as well as in patients taking both a statin and gemfibrozil, which experimental studies have shown can inhibit glucuronidation of some statins (47, 48). Estimates of myopathy with statin therapy vary, but one estimate is approximately 1 in 10,000 treated patients (49). Changes in liver enzymes (transaminases) can occur, particularly at higher doses of statins, but tend to be almost always reversible. In general, the statins have not proven to be significantly hepatotoxic. Recently, physician-reported results in the Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) study suggested a slight increase in development of diabetes in patients taking statins (50). A meta-analysis of statin trials found a 9% greater risk for incident diabetes (equivalent to 1 extra case of diabetes per 255 patients treated with statins for 4 years), which is consistent with the JUPITER trial findings (51). In contrast, another meta-analysis by the CTT collaborators found that cardiovascular risk reduction with statins was equivalent in patients both with and without diabetes, so that after 5 years of statin therapy, 42 major vascular events would be prevented per 1,000 diabetic patients treated with a statin (52).
The proven efficacy and safety of statins have made them first-line agents for LDL-C reduction. Other drugs used for the treatment of dyslipidemia and their effects on serum lipids are listed in Table 5. Statins primarily reduce LDL-C, although they can also modestly increase HDL-C and reduce triglycerides. Bile-acid resins reduce LDL-C to a lesser extent than statins, and their use is limited by gastrointestinal side effects. The cholesterol absorption inhibitor ezetimibe acts in the gastrointestinal tract to reduce LDL-C and is recommended for use in patients who have difficulty reaching their LDL-C targets with statins alone, or who are statin-intolerant. Nicotinic acid and the fibrates primarily act to reduce triglycerides and increase HDL-C, and they can be cautiously combined with statins for the treatment of mixed dyslipidemia. Use of nicotinic acid is often limited by a side effect of flushing, while the statin-fibrate combination, particularly with the fibrate gemfibrozil, is associated with increased risk for rhabdomyolysis. Omega-3 fatty acids from fish have been shown to favorably affect lipid levels and appear to protect against cardiovascular disease. Prescription-strength omega-3 fatty acids are approved for the treatment of severe hypertriglyceridemia (7).
TABLE 5.
Effects of Drug Classes on Serum Lipids (7)
| Drug Class | Total Cholesterol | LDL-C | HDL-C | Triglycerides |
|---|---|---|---|---|
| Statins | ↓ 15%–60% | ↓ 15%–60% | ↑ 5%–15% | ↓ 15%–25% |
| Bile acid resins | ↓ 20% | ↓ 15%–25% | ↑ 3%–5% | Variable |
| Cholesterol absorption inhibitors | ↓ 13% | ↓ 17–20% | ↑ 3% | ↓ 8% |
| Nicotinic acid | ↓ 25% | ↓ 5%–25% | ↑ 15%–35% | ↓ 20%–50% |
| Fibric acid derivatives | ↓ 15% | Variable | ↑ 10%–20% | ↓ 20%–50% |
| Omega-3 fatty acids | N/A | N/A | N/A | ↓ 35%–50% |
INFLAMMATION IN ATHEROSCLEROSIS
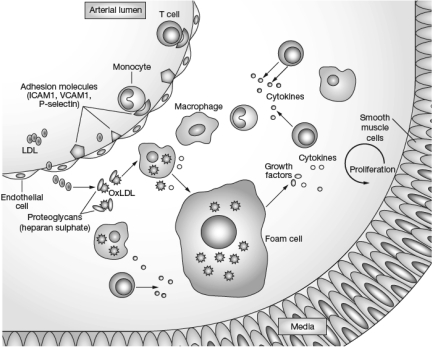
Stimulated by studies conducted by Russell Ross and others beginning in the 1970s and accelerating in the 1990s, research in CVD has increasingly focused on the role of inflammation in the development of the atherosclerotic plaque. The inflammatory reaction within the atherosclerotic plaque has similar characteristics to the reactions seen with polyarteritis nodosa, rheumatoid arthritis, systemic lupus erythematosus and other inflammatory conditions. It is characterized by an accumulation of macrophages and activated T lymphocytes, and by cytokine production leading to an increase in adhesion molecules (intercellular adhesion molecule-1 [ICAM-1], vascular cell adhesion molecule-1 [VCAM-1], and P-selectin), which promote the entry of mononuclear leukocytes (monocytes) into the arterial wall (Figure 10) (53). In the vessel wall, the atherosclerotic process occurs primarily in the intima and to some extent in the inner part of the media. The process is triggered when circulating LDL enters and is retained within the arterial wall. Lipases including lipoprotein lipase, hepatic lipase, secretory phospholipase A2 (sPLA2), and lipoprotein-associated phospholipase A2 (Lp-PLA2) can attack LDL, exposing positively charged lysine and arginine in LDL. These amino acids bind to negatively charged sulfates on glycosaminoglycans or proteoglycans within the arterial wall, resulting in retention of the altered lipoproteins (54). The bound LDL then becomes oxidized or chemically modified when attacked by other lipases.
Fig. 10.
Inflammatory response to atherogenic lipoproteins. (Reprinted with permission from Rev Rheumatol, Sherer Y, Shoenfeld Y, Mechanisms of disease: atherosclerosis in autoimmune diseases. Nat Rev Rheumatol 2006;2(2):99–106. Macmillan Publishers Ltd., copyright 2006.)
After entering the arterial wall, a monocyte becomes a macrophage and contains various receptors, including CD36, that take up oxidized and modified lipoproteins. One of the initial puzzles in understanding the atherosclerotic process within the arterial wall was the relative paucity of LDL receptors on macrophages. The discovery of receptors for oxidized or modified LDL provided an explanation for the way in which LDL contributes to the accumulation of cholesterol and cholesteryl ester within the macrophage. As the macrophages in the arterial wall become filled with lipid, they are converted to foam cells. A series of reactions occurs, including smooth-muscle-cell proliferation and the production of matrix metalloproteinases (MMP), which ultimately break down the elastin and collagen within the wall of the vessel. Fissuring of the vessel wall occurs, with the entry of coagulation factors and adherence of platelets to the arterial wall. Thrombosis results in an MI or cerebrovascular accident. This is a greatly oversimplified description of a complex series of reactions. Many laboratories have contributed to the understanding of this process over the past two decades, with Peter Libby's laboratory at Brigham and Women's Hospital being one of the leading groups in this work, although many others have also contributed (55).
These advances in basic research in the role of inflammation in atherosclerosis are currently being translated into clinical practice. Dr. Paul Ridker and colleagues at the Harvard Medical School-Brigham and Women's Hospital have extensively studied C-reactive protein (CRP), a general measure of inflammation that is produced in the liver in response to interleukin-6. They have described the Reynolds score, which involves additional measurements of CVD risk, including levels of CRP, and may provide some improvement over the Framingham Risk Score assessment (56, 57). The AFCAPS/TexCAPS investigators collaborated with Ridker on a post hoc analysis of the trial and showed that if either their baseline LDL-C or CRP levels were above the median, study participants benefited from lovastatin therapy (58). However, if neither measure was above the median, there was no benefit. This hypothesis-generating analysis provided a rationale for a clinical trial to examine benefit in individuals with elevations in CRP but LDL-C levels not high enough to warrant statin therapy.
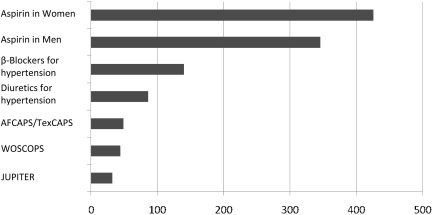
The resulting study was the JUPITER study, a multi-national, randomized, double-blind, placebo-controlled trial (51). To participate, subjects had to be male and at least 50 years of age or female and at least 60 years of age, and without evidence of CVD or diabetes mellitus, since the latter had been defined by the NCEP as a CHD risk equivalent. The enrollees' LDL-C had to be under 130 mg/dL and their high-sensitivity CRP (hsCRP) at least 2 mg/L. The median baseline level of CRP was approximately 4 mg/L. Subjects were randomized to rosuvastatin 20 mg/d or placebo. The primary endpoint was the combination of MI, stroke, unstable angina, death from CVD, and revascularization. The study was planned to last for 5 years, but the independent data monitoring board recommended to the study steering committee that the study be stopped after 1.9 years. The trial results of the JUPITER study demonstrated a 44% relative reduction in the primary endpoint, with the number of patients needed to treat (NNT) over a period of 5 years in order to prevent one event being 25. The rosuvastatin group had a 50% lower median LDL-C level than the placebo group. Figure 11 shows the 5-year NNT for the primary prevention of CVD in middle- aged populations. Although the NNT for the JUPITER study was 25, it was approximately 425 for aspirin in women, approximately 40 for the WOSCOPS, and just under 50 for the AFCAPS/TexCAPS (59).
Fig. 11.
Estimated 5-Year NNT Values for the Primary Prevention of Cardiovascular Disease in Middle-Aged Populations (60).
The observed benefit of rosuvastatin in the JUPITER study was much greater than was predicted on the basis of the results of earlier statin trials. According to one analysis of the JUPITER study data, the risk reduction observed in the study is more consistent with the results of other statin trials when related to the percent reduction in LDL-C as opposed to the absolute reduction in LDL-C (60). In addition, overall benefit may be better predicted by the reduction in absolute rather than relative risk, with the more potent statins producing a greater absolute risk reduction, as one would expect.
In JUPITER, the participants who experienced the greatest reduction in vascular events were those who achieved an LDL-C level below 70 mg/dL and hsCRP below 1 mg/L (61). These findings raise the question of whether CRP itself should be a target of therapy. We cannot answer this question on the basis of the JUPITER data. In individuals who have genetically determined elevations of CRP over a lifetime, increased cardiovascular risk is not observed (62). However, JUPITER was sufficiently powered to answer questions about the benefit of statin therapy in understudied and low-risk subgroups (51). It demonstrated benefit in women, participants older than 70 years, racial/ethnic minorities including blacks and Hispanics, those with a 10-year Framingham Study-based risk of 10% or less, a baseline LDL-C <100 mg/dL, a body mass index (BMI) <25 mg/m2, persons without hypertension, those without metabolic syndrome, and participants who had only an elevated CRP level.
What JUPITER tells us is that individuals with what are considered normal or low levels of LDL-C according to national and international guidelines may be at increased risk for CVD due to elevated CRP levels. We cannot know, on the basis of the JUPITER results alone, what the effect of treatment would be in individuals with low levels of LDL-C and CRP, although the post hoc analysis of AFCAPS/TexCAPS did not show benefit in these individuals. The data from JUPITER also cannot tell us whether CRP is involved in the atherosclerotic process directly, as an active contributor, or whether it is only a marker of inflammation. In addition, because JUPITER was stopped after 1.9 years, we do not have information from this study about the long-term use of rosuvastatin, although many other studies have demonstrated the continued efficacy and safety of this drug over longer periods of time.
On the basis of the results of the JUPITER study, the FDA approved a new indication for rosuvastatin in March 2010 for the primary prevention of CVD “in individuals without clinically evident coronary heart disease but with an increased risk of cardiovascular disease based on age ≥50 years old in men and ≥60 years old in women, hsCRP ≥2 mg/L, and the presence of at least one additional CVD risk factor” (63). This expanded indication could affect approximately 20% of 11 million middle-aged-to-elderly adults, so that 80% of this population would be eligible for statin therapy (64). In coverage of this new indication, the New York Times carried a front-page article with the headline, “Plan to Widen Use of Statins Has Skeptics” (65). However, the JUPITER results and the new rosuvastatin indication should put to rest once and for all any assertion that statins show no evidence of benefit in the primary prevention of CVD in women or in individuals over the age of 70.
FUTURE OF CARDIOVASCULAR PREVENTION
Although LDL-C is the primary target of therapy to reduce cardiovascular risk, other approaches to this are currently being investigated. There are a number of hypothesized mechanisms for protection through HDL, although none has been firmly established. Two major functions of HDL have been proposed. One is participation in reverse cholesterol transport, and the other is exertion of an anti-inflammatory effect (66). The latter has been extensively studied by Navab, Fogelman and associates, who propose that the pro- or anti-inflammatory effects of HDL depend upon the surface constituents of the particle. Additionally, HDL is known to inhibit the oxidation of LDL and has been found to inhibit the expression of adhesion molecules, endothelial cells, and inflammatory cytokines.
Our understanding of HDL metabolism and the process of reverse cholesterol transport is continually evolving (67). High-density lipoprotein is initially secreted by the liver as lipid-poor apo A-I, a discoidal particle containing protein and phospholipid. Apo A-I contains amphipathic helixes having a structure first described by Segrest et al. in 1974 (68). It is hypothesized that discoidal HDL consists of two apo A-I particles wrapped around a phospholipid bilayer like a belt (69). The lipid-poor apo A-I, which migrates as pre-beta HDL or pre-beta A-I with electrophoresis, then accumulates free cholesterol from macrophages by interacting with ATP-binding cassette transporter A1 (ABCA1), and forms a larger particle called nascent pre-β HDL. This can then be converted into spherical α-HDL through the action of lecithin-cholesterol acyltransferase (LCAT), which catalyzes the esterification of the free cholesterol in the nascent pre-β HDL. It should be noted that there is evidence that the ABCA1 transporter in the liver plays a major role in controlling the level of HDL particles circulating in the blood.
Cholesteryl ester from the mature HDL particle can reach the liver in at least two ways. First, HDL can interact with a receptor called SR-BI to facilitate the removal of cholesteryl ester from the HDL particle directly to the liver. Second, cholesteryl ester can be transferred to the apo B-containing particles in exchange for triglyceride. This transfer depends on the activity of a protein called cholesteryl ester transfer protein (CETP). It is not known in humans what the qualitative significance of these two alternative pathways is. If the LDL receptor pathway is highly active, one could argue that it might not be desirable to inhibit CETP.
At any rate, CETP inhibitors raise levels of HDL-C as well as decreasing those of Lp(a). Their effect on cholesterol balance is uncertain. One study showed that the CETP inhibitor torcetrapib did not increase bile acid synthesis or fecal sterol excretion (70). In a subsequent phase III trial, torcetrapib substantially raised concentrations of HDL-C and decreased LDL-C, but the study was terminated because of an increase in cardiovascular death (66). Torcetrapib appeared to have an off-target effect in stimulating aldosterone secretion and raising blood pressure. Two other CETP inhibitors are currently in clinical trials: dalcetrapib, which raises HDL-C but has little effect on LDL-C concentrations, and anacetrapib, which raises HDL-C and lowers LDL-C (71, 72). Another approach directed at HDL involves administering apo A-I, a genetic variant of apo A-I called apo A-I Milano, or apo A-I mimetics. Apo A-I Milano and apo A-I mimetics are thought to interact with the ABCA1 transporter, as does lipid-poor HDL containing apo A-I. In one trial, infusions of apo A-I Milano were given over a 5-week period to patients following an acute coronary syndrome (ACS), and were reported to induce regression of coronary atherosclerosis as determined by intravascular ultrasonography (73). In another recent study, infusions of plasma containing delipidated HDL given to ACS patients were shown to convert α-HDL to pre-beta-like HDL, which is believed to be the most effective form of HDL for lipid removal from arterial plaques (74). Another experimental drug, RVX-208, has been reported to upregulate the transcription of apo A-I and to increase apo A-I and HDL-C levels by this mechanism (75). The dual peroxisome proliferator-activated receptor (PPAR) α/γ agonist aleglitazar, which has been shown to increase HDL-C and reduce triglycerides, is also currently being studied for treatment of type 2 diabetes (76).
Other therapies are being tested to determine whether additional LDL-C reduction is safely obtainable. Whatever additional therapies for dyslipidemia are introduced, it seems apparent that at this point in time they would need to be compatible with the statins and would be prescribed against a background of statin therapy. The statins set a very high bar for efficacy and safety, yet there are many patients whose target levels of LDL-C cannot be reached with statin therapy alone. Patients with severe primary hypercholesterolemia or heterozygous FH, as well as the rare homozygous FH subject, often need add-on therapy to statins. Treatments for patients with homozygous FH can qualify for orphan drug status. Until recently, the FDA has taken the position that if a drug lowers LDL-C by 15% or more and has an acceptable safety record, it is eligible for approval on the basis of LDL-C reduction alone. The FDA appears to have altered this position as it applies to new mechanisms of LDL-C reduction. A commitment to conducting clinical outcomes trials seems to be a requirement for drugs used to treat dyslipidemias other than homozygous FH. It seems unlikely that a new class of drug would be approved on the basis of surrogate outcomes such as LDL-C reduction, intravascular ultrasonography, carotid ultrasonography, or coronary calcium scoring.
As far as reducing LDL-C is concerned, the apo B antisense oligonucleotide mipomersen has been found in phase III studies to reduce cholesterol in patients with homozygous FH as well as in patients with primary hypercholesterolemia (77, 78). Mipomersen and another class of experimental drug, the microsomal triglyceride transfer protein (MTP) inhibitors, act by inhibiting secretion of VLDL. Two MTP inhibitors in development are lomitapide and JTT-130, with lomitapide currently being tested in clinical trials (79). An earlier problem with lomitapide was that it produced fatty liver, but recent studies have indicated that this problem may be addressed by titrating the drug. Another, entirely different approach to reducing LDL-C involves thyroid hormone analogues (80). One of these, eprotirome, is a thyroid hormone receptor-β agonist that works in the liver and is currently in clinical trials. It reportedly has less of a tendency than thyroid hormone to induce cardiac arrhythmias, promote bone loss, or stimulate metabolism. The exact mechanism of action of this class of drugs is unknown, although thyroid hormone analogues may act independently of the LDL receptor and are reported to produce substantial reductions in Lp(a). For the patient with homozygous FH, LDL apheresis is available, and liver transplantation is a possible last resort.
A number of novel anti-inflammatory approaches to the prevention and treatment of atherosclerotic CVD are currently being studied. Darapladib is a reversible oral inhibitor of Lp-PLA2, and varespladib methyl is a sPLA2 inhibitor; both types of drugs have reduced atherosclerosis in experimental models and reduced levels of biomarkers in CHD patients (81). Other potential anti-inflammatory approaches to CVD are succinobucol, LOX-1 gene therapy, and vaccines targeting peptide sequences in apo B. In addition, the Cholesterol Inflammation Reduction Trial, headed by Paul Ridker, proposes to study the effect of low-dose methotrexate in patients with stable coronary artery disease and elevated levels of CRP (82). Additional ongoing clinical trials with various experimental and approved agents include AIM-HIGH, comparing extended-release niacin/simvastatin with simvastatin monotherapy; HPS2-THRIVE, comparing extended-release niacin plus laropiprant, a prostaglandin D2 receptor inhibitor that decreases the flushing response of niacin, with placebo in simvastatin-treated patients; IMPROVE-IT, an outcomes trial comparing ezetimibe/simvastatin with simvastatin in ACS patients; and dal-PLAQUE, comparing the effects of dalcetrapib with those of placebo on atherosclerotic plaque.
An interesting and potentially important new target for therapy directed at reducing CHD risk is PCSK9. First described in France, PCSK9 is a gene that produces a protein that regulates LDL receptor removal from the surface of cells. A gain-of-function mutation can alter the activity of PCSK9 such that there are fewer LDL receptors, producing a phenotype like that in FH, and a loss-of-function mutation can increase the number of LDL receptors, resulting in lifelong hypocholesterolemia (83). In the Dallas Heart Study, African-Americans with PCSK9 mutations that reduced LDL-C by 28% had an 88% reduction in risk for CHD, and Caucasians with mutations that reduced LDL-C by 15% had a 47% decrease in risk for CHD (84). Biotoxicity associated with low LDL-C levels in these individuals has not been described. These findings suggest that reducing LDL-C levels over a lifetime might be expected to result in much greater reductions in CHD than predicted by the 1:1 relation observed in the 5-year clinical trials, by which a 1% relative reduction in risk for CHD accompanies a 1% reduction in LDL-C. Numerous approaches to inhibiting PCSK9 expression, including its inhibition by antisense oligonucleotides, small interference RNAs (siRNAs), antibodies, and other small molecules, are currently in development (85).
The PCSK9 studies not only identified this gene and its protein as potential targets for drug therapy for reducing CHD risk, but also support the case for earlier intervention. The current NCEP guidelines for adults call for cholesterol screening starting at age 20. New pediatric guidelines established by the American Academy of Pediatrics suggest screening children for CHD risk as early as age 2 if they have risk factors for the disease, as well as earlier intervention than has previously been advised (86). The evolution of guidelines supported by the National Heart, Lung and Blood Institute, beginning with the first Adult Treatment Panel (ATP I) in 1988, has moved progressively toward more intensive treatment recommendations. At this time, the most recent update to ATP III, originally released in 2001, was issued in 2004, and it is anticipated that the next set of guidelines, ATP IV, will be released in the next 1–2 years. Some of the questions the panel will have to consider include the age at which to start treatment and what treatment to use, the minimal levels of LDL-C levels at which to start treatment in various subgroups at risk, and the place of CRP in the diagnostic algorithm for CHD risk.
There is certainly much yet to learn about the genetic determinants of CHD. For example, at least three separate studies with Caucasian subjects have identified an allele on chromosome 9p21 that is associated with increased CHD risk (87, 88). None of the major risk factors for CHD or factors involved in lipid metabolism is known to be associated with this particular allele. According to Ruth McPherson and colleagues, 20%–25% of the population are homozygotes for the allele and have a 30–40% greater risk for developing CHD (89). Heterozygotes, who make up 50% of the population, have an approximately 15–20% greater risk for CHD.
With all of these exciting developments, we should not lose sight of what we can accomplish from what we already know about the benefit of lifestyle, diet, exercise, blood pressure control, and lipid-lowering therapies in reducing CHD risk. We have come far in our understanding of the atherosclerotic process since the time of von Rokitansky and Virchow. The development of statins has helped confirm the lipid hypothesis and has revolutionized the field of prevention and treatment of CVD. The primary target of therapy is LDL-C, and numerous clinical trials have demonstrated that reducing LDL-C significantly decreases cardiovascular risk. Advances in basic science have increasingly elucidated the role of inflammation in atherosclerosis, and a major trial has established that reduction of CRP correlates with a reduction in cardiovascular events. Whether CRP should be a therapeutic target remains uncertain. Ongoing research will help clarify the specific roles of inflammation, HDL-C, and non-HDL-C components (VLDL, IDL, chylomicron remnants) in the prevention and treatment of CVD.
Footnotes
Potential Conflicts of Interest: Dr. Gotto is a consultant for AstraZeneca, Kowa, and Merck, and serves on the Board of Directors for Aegerion Pharmaceuticals and Arisaph Pharmaceuticals. He is a member of advisory boards for DuPont and Vatera Capital.
DISCUSSION
Mandell, Charlottesville: This is probably a simple dumb question, but why is obesity bad?
Gotto, New York: Well, there are many different reasons, but one is that adipocytes produce cytokines, which have pro-inflammatory effects. Obesity also adversely affects various cardiovascular risk factors, including blood glucose control, insulin sensitivity, blood pressure, and lipids.
August, New York: Thank you very much, Tony. That was a great talk. I didn't notice in the JUPITER study whether they analyzed the results based on HDL levels. Did they do any analyses based on HDL?
Gotto, New York: Yes, we did, and baseline HDL did not predict cardiovascular events. This was different than in the Treating to New Targets study, which compared treatment with 10 versus 80 mg of atorvastatin and found that HDL was predictive even in patients with an LDL of less than 70 mg/dL. In JUPITER, HDL lost its predictive value in the group in which LDL levels were reduced to 40 mg/dL.
August, New York: So patients with high HDL still get benefit from the statins on those borderline levels of LDL?
Gotto, New York: Yes. They will not get as much absolute benefit as patients with lower HDL, because the lower HDL patients are at higher risk. I think that having a high LDL and a high HDL doesn't cancel out all of the risks from the high LDL, and these patients should be treated.
Hoffman, New York: Patients with myeloproliferative disorders have an unacceptable rate of thrombosis, dying of arterial thromboses, venous thromboses, and intra-abdominal thromboses. These patients characteristically have long-term low cholesterol levels, a chronic inflammatory state, and genetic abnormalities on chromosome 9p. I was wondering if you had any theories about this, and how that predisposition might occur, and what therapeutic interventions could lead to a reduction in this unacceptable rate of thrombosis.
Gotto, New York: Thank you for that question, Ron. That is a very good question and an interesting one that we've given a great deal of thought to, because one of the unexpected results from JUPITER was a significant reduction in venous thrombosis. We don't have an explanation, but we hypothesize that the anti-inflammatory effect may have some relationship to it, and that there may be things that are affected by the degree of reduction of HMG-CoA reductase that may not necessarily be reflected by the degree of LDL reduction.
Alexander, Atlanta: Tony, I look forward very much to seeing the manuscript that comes from this, because this is the most elegant summation of all of these data that I have ever seen. I congratulate you.
Gotto, New York: Phil reminded me last night that it was due by December 31. So thank you.
Alexander, Atlanta: I see, and he comes to look for you if it's not there, as I know well. You mentioned the duration of the JUPITER study, and most of these studies enroll people who have had atherosclerotic disease, although sometimes it may not have clinically manifested itself for decades. Sometimes the studies include those in whom we can't demonstrate it clinically yet at all. You mentioned the fact that we don't know the effect of starting statins even earlier. There are some epidemiologic data on elderly cohorts that just look at lifestyle. For example, a paper in JAMA several years ago looked at patients aged 70 to 90 who follow a Mediterranean diet, don't smoke, exercise normally, and have a couple drinks of spirits or wine a day, and they have an attributable risk for all-cause mortality, specifically cancer and cardiovascular disease, that is decreased by two-thirds. That's kind of a long introduction to asking the question about what we should be looking forward to in the future in terms of treating pre-morbid antecedents of this disease and other diseases, perhaps from a public health point of view.
Gotto, New York: I have two comments. One is that I think that far and away what we have to be looking toward in this country is obesity, which was talked about earlier in this meeting, and the increasing prevalence of its starting earlier in life, as adolescent obesity. So I think that's going to occupy a great deal of our public-health effort. It's an interesting question about where to define the beginning of atherosclerosis. The Bogalusa Heart Study showed that abnormal risk factors or elevated risk factors in childhood and adolescence predicted later events. The study of Korean War casualties found plaques in their coronary arteries. In younger people who die, there are plaques and fatty streaks and sometimes raised lesions in adolescence. The so-called fatty streaks, which are macrophages filled with lipid in the arterial wall, are not benign. They progress under appropriate stimulation—the conventional risk factors, for CVD of a Western industrialized diet and lack of exercise—and develop into more advanced lesions. My question is, where does it actually start? Some studies have shown that treating the hyperlipidemia that's associated with pregnancy results in a reduction in fatty streaks in infants. I don't know really where we should start, but we need to start early with lifestyle. I think, based on the level of risk and the long-term safety effects of medication, that at some point in time we will have to make a decision about other treatments. For children with familial hypercholesterolemia, we start drugs very early.
Lange, San Antonio: Again, a very nice summary. One of the unusual things about the JUPITER Study was that although people in the study had normal LDL and elevated CRP, only about 20% of them were actually receiving aspirin. I've not yet seen the data suggesting or defining whether the patients who were receiving aspirin had a risk reduction through lowering of LDL cholesterol. I guess I'd like you to address the question of whether for someone with a normal LDL and an elevated CRP, aspirin should be the therapy or should they stop taking it?
Gotto, New York: Well, I think the answer to that is that they might be treated with both aspirin and rosuvastatin. Aspirin doesn't have much effect on CRP. JUPITER was conducted in 39 countries, and so aspirin was not mandated as a part of the therapy.
Abboud, Iowa City: Thank you very much, Tony, for a review of the problem. In terms of the inflammatory response, to what extent is there a change in the immune system itself as a result of lipid or the statin influence? Are there different types of T lymphocytes? Is the secretion of inflammatory versus anti-inflammatory cytokines altered? I'm thinking about the immune system itself being affected rather than simply of lymphocytes being attracted into the vasculature and inducing inflammation.
Gotto, New York: That's a very good question, and there have been a number of animal studies looking at differential effects on Th1 and Th2 lymphocytes. There are some effects of the statins that would be predicted to be protective and others that wouldn't, so it's a mixed bag at present. I think it's going to take a lot of work to try to sort out what part of the benefit with statins is due to inhibiting HMG-CoA reductase and then the downstream effects of that inhibition, which subsequently influences various other cytokines and results in an anti-inflammatory effect. It's very complicated. For example, lowering LDL cholesterol with a statin will have an antioxidant effect, not simply because statins may have specific antioxidant properties per se, but because you will have less LDL to oxidize. Identifying the specific anti-inflammatory and antioxidant properties of statins and how they affect the different forms of lymphocytes is going to take a lot more work.
Oates, Nashville: Tony, thank you for the insightful presentation. The JUPITER trial characterized an LDL cholesterol of less than 130 mg/dL as “normal,” and I wonder if in light of all of the data from these trials we should any longer refer to LDL cholesterol levels of 130 mg/dL as normal?
Gotto, New York: I don't think that's normal. I think a level of 100 mg/dL is a desirable level for all adults, but it could be lower than that. We picked 130 mg/dL because that was as high as we could go and still have a placebo-controlled study by any national or international guideline. Personally, I think 130 mg/dL is too high, and in patients on whom I still consult, I try to get everybody, unless they have other risk factors, down to an LDL cholesterol of 70 mg/dL.
REFERENCES
- 1.Rokitansky C. A manual of pathological anatomy. Philadelphia: Blanchard and Lea; 1855. [Google Scholar]
- 2.Mayerl C, Lukasser M, Sedivy R, et al. Atherosclerosis research from past to present-on the track of two pathologists with opposing views. Virchows Arch. 2006;449(1):96–103. doi: 10.1007/s00428-006-0176-7. [DOI] [PubMed] [Google Scholar]
- 3.Gotto AM., Jr Evolving concepts of dyslipidemia, atherosclerosis, and cardiovascular disease. The Louis F. Bishop Lecture. J Am Coll Cardiol. 2005;46(7):1219–24. doi: 10.1016/j.jacc.2005.06.059. [DOI] [PubMed] [Google Scholar]
- 4.Stedman's Medical Dictionary. 28th ed. Philadelphia: Lippincott Williams and Wilkins; 2005. Atherosclerosis. [Google Scholar]
- 5.Anichkov NN, Chalatow S. Ueber experimentelle Cholesterinsteatose und ihre Bedeutung für die Entstehung einiger pathologischer Prozesse. Zentralbl Allg Pathol. 1913;24:1–9. [Google Scholar]
- 6.Steinberg D. Amsterdam: Academic Press; 2007. The Cholesterol Wars: The Skeptics vs the Preponderance of Evidence; pp. 15–20. [Google Scholar]
- 7.Gotto A, Pownall H. Manual of Lipid Disorders. Philadelphia: Lippincott Williams & Wilkins; 2003. pp. 2–10.pp. 221–242.pp. 299–354. [Google Scholar]
- 8.Macheboeuf M. Recherches sur les phosphoaminolipides et les stérides du serum et du plasma sanguins. Bull Soc Chem Biol. 1929;11:485–503. [Google Scholar]
- 9.Cohn EJ, Strong LE, Hughes WL, Jr., et al. Preparation and properties of serum and plasma lipoproteins. IV. A system for the separation into fractions of the protein and lipoprotein components of biological tissues and fluids. J Am Chem Soc. 1946;68:459–75. doi: 10.1021/ja01207a034. [DOI] [PubMed] [Google Scholar]
- 10.Gofman JW, Lindgren FT, Elliott H. Ultracentrifugal studies of lipoproteins of human serum. J Biol Chem. 1949;179:973–9. [PubMed] [Google Scholar]
- 11.Russ EM, Eder HA, Barr DP. Protein-lipid relationships in human plasma. I–II. Am J Med. 1951;11:468–79. 480–93. doi: 10.1016/0002-9343(51)90182-9. [DOI] [PubMed] [Google Scholar]
- 12.Choi BG, Badimon JJ, Moreno PR, Fuster V. Lipoprotein metabolism and vascular biology. In: Davidson M, Toth PP, Maki KC, editors. Therapeutic Lipidology. Totowa, NJ: Humana Press; 2007. pp. 1–22. [Google Scholar]
- 13.Müller C. Angina pectoris in hereditary xanthomatosis. Arch Intern Med. 1939;64:675–700. [Google Scholar]
- 14.Khachadurian AK. The inheritance of essential familial hypercholesterolemia. Am J Med. 1964;37:402–7. doi: 10.1016/0002-9343(64)90196-2. [DOI] [PubMed] [Google Scholar]
- 15.Verschuren WM, Jacobs DR, Bloemberg BP, et al. Serum total cholesterol and long-term coronary heart disease mortality in different cultures. Twenty-five-year follow-up of the seven countries study. JAMA. 1995;274(2):131–6. [PubMed] [Google Scholar]
- 16.Keys A, Anderson JT, Grande F. Serum cholesterol response to changes in the diet. IV. Particular saturated fatty acids in the diet. Metabolism. 1965;14(7):776–87. doi: 10.1016/0026-0495(65)90004-1. [DOI] [PubMed] [Google Scholar]
- 17.Gofman JW, Hanig M, Jones HB, et al. Evaluation of serum lipoproteins and cholesterol measurements as predictors of clinical complications of atherosclerosis: report of a cooperative study of lipoproteins and atherosclerosis. Circulation. 1956;14:689–741. [PubMed] [Google Scholar]
- 18.Kannel WB, Dawber TR, Kagan A, Revotskie N, Stokes J. Factors of risk in the development of coronary heart disease-six year follow-up experience. The Framingham Study. Ann Intern Med. 1961;55:33–50. doi: 10.7326/0003-4819-55-1-33. [DOI] [PubMed] [Google Scholar]
- 19.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High-density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977;62(5):707–14. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- 20.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 21.Fredrickson DS, Levy RI, Lees RS. Fat transport in lipoproteins-an integrated approach to mechanisms and disorders. N Engl J Med. 1967;276:34–44. 94–103, 148–56, 215–25, 273–81, 321–7. doi: 10.1056/NEJM196701052760107. [DOI] [PubMed] [Google Scholar]
- 22.Havel RJ, Eder HA, Bragdon JH. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955;34(9):1345–53. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lees RS, Hatch FT. Sharper separation of lipoprotein species by paper electrophoresis in albumin-containing buffer. J Lab Clin Med. 1963;61:518–28. [PubMed] [Google Scholar]
- 24.The Lipid Research Clinics Coronary Primary Prevention Trial results. I–II. JAMA. 1984;251:351–64. 365–74. doi: 10.1001/jama.1984.03340270029025. [DOI] [PubMed] [Google Scholar]
- 25.Goldstein JL, Brown MS. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- 26.Wu K C-W, Cooper AD. Postprandial lipoproteins and atherosclerosis. Front Biosci. 2001;6:D332–354. doi: 10.2741/yu. [DOI] [PubMed] [Google Scholar]
- 27.Brown MS, Goldstein JL, Krieger M, Ho YK, Anderson RG. Reversible accumulation of cholesteryl esters in macrophages incubated with acetylated lipoproteins. J Cell Biol. 1979;82:597–613. doi: 10.1083/jcb.82.3.597. Goldstein JL, Ho YK, Basu SK, Brown MS. Binding site on macrophages that mediate uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci 1979;76:333–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown MS, Goldstein JL. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;77:2214–18. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- 29.Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89(3):331–40. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 30.Radhakrishnan A, Ikeda Y, Kwon HJ, Brown MS, Goldstein JL. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: oxysterols block transport by binding to Insig. Proc Natl Acad Sci USA. 2007;104(16):6511–8. doi: 10.1073/pnas.0700899104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun LP, Seemann J, Goldstein JL, Brown MS. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: Insig renders sorting signal in Scap inaccessible to COPII proteins. Proc Natl Acad Sci USA. 2007;104(16):6519–26. doi: 10.1073/pnas.0700907104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Endo A, Kuroda M, Tanzawa K. Competitive inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase by ML-236A and ML-236B fungal metabolites, having hypocholesterolemic activity. FEBS Lett. 1976;72:323–26. doi: 10.1016/0014-5793(76)80996-9. [DOI] [PubMed] [Google Scholar]
- 33.Alberts AW, Chen J, Kuron G, et al. Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc Natl Acad Sci. 1980;77(7):3957–61. doi: 10.1073/pnas.77.7.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oliver M. Dietary cholesterol, plasma cholesterol and coronary heart disease. Br Heart J. 1976;38:214–8. [Google Scholar]
- 35.Oliver M. Lancet; Consensus or nonconsensus conferences on coronary heart disease; 1985. pp. 1087–9. [DOI] [PubMed] [Google Scholar]
- 36.Muldoon MF, Manuck SB, Matthews KA. Lowering cholesterol concentrations and mortality: a quantitative review of primary prevention trials. BMJ. 1990;301(6747):309–14. doi: 10.1136/bmj.301.6747.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moore TJ. The cholesterol myth. Atlantic Monthly. 1989 Sep;264(3):37. [Google Scholar]
- 38.Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–9. [PubMed] [Google Scholar]
- 39.Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–7. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 40.Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: Results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279:1615–22. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 41.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomized placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 42.Cannon CP. The IDEAL cholesterol: lower is better. JAMA. 2005;294(19):2492–4. doi: 10.1001/jama.294.19.2492. [DOI] [PubMed] [Google Scholar]
- 43.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–78. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 44.Cholesterol Treatment Trialists' Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data fromo 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–81. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robinson JG, Smith B, Maheshwari N, et al. Pleiotropic effects of statins: benefit beyond cholesterol reduction? A meta-regression analysis. J Am Coll Cardiol. 2005;46:1855–62. doi: 10.1016/j.jacc.2005.05.085. [DOI] [PubMed] [Google Scholar]
- 46.Grundy SM, Cleeman JI, Merz CNB, Brewer HB, Clark LT, Hunninghake DB, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol. 2004;44:720–32. doi: 10.1016/j.jacc.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 47.Prueksaritanont T, Zhao J, Ma B, Roadcap B, et al. Mechanistic studies on metabolic interactions between gemfibrozil and statins. J Pharm Exp Ther. 2002;301(3):1042–51. doi: 10.1124/jpet.301.3.1042. [DOI] [PubMed] [Google Scholar]
- 48.Fujino H, Saito T, Tsunenari Y, Kojima J. Effect of gemfibrozil on the metabolism of pitavastatin-determining the best animal model for human CYP and UGT activities. Drug Metabol Drug Interact. 2004;20(1–2):25–42. doi: 10.1515/dmdi.2004.20.1-2.25. [DOI] [PubMed] [Google Scholar]
- 49.Armitage J. The safety of statins in clinical practice. Lancet. 2007;370(9601):1781–90. doi: 10.1016/S0140-6736(07)60716-8. [DOI] [PubMed] [Google Scholar]
- 50.Ridker PM, Danielson E, Fonseca FAH, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 51.Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomized statin trials. Lancet. 2010;375(9716):735–42. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 52.Cholesterol Treatment Trialists' (CTT) Collaborators. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117–25. doi: 10.1016/S0140-6736(08)60104-X. [DOI] [PubMed] [Google Scholar]
- 53.Sherer Y, Shoenfeld Y. Mechanisms of disease: atherosclerosis in autoimmune diseases. Nat Clin Pract Rheumatol. 2005;2(2):99–106. doi: 10.1038/ncprheum0092. [DOI] [PubMed] [Google Scholar]
- 54.Gustafsson M, Borén J. Mechanism of lipoprotein retention by the extracellular matrix. Curr Opin Lipidol. 2004;15:505–14. doi: 10.1097/00041433-200410000-00003. [DOI] [PubMed] [Google Scholar]
- 55.Libby P, Ridker PM, Hansson GK. Leducq Transatlantic Network on Atherothrombosis. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54(23):2129–38. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297(6):611–9. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 57.Ridker PM, Paynter NP, Rifai N, Gaziano JM, Cook NR. C-reactive protein and parental history improve global cardiovascular risk prediction: the Reynolds Risk Score for men. Circulation. 2008;118(22):2243–51. doi: 10.1161/CIRCULATIONAHA.108.814251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ridker PM, Rifai N, Clearfield M, Downs JR, Weis JS, Gotto AM. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. 2001;344(26):1959–65. doi: 10.1056/NEJM200106283442601. [DOI] [PubMed] [Google Scholar]
- 59.Ridker PM, MacFadyen JG, Fonseca FAH, et al. Number needed to treat with rosuvastatin to prevent first cardiovascular events and death among men and women with low low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein. Circ Cardiovasc Qual Outcomes. 2009;2(6):616–23. doi: 10.1161/CIRCOUTCOMES.109.848473. [DOI] [PubMed] [Google Scholar]
- 60.Stewart RAH. Predicting benefit from statins by C-reactive protein, LDL-cholesterol or absolute cardiovascular risk. Future Cardiol. 2009;5(3):231–236. doi: 10.2217/fca.09.8. [DOI] [PubMed] [Google Scholar]
- 61.Ridker PM, Danielson E, Fonseca FAH, Genest J, Gotto AM, Kastelein JJP, et al. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet. 2009;373(9670):1175–82. doi: 10.1016/S0140-6736(09)60447-5. [DOI] [PubMed] [Google Scholar]
- 62.Elliott P, Chambers JC, Zhang W, Clarke R, Hopewell JC, Peden JF, et al. Genetic loci associated with C-reactive protein levels and risk of coronary heart disease. JAMA. 2009;302(1):37–48. doi: 10.1001/jama.2009.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilmington, DE: AstraZeneca; 2010. Feb, Product information. Crestor (rosuvastatin) [Google Scholar]
- 64.Spatz ES, Canavan ME, Desai MM. From here to JUPITER: identifying new patients for statin therapy using data from the 1999-2004 National Health and Nutrition Examination Survey. Circ Cardiovasc Qual Outcomes. 2009;2(1):41–8. doi: 10.1161/CIRCOUTCOMES.108.832592. [DOI] [PubMed] [Google Scholar]
- 65.Wilson D. “Plan to Widen Use of Statins Has Skeptics.”. New York Times. 2010 Mar 31; [Google Scholar]
- 66.Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ Res. 2004;95(8):764–72. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 67.Nissen SE, Tardif JC, Nicholls SJ, et al. Effect of torcetrapib on the progression of coronary atherosclerosis. N Engl J Med. 2007;356(13):1304–16. doi: 10.1056/NEJMoa070635. [DOI] [PubMed] [Google Scholar]
- 68.Segrest JP, Jackson RL, Morrisett JD, Gotto AM. A molecular theory of lipid-protein interactions in the plasma lipoproteins. FEBS Lett. 1974;38(3):247–58. doi: 10.1016/0014-5793(74)80064-5. [DOI] [PubMed] [Google Scholar]
- 69.Bhat S, Sorci-Thomas MG, Alexander ET, Samuel MP, Thomas MJ. Intermolecular contact between globular N-terminal fold and C-terminal domain of ApoA-I stabilizes Its lipid-bound conformation. J Biol Chem. 2005;280(3):33015–25. doi: 10.1074/jbc.M505081200. [DOI] [PubMed] [Google Scholar]
- 70.Brousseau ME, Diffenderfer MR, Millar JS, et al. Effects of cholesteryl ester transfer protein inhibition on high-density lipoprotein subspecies, apolipoprotein A-I metabolism, and fecal sterol excretion. Arterioscler Thromb Vasc Biol. 2005;25(5):1057–64. doi: 10.1161/01.ATV.0000161928.16334.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Robinson JG. Dalcetrapib: a review of Phase II data. Exp Opin Invest Drugs. 2010;19(6):795–805. doi: 10.1517/13543784.2010.488219. [DOI] [PubMed] [Google Scholar]
- 72.Cannon CP, Shah S, Dansky HM, et al. Safety of anacetrapib in patients with or at high risk for coronary heart disease. N Engl J Med. 2010 Nov 17; doi: 10.1056/NEJMoa1009744. [epub ahead of print]. PMID: 21082868. [DOI] [PubMed] [Google Scholar]
- 73.Nicholls SJ, Tuzcu EM, Sipahi I, Schoenhagen P, Crowe T, et al. Relationship between atheroma regression and change in lumen size after infusion of apolipoprotein A-I Milano. J Am Coll Cardiol. 2006;47(5):992–7. doi: 10.1016/j.jacc.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 74.Waksman R, Torguson, Kent KM, et al. A first-in-man, randomized, placebo-controlled study to evaluate the safety and feasibility of autologous delipidated high-density lipoprotein plasma infusions in patients with acute coronary syndrome. J Am Coll Cardiol. 2010;55(24):2727–35. doi: 10.1016/j.jacc.2009.12.067. [DOI] [PubMed] [Google Scholar]
- 75.Bailey D, Jahagirdar R, Gordon A, et al. RVX-208: a small molecule that increases apolipoprotein A-I and high-density lipoprotein cholesterol in vitro and in vivo. J Am Coll Cardiol. 2010;55(23):2580–9. doi: 10.1016/j.jacc.2010.02.035. [DOI] [PubMed] [Google Scholar]
- 76.Henry RR, Lincoff AM, Mudaliar S, et al. Effect of the dual peroxisome proliferator-activated receptor-alpha/gamma agonist aleglitazar on risk of cardiovascular disease in patients with type 2 diabetes (SYNCHRONY): a phase II, randomized, dose-ranging study. Lancet. 2009;374(9684):126–35. doi: 10.1016/S0140-6736(09)60870-9. [DOI] [PubMed] [Google Scholar]
- 77.Akdim F, Visser ME, Tribble DL, et al. Effect of mipomersen, an apolipoprotein B synthesis inhibitor, on low-density lipoprotein cholesterol in patients with familial hypercholesterolemia. Am J Cardiol. 2010;105(10):1413–9. doi: 10.1016/j.amjcard.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 78.Akdim F, Stroes ESG, Sijbrands EJG, et al. Efficacy and safety of mipomersen, an antisense inhibitor of apolipoprotein B, in hypercholesterolemic subjects receiving stable statin therapy. J Am Coll Cardiol. 2010;55:1611–18. doi: 10.1016/j.jacc.2009.11.069. [DOI] [PubMed] [Google Scholar]
- 79.Rizzo M. Lomitapide, a microsomal triglyceride transfer protein inhibitor for the treatment of hypercholesterolemia. IDrugs. 2010;13(2):103–11. [PubMed] [Google Scholar]
- 80.Baxter JD, Webb P. Thyroid hormone mimetics: potential applications in atherosclerosis, obesity and type 2 diabetes. Nat Rev Drug Discov. 2009;8(4):308–20. doi: 10.1038/nrd2830. [DOI] [PubMed] [Google Scholar]
- 81.Rosenson RS. Phospholipase A2 inhibition and atherosclerotic vascular disease: prospects for targeting secretory and lipoprotein-associated phospholipase A2 enzymes. Curr Opin Lipidol. 2010 Aug 25; doi: 10.1097/MOL.0b013e32833eb581. [epub ahead of print] PMID: 20739882. [DOI] [PubMed] [Google Scholar]
- 82.Ridker PM. Testing the inflammatory hypothesis of atherothrombosis: scientific rationale for the Cardiovascular Inflammation Reduction Trial (CIRT) J Thromb Haemost. 2009;7(suppl 1):332–9. doi: 10.1111/j.1538-7836.2009.03404.x. [DOI] [PubMed] [Google Scholar]
- 83.Ouguerram K, Chetiveaux M, Zair Y, et al. Apolipoprotein B100 metabolism in autosomal-dominant hypercholesterolemia related to mutations in PCSK9. Arterioscler Thromb Vasc Biol. 2004;24(8):1448–53. doi: 10.1161/01.ATV.0000133684.77013.88. [DOI] [PubMed] [Google Scholar]
- 84.Cohen JC, Boerwinkle E, Mosley TH, Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354(12):1264–72. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 85.Li H, Li H, Ziegler N, et al. Recent patents on PCSK9: a new target for treating hypercholesterolemia. Recent Pat DNA Gene Seq. 2009;3(3):201–12. doi: 10.2174/187221509789318388. [DOI] [PubMed] [Google Scholar]
- 86.Daniels SR, Greer FR the Committee on Nutrition. Lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122:198–208. doi: 10.1542/peds.2008-1349. [DOI] [PubMed] [Google Scholar]
- 87.Samani NJ, Erdmann J, Hall AS, Hengstenberg C, Mangino M, Mayer B, et al. Genomewide association analysis of coronary artery disease. N Engl J Med. 2007;357(5):443–53. doi: 10.1056/NEJMoa072366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Helgadottir A, Thorleifsson G, Manolescu A, et al. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science. 2007;316(5830):1491–3. doi: 10.1126/science.1142842. [DOI] [PubMed] [Google Scholar]
- 89.McPherson R, Pertsemlidis A, Kavaslar N, et al. A common allele on chromosome 9 associated with coronary heart disease. Science. 2007;316(5830):1488–91. doi: 10.1126/science.1142447. [DOI] [PMC free article] [PubMed] [Google Scholar]