Abstract
Terrestrial life would be miserable without the ability to concentrate urine. Production of concentrated urine requires complex interactions among the nephron segments and vasculature in the kidney medulla. In addition to water channels (aquaporins) and sodium transporters, urea transporters are critically important to the theories proposed to explain the physiologic processes occurring when urine is concentrated. Vasopressin (anti-diuretic hormone) is the key hormone regulating the production of concentrated urine. Vasopressin rapidly increases water and urea transport in the terminal inner medullary collecting duct (IMCD). Vasopressin rapidly increases urea permeability in the IMCD through increases in phosphorylation and apical plasma-membrane accumulation of the urea transporter A1 (UT-A1). Vasopressin acts through two cAMP-dependent signaling pathways in the IMCD: protein kinase A and exchange protein activated by cAMP Epac. Protein kinase A phosphorylates UT-A1 at serines 486 and 499. In summary, vasopressin regulates urea transport acutely by increasing UT-A1 phosphorylation and the apical plasma-membrane accumulation of UT-A1 through two cAMP-dependent pathways.
Urea is a highly polar molecule, much like water. The urea molecule has a C O double bond between two NH2 groups (O
O double bond between two NH2 groups (O C
C NH2)2), and is a small molecule with a molecular weight of 60 Da and a radius of 2 Å. Urea has a low permeability of 4 × 10−6 cm/s through artificial lipid bilayers (1) that lack any transport proteins to facilitate its transfer, as would be expected for a highly polar molecule. Although the permeability of urea across lipid bilayers is quite low, it is not zero, and given enough time, urea will diffuse across cell membranes and achieve equilibrium. That is why most textbooks state that urea is freely permeable across cell membranes and not osmotically active.
NH2)2), and is a small molecule with a molecular weight of 60 Da and a radius of 2 Å. Urea has a low permeability of 4 × 10−6 cm/s through artificial lipid bilayers (1) that lack any transport proteins to facilitate its transfer, as would be expected for a highly polar molecule. Although the permeability of urea across lipid bilayers is quite low, it is not zero, and given enough time, urea will diffuse across cell membranes and achieve equilibrium. That is why most textbooks state that urea is freely permeable across cell membranes and not osmotically active.
In contrast, the transit of tubule fluid through the kidney's inner medullary collecting duct (IMCD) is too fast to allow urea concentrations to reach equilibrium solely by passive diffusion. Similarly, the transit of red blood cells through the vasa recta is too fast to allow urea concentrations to reach equilibrium solely by passive diffusion. Urea transport across the IMCD or red blood cells is high because of the presence of specific urea transporter proteins (2, 3). In addition, the ability of vasopressin to regulate urea transport across the IMCD argues against urea being transported simply by passive diffusion (2). Urea is recognized to be osmotically active in two clinical situations: it acts as an osmotic diuretic during post-obstructive diuresis; and it may contribute to the dialysis disequilibrium syndrome that accompanied overly rapid dialysis before the advent of current dialysis technology.
In the 1980s, physiologic evidence established the concept of urea transporters in the kidney and red blood cells (4, 5). The initial publication proposing a urea transporter protein in the IMCD appeared in 1987 (2). Subsequently, two genes and several cDNA isoforms for urea transporters have been cloned (Table 1).
TABLE 1.
Regulation and Location of the Cloned Mammalian Urea Transporters
| Gene | Isoform | RNA (kb) | Protein (kDa) | AVP | Location |
|---|---|---|---|---|---|
| Slc14a1 | UT-B1 | 3.8 | 43 | DVR, RBC† | |
| UT-B2 | 3.7 | 43–54 | No | Bovine rumen | |
| Slc14a2 | UT-A1 (UT-A1b#) | 4.0 (3.5) | 117, 97 | Yes | IMCD (medulla*) |
| UT-A2 (UT-A2b#) | 2.9 (2.5) | 55 | No | tDL, liver (medulla*, heart) | |
| UT-A3 (UT-A3b#) | 2.1 (3.7) | 67, 44 | Yes | IMCD (medulla*) | |
| UT-A4§ | 2.5 | 43 | Yes | medulla* | |
| UT-A5‡ | 1.4 | testis | |||
| UT-A6ψ | 1.8 | colon |
Original citations are reviewed in (4, 5, 20). Abbreviations: AVP, arginine vasopressin. Urea flux is stimulated by vasopressin; DVR, descending vasa recta; IMCD, intermedullary collecting duct; RBC, red blood cells; tDL, thin descending limb of the renal tubule; †, also expressed in several other tissues and endothelial cells; (#), human RNA form with additional 3′ untranslated regions; medulla* (exact tubular location unknown); §, cloned from rat only; ‡, cloned from mouse only; and ψ, cloned from human only.
THE ROLE OF UREA IN THE URINARY CONCENTRATING MECHANISM
The search for a urea transporter protein was based on the key role of urea in the urinary concentrating mechanism. In 1934, Gamble and colleagues described “an economy of water in renal function referable to urea” (6). Their findings were recently verified using genetically engineered mice that lack the UT-A1 and UT-A3 urea transport proteins (7). Many studies show that maximal urine concentrating ability is decreased in protein-deprived humans and in several animal species (4, 5). Genetically engineered mice lacking UT-A1/UT-A3 (8–10), as well as mice lacking either UT-A2 (11) or UT-B (12–14), have decreased maximal urine concentrating ability. Although the mechanism by which the inner medulla concentrates urine remains controversial (15, 16), any solution to this question needs to include an effect derived from urea and urea transport proteins.
The most widely accepted mechanism for urine concentration in the inner medulla is the passive mechanism, a hypothesis proposed by Kokko and Rector (17) and Stephenson (18) in 1972. This hypothesis requires that the inner medullary interstitial urea concentration exceed the urea concentration in the lumen of the thin ascending limb of the renal tubule. If an insufficient quantity of urea is delivered to the deep inner medulla, then the chemical gradients necessary for passive NaCl reabsorption from the thin ascending limb cannot be established and urine concentrating ability is reduced. The major mechanism for delivering urea to the inner medullary interstitium is urea reabsorption from the terminal IMCD (19), mediated by the UT-A1 and UT-A3 urea transporter proteins (5, 20). Figure 1 shows the location of key transport proteins involved in urine concentration.
Fig. 1.
Diagram showing the locations of the chief urea transporters (UT-A1, UT-A2, UT-A3), aquaporins (AQP2-4), and sodium co-transporter (NKCC2) involved in urine concentration. The major regions of the kidney are indicated on the right. NaCl is actively reabsorbed across the thick ascending limb by the apical plasma-membrane Na-K-2C1 co-transporter (NKCC2). Water is reabsorbed across the descending limb segments by AQP1 water channels in both apical and basolateral plasma membranes. Water is reabsorbed across the apical plasma membrane of the entire collecting duct by AQP2 water channels when vasopressin is present. Water is reabsorbed across the basolateral plasma membrane by AQP3 water channels in the cortical and outer medullary collecting ducts and by both AQP3 and AQP4 water channels in the IMCD. Urea is concentrated within the collecting-duct lumen (by water reabsorption) until it reaches the terminal IMCD, where it is reabsorbed by the urea transporters UT-A1 and UT-A3.
The UT-A1 Urea Transport Protein
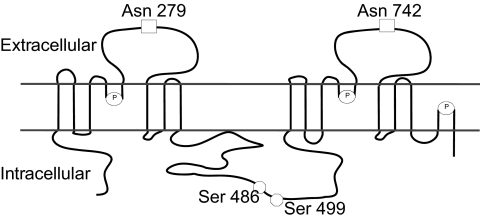
UT-A1 (Figure 2) is expressed in the apical plasma membrane of the IMCD in rodents (5, 20) and humans (21). When UT-A1 is stably transfected into polarized epithelial cells grown on permeable supports, it is also expressed in the apical plasma membrane in both UT-A1-Madin-Darby canine kidney (MDCK) cells (22–24) and UT-A1-mouse inner medullary collecting duct (mIMCD3) cells (25). UT-A1 has two N-linked glycosylation sites that reside in extracellular domains: Asn 279 and Asn 742 (24). As discussed below, UT-A1 has two protein kinase A (PKA) phosphorylation sites: Ser 486 and Ser 499 (26).
Fig. 2.
Proposed membrane structure of UT-A1 (4, 5) showing the two established N-glycosylation sites (squares) (24) and the two established PKA phosphorylation sites (circles) (26). Also shown are the prolines that are putative β-turn sites (ovals containing P) (4, 5).
Rapid Regulation of UT-A1 by Vasopressin
Vasopressin increases urea permeability in a perfused rat terminal IMCD within 5–10 minutes (27). Vasopressin binds to V2-receptors in the basolateral plasma membrane, stimulates adenylyl cyclase, generates cAMP, and increases urea transport (5, 20). Vasopressin also increases urea flux in cells that are stably transfected with UT-A1: UT-A1-MDCK cells (22, 23) and UT-A1-mIMCD3 cells (25).
Vasopressin increases the phosphorylation of both glycoprotein forms of UT-A1, which are detected at 117 and 97 kDa on Western blotting, with a similar time course to the increase in urea transport in perfused tubules (28). Vasopressin also increases UT-A1 phosphorylation in UT-A1-MDCK cells (22, 23) and UT-A1-mIMCD3 cells (25). Cyclic AMP, forskolin, and 1-deamino-8-D-arginine vasopressin (dDAVP) (a selective V2-vasopressin receptor agonist) also increase UT-A1 phosphorylation, both in the rat IMCD (28) and in UT-A1-MDCK cells (23). In addition to stimulating PKA, vasopressin/cAMP stimulates Epac, the exchange protein activated by cAMP (29, 30). Activating Epac increases UT-A1 phosphorylation in IMCD suspensions and urea permeability in perfused tubules (31).
Vasopressin also increases the plasma-membrane accumulation of UT-A1 in the rat IMCD (32, 33). In contrast, vasopressin does not increase the plasma membrane accumulation of UT-A1 in IMCDs from Brattleboro rats, which have central diabetes insipidus, or from rats in which endogenous vasopressin levels have been suppressed by 2 weeks of water diuresis (33, 34). When forskolin instead of vasopressin, is used to stimulate cAMP, plasma-membrane accumulation of UT-A1 is increased in the IMCD from 2-week water diuresed rats (33). Chronically diuretic animals have a blunted cAMP response to vasopressin, regardless of the cause (35–37). Using forskolin to directly stimulate adenylyl cyclases results in higher levels of cAMP production, which may explain the increase in UT-A1 accumulation in the plasma membrane in 2-week water diuresed rats (33). Activating Epac also increases plasma membrane accumulation of UT-A1 in the rat IMCD (31).
Apical plasma-membrane accumulation of UT-A1 is increased by vasopressin or by forskolin in UT-A1-MDCK cells (24, 33) or UT-A1-mIMCD3 cells (25). PKA phosphorylates UT-A1 at Ser 486 and Ser 499 (26). Mutation of both Ser 486 and Ser 499, but not either one alone, eliminates forskolin's ability to stimulate both UT-A1 accumulation in the apical plasma membrane and urea transport, indicating that at least one of these serines must be phosphorylated (25, 26). A phospho-specific antibody to Ser 486-UT-A1 shows that Ser 486-UT-A1 is primarily expressed in the apical plasma membrane in rats, and confirms that vasopressin increases UT-A1 accumulation in the apical plasma membrane (25).
VASOPRESSIN REGULATES BOTH UREA AND WATER TRANSPORT
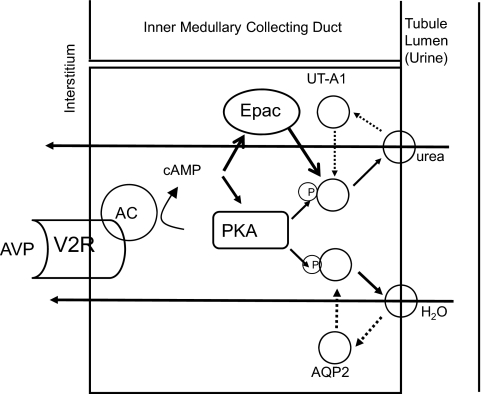
The kidney must independently regulate urea and water excretion in order to vary urine osmolality and maintain (or restore) plasma osmolality within (to) the normal range. Although urea and water permeabilities often change together, there are situations in which they are regulated independently of one another. Since vasopressin is the primary hormone regulating both urea and water excretion through the V2-vasopressin receptor in the IMCD, there must be differences in the vasopressin-mediated signaling pathways or additional signals or signaling pathways that regulate urea versus water permeability (Figure 3). The mechanism for vasopressin-regulation of water permeability seems clear: vasopressin binds to the V2-receptor, increases cAMP production, activates PKA, phosphorylates AQP2 at serines 256, 261, 264, and 269, and increases AQP2 accumulation in the apical plasma membrane (38–42). The mechanism for urea transporter regulation by vasopressin is only partially explained: vasopressin binds to the V2-receptor, increases cAMP production, activates PKA (which phosphorylates UT-A1 at serines 486 and 499) and Epac, and increases UT-A1 accumulation in the apical plasma membrane (26, 28, 31–33, 43). In addition, protein kinase C activation (by hypertonicity or angiotensin II) also increases urea permeability in the rat IMCD (43, 44).
Fig. 3.
Urea and water reabsorption in principal cells of the inner medullary collecting duct. For water, vasopressin (AVP) binds to the V2-receptor (V2R) on the basolateral plasma membrane, activates adenylyl cyclase (AC), increases intracellular cyclic AMP (cAMP), and stimulates protein kinase A (PKA) activity. Cytoplasmic vesicles carrying the AQP2 water-channel protein are phosphorylated and inserted into the luminal membrane in response to vasopressin, thereby increasing the water permeability of this membrane (45). When vasopressin stimulation ends, water channels are retrieved by an endocytic process, and water permeability returns to its low basal rate. The process is similar for urea, except that cAMP stimulates both PKA and Epac, the exchange protein activated by cAMP (31, 33). UT-A1 is ubiquitinated and degraded in the proteasome (46). Although not shown, the basolateral plasma membrane contains AQP3 and AQP4 water channels and the UT-A3 urea transporter, thereby completing the transcellular pathways for water and urea reabsorption.
SUMMARY
Urea is transported by a specific urea transport protein, UT-A1, in the apical plasma membrane of the IMCD. Vasopressin increases UT-A1 phosphorylation and apical plasma membrane accumulation of UT-A1. Mutation of both Ser 486 and Ser 499, but not either single mutation alone, blocks forskolin stimulated plasma-membrane accumulation of UT-A1 and urea transport. The Ser 486-phosphorylated form of UT-A1 is primarily localized in the apical plasma membrane.
Vasopressin, acting through cAMP, regulates urea transport by signaling through both PKA and Epac (non-PKA) pathways. This provides a mechanism to increase both water (AQP2) and urea (UT-A1) reabsorption in parallel (through PKA) and urea (UT-A1) absorption alone (via Epac) in IMCD cells through the V2-vasopressin receptor.
ACKNOWLEDGEMENT
This work was supported by grant R01-DK41707 from the NIH.
Footnotes
Potential Conflicts of Interest: None disclosed.
DISCUSSION
Stokes, Iowa City: Jeff, that's a very nice body of work. I do have one rather physiologically irrelevant question. You mentioned that there would be two separate pathways whereby vasopressin would activate urea transport and water transport by inserting the appropriate carriers in the apical membrane. But, I can't think of a physiologic circumstance in which the body would want to reabsorb urea and not water or reabsorb water and not urea. Can you think of a situation in which that would occur?
Sands, Atlanta: In general, you're right in saying that most of the time you would want to reabsorb the water and urea in parallel. You also want to reabsorb the water in the more proximal portions of the kidney, in the cortical collecting duct and the outer medullary collecting duct, both because the blood flow is better and you can return the water to the circulation more effectively. Also, as you reabsorb water in the very deep inner medulla, you are sort of diluting the gradients that you need to reabsorb the water in general. Thus, you may want to preferentially reabsorb the water in the more proximal locations and preferentially reabsorb the urea more deeply. You may also have such a situation in conditions where there are high levels of glucocorticoids, where you have a highly catabolic state and you may have changes in urea delivery for other reasons, because most of urea in the kidney is there to excrete nitrogenous waste and only a small fraction is for concentrating ability. There could be conditions in which you would want to be able to regulate the urea and decouple it from the water in those settings.
Luke, Cincinnati: Thank you, Jeff. You've made yourself the world expert in this area. Two questions: In hyponatremic encephalopathy, it has been observed that a high level of urea seems to be protective. I've always suspected there was some change in urea transport related to that. Do you have any comments, on that? And secondly, do the vaptans do anything to urea transport?
Sands, Atlanta: In terms of the second question, I am not aware of any data on the vaptans in terms of urea transport. I don't think it's been looked at in experimental studies and I'm not sure even in the human studies how carefully that's been looked at. In terms of urea transporters in the brain, there are some transporters there. The UT-B form is present in the brain, and whether those are up-regulated or down-regulated or in some way altered in order to explain the findings you suggest in hyponatremia has not been looked at. I had thought that perhaps the old dialysis disequilibrium syndrome, in which if you dialyze patients too rapidly, the urea can't get out quickly enough, we would see some abnormalities in UT-B. In collaboration with Bill Mitch, who was using the 5/6 nephrectomy model, we had looked at that question and not seen anything in that model. But I don't think anyone has modeled hyponatremia to see whether UT-B in particular is altered and contributes to that clinical observation.
Suthanthiran, New York: Jeff, thank you for that very nice description. We always teach people that when there is prerenal azotemia, the urea-to-creatinine ratio is almost 20:1, whereas in intrarenal failure the ratio is almost 1:10 instead of 1:20. Do you see, in your model, that when a patient has prerenal azotemla and the urea disproportionately increases, that there is a role for vasopressin and up-regulation of these receptors, or is it simply a question of a transit time, and that's why there is reabsorption, or are both situations possible?
Sands, Atlanta: It is thought that most of the urea that is absorbed in prerenal azotemia is actually coming from the proximal tubule, not from this distal portion of the nephron. In terms of whether there are urea transporters in the proximal tubule, most of the evidence at present would say no. In some modeling studies that I had done back in the 1980s with Mark Knepper, it struck us that mathematically there seemed to be a need for a proximal-tubule urea transporter, but at present there is no definitive evidence for that. So I think that what happens with vasopressin in prerenal azotemia is that there is almost certainly some elaboration of vasopression and stimulation of these distal transporters. But in terms of explaining the BUN-to-creatinine ratios that we classically teach about, I don't think that's what's contributing. I think it's the classical teaching of increased proximal reabsorption due to a low glomerular filtration rate.
Hochburg, Baltimore: In addition to an increased excretion of dilute urine this evening, following our average consumption of 3.2 alcoholic beverages per hour, we will probably all experience some transient hyperuricemia. Although part of that may be attributable to the type of alcohol we consume, some of it is obviously related to the effects on urate excretion, and we know now that there are a number of urate as opposed to urea transporters in the kidney, which are related to serum urate levels and the development of gout. Can you tell us whether vasopressin has an effect on those?
Sands, Atlanta: That's a great question, and I'm not aware of any data on whether vasopressin has been looked at to see whether it affects urate transporters.
Stokes, Iowa City: There are data on that question, and they come from patients who have inappropriate secretion of vasopressin. One of the points about that is that they have very low uric acid levels. I can't remember the name of the author, but somebody did a study in which they compared the same degree of hyponatremia produced by DDAVP, and they did not find the same suppression of urate. So the implication was that vasopression did have some effect on urate transport in the proximal tubule, but nobody knows exactly what the mechanism is for that.
REFERENCES
- 1.Galluci E, Micelli S, Lippe C. Non-electrolyte permeability across thin lipid membranes. Arch Int Physiol Biochim. 1971;79:881–7. doi: 10.3109/13813457109104847. [DOI] [PubMed] [Google Scholar]
- 2.Sands JM, Nonoguchi H, Knepper MA. Vasopressin effects on urea and H2O transport in inner medullary collecting duct subsegments. Am J Physiol. 1987;253:F823–32. doi: 10.1152/ajprenal.1987.253.5.F823. [DOI] [PubMed] [Google Scholar]
- 3.Wieth JO, Funder J, Gunn RB, Brahm J. Passive transport pathways for chloride and urea through the red cell membrane. In: Bolis K, Bloch K, Luria SE, Lynen F, editors. Comparative Biochemistry and Physiology of Transport. Amsterdam: Elsevier/North-Holland; 1974. pp. 317–37. [Google Scholar]
- 4.Sands JM, Timmer RT, Gunn RB. Urea transporters in kidney and erythrocytes. Am J Physiol. 1997;273(3):F321–39. doi: 10.1152/ajprenal.1997.273.3.F321. [DOI] [PubMed] [Google Scholar]
- 5.Sands JM, Layton HE. The physiology of urinary concentration: an update. Semin Nephrol. 2009;29(3):178–95. doi: 10.1016/j.semnephrol.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gamble JL, McKhann CF, Butler AM, Tuthill E. An economy of water in renal function referable to urea. Am J Physiol. 1934;109:139–54. [Google Scholar]
- 7.Fenton RA, Chou CL, Sowersby H, Smith CP, Knepper MA. Gamble's “economy of water” revisited: studies in urea transporter knockout mice. Am J Physiol Renal Physiol. 2006;291(1):F148–54. doi: 10.1152/ajprenal.00348.2005. [DOI] [PubMed] [Google Scholar]
- 8.Fenton RA, Chou C-L, Stewart GS, Smith CP, Knepper MA. Urinary concentrating defect in mice with selective deletion of phloretin-sensitive urea transporters in the renal collecting duct. Proc Natl Acad Sci USA. 2004;101(19):7469–74. doi: 10.1073/pnas.0401704101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fenton RA, Flynn A, Shodeinde A, Smith CP, Schnermann J, Knepper MA. Renal phenotype of UT-A urea transporter knockout mice. J Am Soc Nephrol. 2005;16(6):1583–92. doi: 10.1681/ASN.2005010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacob VA, Harbaugh CM, Dietz JR, et al. Magnetic resonance imaging of urea transporter knockout mice shows renal pelvic abnormalities. Kidney Int. 2008;74(9):1202–8. doi: 10.1038/ki.2008.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uchida S, Sohara E, Rai T, Ikawa M, Okabe M, Sasaki S. Impaired urea accumulation in the inner medulla of mice lacking the urea transporter UT-A2. Mol Cell Biol. 2005;25(16):7357–63. doi: 10.1128/MCB.25.16.7357-7363.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang B, Bankir L, Gillespie A, Epstein CJ, Verkman AS. Urea-selective concentrating defect in transgenic mice lacking urea transporter UT-B. J Biol Chem. 2002;277:10633–7. doi: 10.1074/jbc.M200207200. [DOI] [PubMed] [Google Scholar]
- 13.Yang B, Verkman AS. Analysis of double knockout mice lacking aquaporin-1 and urea transporter UT-B. J Biol Chem. 2002;277(39):36782–6. doi: 10.1074/jbc.M206948200. [DOI] [PubMed] [Google Scholar]
- 14.Klein JD, Sands JM, Qian L, Wang X, Yang B. Upregulation of urea transporter UT-A2 and water channels AQP2 and AQP3 in mice lacking urea transporter UT-B. J Am Soc Nephrol. 2004;15(5):1161–7. doi: 10.1097/01.asn.0000125617.19799.72. [DOI] [PubMed] [Google Scholar]
- 15.Fenton RA, Knepper MA. Urea and renal function in the 21st century: insights from knockout mice. J Am Soc Nephrol. 2007;18(3):679–88. doi: 10.1681/ASN.2006101108. [DOI] [PubMed] [Google Scholar]
- 16.Sands JM. Critical role of urea in the urine-concentrating mechanism. J Am Soc Nephrol. 2007;18(3):670–1. doi: 10.1681/ASN.2006121314. [DOI] [PubMed] [Google Scholar]
- 17.Kokko JP, Rector FC. Countercurrent multiplication system without active transport in inner medulla. Kidney Int. 1972;2:214–23. doi: 10.1038/ki.1972.97. [DOI] [PubMed] [Google Scholar]
- 18.Stephenson JL. Concentration of urine in a central core model of the renal counterflow system. Kidney Int. 1972;2:85–94. doi: 10.1038/ki.1972.75. [DOI] [PubMed] [Google Scholar]
- 19.Sands JM, Knepper MA. Urea permeability of mammalian inner medullary collecting duct system and papillary surface epithelium. J Clin Invest. 1987;79:138–47. doi: 10.1172/JCI112774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sands JM, Layton HE. The urine concentrating mechanism and urea transporters. In: Alpern RJ, Hebert SC, editors. The Kidney: Physiology and Pathophysiology. eds. 4. San Diego: Academic Press; 2008. pp. 1143–78. [Google Scholar]
- 21.Bagnasco SM, Peng T, Janech MG, Karakashian A, Sands JM. Cloning and characterization of the human urea transporter UT-A1 and mapping of the human Slc14a2 gene. Am J Physiol Renal Physiol. 2001;281:F400–6. doi: 10.1152/ajprenal.2001.281.3.F400. [DOI] [PubMed] [Google Scholar]
- 22.Fröhlich O, Klein JD, Smith PM, Sands JM, Gunn RB. Urea transport in MDCK cells that are stably transfected with UT-A1. Am J Physiol Cell Physiol. 2004;286(6):C1264–70. doi: 10.1152/ajpcell.00499.2003. [DOI] [PubMed] [Google Scholar]
- 23.Fröhlich O, Klein JD, Smith PM, Sands JM, Gunn RB. Regulation of UT-A1-mediated transepithelial urea flux in MDCK cells. Am J Physiol Cell Physiol. 2006;291:C600–6. doi: 10.1152/ajpcell.00413.2005. [DOI] [PubMed] [Google Scholar]
- 24.Chen G, Fröhlich O, Yang Y, Klein JD, Sands JM. Loss of N-linked glycosylation reduces urea transporter UT-A1 response to vasopressin. J Biol Chem. 2006;281(37):27436–42. doi: 10.1074/jbc.M605525200. [DOI] [PubMed] [Google Scholar]
- 25.Klein JD, Blount MA, Fröhlich O, et al. Phosphorylation of UT-A1 on serine 486 correlates with membrane accumulation and urea transport activity in both rat IMCDs and cultured cells. Am J Physiol Renal Physiol. 2010;298(4):F935–40. doi: 10.1152/ajprenal.00682.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blount MA, Mistry AC, Fröhlich O, et al. Phosphorylation of UT-A1 urea transporter at serines 486 and 499 is important for vasopressin-regulated activity and membrane accumulation. Am J Physiol Renal Physiol. 2008;295(1):F295–9. doi: 10.1152/ajprenal.00102.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wall SM, Suk Han J, Chou C-L, Knepper MA. Kinetics of urea and water permeability activation by vasopressin in rat terminal IMCD. Am J Physiol Renal Fluid Electrolyte Physiol. 1992;262:F989–98. doi: 10.1152/ajprenal.1992.262.6.F989. [DOI] [PubMed] [Google Scholar]
- 28.Zhang C, Sands JM, Klein JD. Vasopressin rapidly increases the phosphorylation of the UT-A1 urea transporter activity in rat IMCDs through PKA. Am J Physiol Renal Physiol. 2002;282(1):F85–90. doi: 10.1152/ajprenal.0054.2001. [DOI] [PubMed] [Google Scholar]
- 29.Bos JL. Epac: a new cAMP target and new avenues in cAMP research. Nat Rev Mol Cell Biol. 2003;4:733–8. doi: 10.1038/nrm1197. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Konings IBM, Zhao J, Price LS, de Heer E, Deen PMT. Renal expression of exchange protein directly activated by cAMP (Epac) 1 and 2. Am J Physiol Renal Physiol. 2008;295:F525–33. doi: 10.1152/ajprenal.00448.2007. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Klein JD, Blount MA, et al. Epac regulation of the UT-A1 urea transporter in rat IMCDs. J Am Soc Nephrol. 2009;20(3):2018–24. doi: 10.1681/ASN.2008121225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blount MA, Klein JD, Martin CF, Tchapyjnikov D, Sands JM. Forskolin stimulates phosphorylation and membrane accumulation of UT-A3. Am J Physiol Renal Physiol. 2007;293(4):F1308–13. doi: 10.1152/ajprenal.00197.2007. [DOI] [PubMed] [Google Scholar]
- 33.Klein JD, Fröhlich O, Blount MA, Martin CF, Smith TD, Sands JM. Vasopressin increases plasma membrane accumulation of urea transporter UT-A1 in rat inner medullary collecting ducts. J Am Soc Nephrol. 2006;17:2680–6. doi: 10.1681/ASN.2006030246. [DOI] [PubMed] [Google Scholar]
- 34.Inoue T, Terris J, Ecelbarger CA, Chou C-L, Nielsen S, Knepper MA. Vasopressin regulates apical targeting of aquaporin-2 but not of UT1 urea transporter in renal collecting duct. Am J Physiol. 1999;276(4):F559–66. doi: 10.1152/ajprenal.1999.276.4.F559. [DOI] [PubMed] [Google Scholar]
- 35.Cotte N, Balestre MN, Phalipou S, et al. Identification of residues responsible for the selective binding of peptide antagonists and agonists in the V2 vasopressin receptor. J Biol Chem. 1998;273(45):29462–8. doi: 10.1074/jbc.273.45.29462. [DOI] [PubMed] [Google Scholar]
- 36.Seamon KB, Padgett W, Daly JW. Forskolin–unique diterpene activator of adenylate cyclase in membranes and intact cells. Proc Natl Acad Sci USA. 1981;87:3363–7. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim D-U, Sands JM, Klein JD. Changes in renal medullary transport proteins during uncontrolled diabetes mellitus in rats. Am J Physiol Renal Physiol. 2003;285(2):F303–9. doi: 10.1152/ajprenal.00438.2002. [DOI] [PubMed] [Google Scholar]
- 38.Hoffert JD, Fenton RA, Moeller HB, et al. Vasopressin-stimulated increase in phosphorylation at Ser269 potentiates plasma membrane retention of aquaporin-2. J Biol Chem. 2008;283(36):24617–27. doi: 10.1074/jbc.M803074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffert JD, Pisitkun T, Wang GH, Shen RF, Knepper MA. Dynamics of aquaporin-2 serine-261 phosphorylation in response to short-term vasopressin treatment in collecting duct. Am J Physiol Renal Physiol. 2007;292(2):F691–700. doi: 10.1152/ajprenal.00284.2006. [DOI] [PubMed] [Google Scholar]
- 40.Hoffert JD, Pisitkun T, Wang G, Shen R-F, Knepper MA. Quantitative phosphoproteomics of vasopressin-sensitive renal cells: regulation of aquaporin-2 phosphorylation at two sites. Proc Natl Acad Sci USA. 2006;103(18):7159–64. doi: 10.1073/pnas.0600895103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fenton RA, Moeller HB, Hoffert JD, Yu MJ, Nielsen S, Knepper MA. Acute regulation of aquaporin-2 phosphorylation at Ser-264 by vasopressin. Proc Natl Acad Sci USA. 2008;105(8):3134–9. doi: 10.1073/pnas.0712338105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wade JB, Stetson DL, Lewis SA. ADH action: evidence for a membrane shuttle mechanism. Ann NY Acad Sci. 1981;372:106–17. doi: 10.1111/j.1749-6632.1981.tb15464.x. [DOI] [PubMed] [Google Scholar]
- 43.Kato A, Klein JD, Zhang C, Sands JM. Angiotensin II increases vasopressin-stimulated facilitated urea permeability in rat terminal IMCDs. Am J Physiol Renal Physiol. 2000;279(5):F835–40. doi: 10.1152/ajprenal.2000.279.5.F835. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Liedtke CM, Klein JD, Sands JM. Protein kinase C regulates urea permeability in the rat inner medullary collecting duct. Am J Physiol Renal Physiol. 2010;299 doi: 10.1152/ajprenal.00322.2010. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nielsen S, Frokiaer J, Marples D, Kwon ED, Agre P, Knepper M. Aquaporins in the kidney: from molecules to medicine. Physiol Rev. 2002;82:205–44. doi: 10.1152/physrev.00024.2001. [DOI] [PubMed] [Google Scholar]
- 46.Chen G, Huang H, Fröhlich O, et al. MDM2 E3 ubiquitin ligase mediates UT-A1 urea transporter ubiquitination and degradation. Am J Physiol Renal Physiol. 2008;295(5):F1528–34. doi: 10.1152/ajprenal.90482.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]