Abstract
The stochastic and elite models have been proposed for the mechanism of induced pluripotent stem (iPS) cell generation. In this study we report a system that supports the elite model. We previously identified multilineage-differentiating stress-enduring (Muse) cells in human dermal fibroblasts that are characterized by stress tolerance, expression of pluripotency markers, self-renewal, and the ability to differentiate into endodermal-, mesodermal-, and ectodermal-lineage cells from a single cell. They can be isolated as stage-specific embryonic antigen-3/CD105 double-positive cells. When human fibroblasts were separated into Muse and non-Muse cells and transduced with Oct3/4, Sox2, Klf4, and c-Myc, iPS cells were generated exclusively from Muse cells but not from non-Muse cells. Although some colonies were formed from non-Muse cells, they were unlike iPS cells. Furthermore, epigenetic alterations were not seen, and some of the major pluripotency markers were not expressed for the entire period during iPS cell generation. These findings were confirmed further using cells transduced with a single polycistronic virus vector encoding all four factors. The results demonstrate that in adult human fibroblasts a subset of preexisting adult stem cells whose properties are similar in some respects to those of iPS cells selectively become iPS cells, but the remaining cells make no contribution to the generation of iPS cells. Therefore this system seems to fit the elite model rather than the stochastic model.
Keywords: ES cells, mesenchymal stem cells, TRA-1-81
Cell therapy is expected to be an efficient method for replenishment of lost cells in many intractable diseases, and several types of cells are considered candidate sources. Adult human stem cells, whose primary roles in a living organ are to maintain and repair tissues, typically generate cell types of the tissue in which they reside, and thus the range of their differentiation capabilities is considered limited (1). On the other hand, because they are innate stem cells and not artificial, they are considered relatively safe for clinical use. In fact, some somatic stem cells, such as hematopoietic stem cells, already have been used for bone marrow transplants (2).
hESCs, pluripotent stem cells established from the inner cell mass of blastocysts, have attracted attention since the first report in 1998 because of their great ability to differentiate into cells with characteristics of all three germ layers (3). However, there are concerns about the use of human fertilized eggs as a source and about the risk of tumor formation. Induced pluripotent stem (iPS) cells, whose properties are similar to those of ES cells but differ from ES cells with respect to epigenetic modification, lifespan, and differentiation potential, can be generated from adult human cells and are thus attracting increasing attention as a pluripotent stem cell without major ethical concerns (4, 5).
Two theories, the stochastic and elite models, have been proposed regarding the mechanism of iPS cell generation (6). Although iPS cells are generated from various cell types, the differentiation stage of the starting cells and the use of certain cell populations or cell types are reported to affect their generation efficiency (7–12). In any case, the stochastic model purports that every cell type has the potential to be reprogrammed to become an iPS cell by introducing factors such as Oct3/4, Sox2, Klf4, c-Myc, Nanog, and Lin28 (4, 13), and this model is widely accepted. On the other hand, the elite model proposes that iPS cell generation occurs only from a subset of cells (6). The validity of the elite model is still debated (11, 14, 15).
Here we report a system that supports the elite model. As mentioned above, adult human stem cells generally are considered to generate cell types of the tissue in which they reside (1). We previously reported a type of stem cell found in adult human mesenchymal cells such as dermal fibroblasts and bone marrow stromal cells (BMSCs) that is able to differentiate not only into cells of the same mesodermal lineage but also into cells of ectodermal and endodermal lineage (16). The cells are stress tolerant, express pluripotency markers such as Nanog, Oct3/4, and Sox2, can be isolated as cells positive for stage-specific embryonic antigen-3 (SSEA-3), and are able to self-renew and to generate cells representative of all three germ layers from a single cell. The cells are not tumorigenic, a trait that is consistent with their residence in adult tissues. We reported these stem cells as “multilineage-differentiating stress-enduring” (Muse) cells (16).
In the present study, we found that in adult human fibroblasts iPS cells are generated exclusively from Muse cells. When Muse cells were transduced with the four factors Oct3/4, Sox2, Klf4, and c-Myc and were subjected to iPS cell generation, genes related to cell-cycle progression were up-regulated, and the Muse cells successfully became iPS cells. In contrast, when Muse cells were removed from human fibroblasts before transduction with the four factors, the remaining cell population (namely, non-Muse cells) showed only a limited response. After receiving the four factors, non-Muse cells did not express some of the major factors of iPS cells, including endogenous Sox2 and Nanog, for the entire period during iPS cell generation, did not exhibit remarkable epigenetic alterations in the promoter regions of Oct3/4 and Nanog, and failed to generate iPS cells. These results were confirmed using cells transduced with a single polycistronic virus vector encoding all four factors.
Our results suggest that a subset of preexisting adult stem cells among human dermal fibroblasts that originally possess properties similar in some respects to those of iPS cells selectively become iPS cells and that the remaining cells make no contribution to the generation of iPS cells. Therefore, this system seems to fit the elite model rather than the stochastic model of iPS generation. These findings help clarify the mechanisms of iPS cell generation and will contribute to the establishment of an efficient system for generating iPS cells.
Results
Muse Cells in Human Dermis and in Cultured Dermal Skin Fibroblasts.
Previously, we reported that Muse cells can be isolated from naive human cultured dermal fibroblasts and human BMSCs as cells positive for SSEA-3 [a marker for human pluripotent stem cells such as hESCs that recognizes glycolipids expressed on the cell surface (3, 16)] or directly from bone marrow aspirates as cells double-positive for SSEA-3/CD105 by FACS (16).
In naive human fibroblasts, the ratio of SSEA-3+ cells was affected by the frequency of subculture and cell density. In our experiment, we used cells cultured at ∼70–80% confluency, which resulted in ∼1–2% of the cells being SSEA-3+ (Fig. 1A).
Fig. 1.
Muse cells in human dermal fibroblasts. (A and B) Examples of flow cytometry analysis of SSEA-3 (A) and CD105 (B) expression in naive human cultured fibroblasts. (C–F) Flow cytometry analysis of SSEA-3+ cells. Because naive fibroblasts showed no reactivity to NG2 (C), CD34 (D), von Willebrand factor (vWF) (E), or CD31 (F), SSEA-3+ cells were negative for these markers also. (G–I) A small percentage of naive fibroblasts were positive for CD117, CD146, and CD271. However, these cells were SSEA-3−. (J) RT-PCR shows that, although SSEA-3− cells (non-Muse cells) showed weak signals for Snai1 and Slug, SSEA-3+ cells (Muse cells) were negative for all markers. Human embryo (embryo) served as the positive control. (K) (Left) H&E staining of adult human skin and (Right) immunohistochemistry of SSEA-3+ cells in boxed areas 1–3. Positive cells were observed in the connective tissue distributed in dermis (1) and sweat gland (2) (Upper Right) and around small blood vessels (3) (Lower Right). (L and M) SSEA-3 immunohistochemistry of adult human skin combined with counterstaining (hematoxylin staining) in the neighboring section. (Scale bars, 50 μm.)
Because the adult dermis contains several types of stem or progenitor cells, such as skin-derived precursors (SKPs), neural crest-derived stem cells (NCSCs), melanoblasts, perivascular cells (PCs), endothelial progenitors (EPs), and adipose-derived stem cells (ADSCs) (17–24), cultured human fibroblasts harvested from the dermis may contain these cells. We assayed for the expression of various markers in Muse cells, such as NG2 (a marker for PCs), CD34 (EPs and ADSCs), von Willebrand factor (EPs), CD31 (EPs), CD117 (melanoblasts), CD146 (PCs and ADSCs), CD271 (NCSCs), Sox10 (NCSCs), Snai1 (marker for SKPs), Slug (SKPs), Tyrp1 (melanoblasts), and Dct (melanoblasts). None of these markers was detected in Muse cells by flow cytometry analysis or by RT-PCR (Fig. 1 C–J), suggesting that Muse cells differ from these stem or progenitor cells found in the adult human dermis.
We also examined the localization of SSEA-3+ cells in the human skin tissue. SSEA-3+ cells were detected in the connective tissues distributed in the dermis and hypodermis, and in most cases they were scattered sparsely in the connective tissue and did not associate with particular structures such as blood vessels or dermal papilla (Fig. 1 K–M). We further confirmed by flow cytometry analysis that all SSEA-3+ cells expressed the mesenchymal markers CD29, CD90 (SI Results and Fig. S1 A and B), and CD105 (Fig. 1B). Although CD105 is a well-established marker for mesenchymal cells, flow cytometry assay demonstrated that not all human skin fibroblasts used in this study were positive for CD105 (96.7 ± 0.20%) (Fig. S1C). Therefore, the precision of Muse cell isolation will be improved by combining CD105 labeling with single SSEA-3 labeling, and we routinely used these two markers for the isolation of Muse cells (SSEA-3+/CD105+) even from mesenchymal cells such as adult human skin fibroblasts.
Character of Muse Cells.
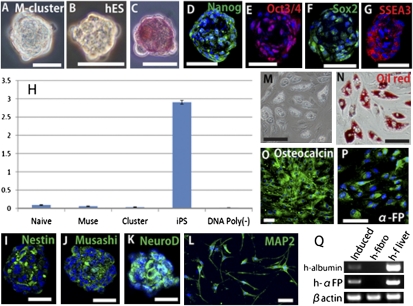
We separated cultured naive human fibroblasts into Muse cells and non-Muse cells (SSEA-3− cells) and subjected both populations to single-cell suspension culture after limiting dilution. At day 10, 63.3 ± 5.4% of Muse cells generated cell clusters whose appearance was similar to those of hESC-derived embryoid bodies formed in suspension culture (Fig. 2 A and B). In contrast, non-Muse cells did not generate clusters. Cell clusters >25 μm in a diameter were consistently positive for alkaline phosphatase (ALP) staining (Fig. 2C) and for the pluripotency markers Nanog, Oct3/4, Sox2, and SSEA-3 (Fig. 2 D–G). These results showed the same tendency as in the previous report (16). These cell clusters were named “Muse cell-derived clusters” (M-clusters) and were analyzed further (16).
Fig. 2.
Character of Muse cells and M-clusters. (A) M-cluster formed in single-cell suspension culture. (B) Cell cluster formed by hESC (hES) in suspension culture. (C) ALP staining of an M-cluster. (D–G) Immunocytochemistry of (D) Nanog, (E) Oct3/4, (F) Sox2, and (G) SSEA-3 in M-clusters. (H) Telomerase activity in naive fibroblasts (Naive), Muse cells (Muse), M-clusters (Cluster), and Fibro-iPSs (iPS). DNA Poly(−) represents a negative control. (I–Q) Controlled differentiation of Muse cells. Neural induction produced spheres that expressed nestin (I), Musashi (J), and NeuroD (K). (L) MAP-2+ cells after differentiation. (M and N) Induced adipocytes contained lipid droplets (M) that could be stained with oil red O (oil red) (N). Induced osteoblasts expressed osteocalcin (O). (P and Q) Induced hepatocytes were positive for human α-FP (P). (Q) RT-PCR analysis after hepatocyte induction (Induced) detected human albumin (h-albumin) and human α-FP (h-αFP). Human naive fibroblasts (h-fibro) served as a negative control, and human fetal liver (h-f liver) served as a positive control. (Scale bars: A–G, and L, 30 μm; I–K and M–O, 50 μm.)
Although the expression of pluripotency markers was detected by immunocytochemistry, their expression level as determined by quantitative RT-PCR (qPCR) generally was very low compared with popular pluripotent stem cells such as hESCs and iPS cells generated from human fibroblasts (Fibro-iPSs) (Table 1). For example, the expression of Oct3/4 was approximately one sixtieth and the expression of Nanog and Sox2 was less than one-thousandth that of iPS cells.
Table 1.
Gene expression in iPS cell generation
 |
Gene expression profiles in Muse cells, Muse cell-derived iPS cells (M-iPS), hESCs (hES), Fibro iPSs, and non-Muse cell–derived colonies (non-M col) in qPCR. The expression level of each mRNA was normalized with that of β-actin by the ΔΔCt method and compared with that of intact non-Muse cells. Red or blue color indicates the increase or decrease of the expression level of each gene, respectively.
We repeatedly confirmed the ability of M-clusters to self-renew with normal karyotypes up to the third generation (SI Results and Fig. S2) and in vitro differentiation into ectodermal, mesodermal, and endodermal cells from a single cell (SI Results and Fig. S3), as reported previously (16). We also injected M-clusters into immunodeficient mouse testes; as reported previously, those testes did not develop teratomas for up to 6 mo (16). Furthermore, both Muse cells and M-clusters showed low telomerase activity (Fig. 2H), suggesting that Muse cells have limited replication potential.
We assessed whether the differentiation of Muse cells can be controlled by certain induction systems. A single M-cluster formed in single-cell suspension culture after limiting dilution was expanded on adherent culture. The expanded cells were divided into four fractions, and each fraction was subjected to conditions that induced differentiation into neural cells (ectoderm) (25), adipocytes (mesoderm) (26, 27), osteoblasts (mesoderm) (26, 27), or hepatocytes (endoderm) (28). Neural induction generated spheres containing cells positive for the neural stem cell markers nestin, Musashi, and NeuroD (Fig. 2 I–K), which further differentiated into MAP-2+ or GFAP+ cells when cultured in differentiation medium (Fig. 2L; 89 ± 5.7% positive for either MAP-2 or GFAP). Adipocyte induction produced cells with lipid droplets that stained with oil red O (90 ± 4.9%; Fig. 2 M and N). Osteoblast induction produced cells positive for osteocalcin (97 ± 3.5%) (Fig. 2O). Hepatocyte induction generated cells positive for human α-fetoprotein (α-FP) (Fig. 2P; 87 ± 7.6%), and RT-PCR confirmed the expression of human albumin and human α-FP (Fig. 2Q). We also confirmed that untreated naive Muse cells do not express any of these markers (SI Results and Fig. S4). These results demonstrate that Muse cells can be directed to differentiate into ectodermal, mesodermal, and endodermal cells with high efficiency under the control of specific induction systems.
Selective Generation of iPS Cells from Muse Cells.
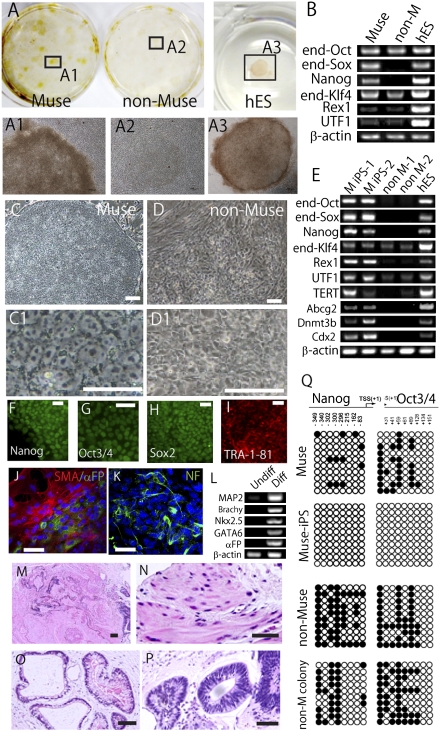
Because Muse cells already possess properties similar to iPS cells, such as the expression of pluripotency markers and the ability to differentiate into cells representative of all three germ layers, they might have a higher propensity to become iPS cells. Muse and non-Muse cells from adult human fibroblasts were transduced with Oct3/4, Sox2, Klf4, and c-Myc by retrovirus and subjected to iPS cell generation according to the original method reported by Takahashi et al. (4). The transduction efficiency for the two populations was almost identical (SI Materials and Methods). Transduced cells then were transferred onto inactivated mouse embryonic fibroblasts (MEFs) at a density of 1 × 105 cells per dish (4). After 30 d, colony generation was observed in both populations. Muse cells, however, formed nearly eight times more colonies than non-Muse cells. Furthermore, only Muse cells formed colonies with hESC-like morphology that were positive for the human pluripotent stem cell marker TRA-1–81, a marker for identifying promising iPS colonies (29). In contrast, none of the colonies derived from non-Muse cells were TRA-1–81+ (Fig. 3A). At this time point (just before colony pickup), all cells and colonies on a dish were collected together and subjected to RT-PCR. Endogenous Sox2 and Nanog were detected in cells and colonies derived from Muse cells but not in those derived from non-Muse cells (Fig. 3B).
Fig. 3.
Generation of iPS cells from Muse cells. (A) (Upper)After 30-d culture on MEFs, colonies derived from Muse cells were TRA-1–81+, whereas those derived from non-Muse cells were TRA-1–81−. hESCs (hES) were the positive control. (Lower) Enlarged views of boxed areas 1–3 are shown in A1–A3. (B) RT-PCR for endogenous Oct3/4 (end-Oct), endogenous Sox2 (end-Sox), Nanog, endogenous Klf4 (end-Klf4), Rex1, and UTF1 Muse and non-Muse (non-M) cells after 30-d culture on MEFs. hESCs (hES) were the positive control. (C and D) The appearance of Muse-iPS cells (C and C1) and non-Muse colony (D and D1) after colony pickup. (E) RT-PCR for endogenous Oct3/4 (end-Oct), endogenous Sox2 (end-Sox), Nanog, endogenous Klf4 (end-Klf4), Rex1, UTF1, TERT, Abcg2, Dnmt3b, and Cdx2 in Muse-iPS cells (two representative clones: M iPS-1 and M iPS -2) and non-Muse colonies (two representative clones: non–M-1 and non–M-2) after colony pickup. (F–I) Immunocytochemistry of (F) Nanog, (G) Oct3/4, (H) Sox2, and (I) TRA-1–81 in Muse-iPS cells and immunocytochemistry of cells expanded from Muse-iPS cell–derived embryoid bodies showing the expression of (J) SMA (α-SMA; red) and α-FP; (green) and (K) neurofilament (NF; green). (L) RT-PCR of differentiation markers in untreated Muse-iPS cells (Undiff) and embryoid body derived from Muse-iPS cells (Diff). (M–P) H&E staining of teratoma (M) that formed in the testes of immunodeficient mice 12 wk after injection of Muse-iPS cells. Mesodermal (N), endodermal (O), and ectodermal (P) tissues were recognized within the teratoma. (Q) Bisulfite sequencing of the promoter regions of Nanog and Oct3/4 in Muse cells, Muse-iPS cells, non-Muse cells, and non-Muse colonies (non-M colony). Empty and filled circles represent unmethylated and methylated cytosines, respectively. (Scale bar: C and D, 100 μm; C1 and D1, 50 μm; F–K, 50 μm; M–P, 100 μm.)
All tightly packed colonies generated from Muse and non-Muse cells were picked and passaged in individual wells to establish iPS cell lines. Only colonies from Muse cells (Fig. 3C and C1), and not those from non-Muse cells (Fig. 3D and D1), had hESC-like, flat growth morphology. After three passages, all colonies were subjected individually to RT-PCR. Because the identification of iPS cells based on only a single-factor assay is considered insufficient for evaluation (30), we identified colonies that expressed all three factors (endogenous Oct3/4, endogenous Sox2, and Nanog) in RT-PCR as iPS cells. This analysis revealed that only colonies originating from Muse cells generated iPS cells (referred to hereafter as “Muse-iPS cells”), but none of the colonies originating from non-Muse cells (non-Muse colonies) generated iPS cells (Fig. 3E). In addition to not expressing endogenous Oct3/4, endogenous Sox2, and Nanog, non-Muse colonies also did not express Rex1, Abcg2, Dnmt3b, or, especially, Cdx2, which are not expressed in the first step of iPS cell generation but are expressed in the fully reprogrammed iPS cells (31), suggesting that non-Muse cells arrested at an early stage of iPS cell generation.
Immunocytochemistry confirmed the expression of Nanog, Oct3/4, Sox2, and TRA-1–81 in Muse-iPS cells (Fig. 3 F–I). When naive human skin fibroblast populations were used, iPS cell lines were generated with an efficiency of ∼0.001%. Muse-iPS cells, however, formed with an efficiency of 0.03%, showing that Muse cells generate iPS cells 30 times more efficiently than naive fibroblasts.
It is possible that the lack of iPS cells generated from non-Muse cells was caused by unsuccessful transduction of one or more of the four retroviral vectors encoding Oct3/4, Sox2, Klf4, and c-Myc. Recently, a single polycistronic viral vector encoding all four factors was applied to iPS cell generation (32). Therefore, we examined whether non-Muse cells generate iPS cells by transduction with a single Oct3/4-Klf4-Sox2-c-Myc-GFP–expressing vector. Transduced cells were isolated by FACS based on GFP expression and subjected to the iPS cell generation procedure. The expression of all four individual proteins (Oct3/4, Sox2, Klf4, and c-Myc) was confirmed by Western blot analysis (32). Muse cells generated Muse-iPS cells, but non-Muse cells did not. The expression of endogenous Sox2 and Nanog in non-Muse cells was not recognized in RT-PCR just before the colony pickup. Also, endogenous Oct3/4, endogenous Sox2, and Nanog, as well as Rex1, Abcg2, Dnmt3b, and Cdx2, were not expressed even after the colony pickup; these results are similar to those obtained with the transduction of four retroviral vectors encoding Oct3/4, Sox2, Klf4, and c-Myc. As a result, non-Muse cells did not generate iPS cells even after transduction with a single polycistronic viral vector encoding all four factors.
We further evaluated the characteristics of Muse-iPS cells. Immunocytochemistry and RT-PCR analysis of embryoid bodies that developed in vitro from Muse-iPS cells showed cells that differentiated into ectodermal cells expressing neurofilament and MAP-2; mesodermal cells expressing smooth muscle actin (SMA), brachyury, and Nkx2.5; and endodermal cells expressing α-FP and GATA-6 (Fig. 3 J–L). Furthermore, in contrast to Muse cells, injection of Muse-iPS cells into the testes of immunodeficient mice resulted in teratoma formation (Fig. 3 M–P).
In qPCR, Muse cells, Muse-iPS cells, hESCs, and Fibro-iPSs showed a higher expression pattern of genes related to pluripotency and an undifferentiated cell state than seen in non-Muse cells (Table 1). The expression pattern and the level of each gene were almost the same among Muse-iPS cells, hESCs, and Fibro-iPSs. The expression levels of many of those genes, including Nanog, Oct3/4, and Sox2, were higher in Muse-iPS cells than in Muse cells. In contrast, non-Muse colonies did not show extensive increase in the expression levels of these genes after receiving the four factors. Genes related to cell-cycle progression were mostly up-regulated in Muse-iPS cells as compared with Muse cells. In non-Muse colonies, some of the genes were up-regulated as compared with non-Muse cells, but the up-regulation was not as extensive as in Muse-iPS cells (Table 1).
Cytosine guanine dinucleotides in the promoter region of Nanog and Oct3/4 were partly methylated in Muse cells and became completely demethylated in Muse-iPS cells (Fig. 3Q). This change in the methylation level of the promoter region may, in part, reflect the higher expression level of Nanog and Oct3/4 in Muse-iPS cells than in Muse cells, as demonstrated with qPCR. On the other hand, the Nanog and Oct3/4 promoter regions were more methylated in naive non-Muse cells than in Muse cells, and the demethylation of the promoter region of Nanog and Oct3/4 observed in Muse-iPS cells was not observed in non-Muse colonies (Fig. 3Q).
Discussion
Muse cells are preexisting adult stem cells whose properties are similar in some respects to those of iPS cells, although they also differ from iPS cells in some respects, including nontumorigenicity and low telomerase activity. We separated Muse and non-Muse cells from fibroblasts and demonstrated that iPS cells were generated exclusively from Muse cells. Thus, Muse cells are a primary cell source for iPS cell generation in adult human fibroblasts.
Byrne et al. (11) reported the very important finding that only SSEA-3+ cells can generate iPS cells in human fibroblasts. However, because the characteristics of the original SSEA-3+ cells were not fully elucidated, the process of iPS cell generation from this cell population has remained obscure (i.e., whether the trigerminal differentiation or self-renewal abilities were newly acquired by these cells only after transduction of the four Yamanaka factors or whether the cells originally possessed these abilities). In the present study, we identified Muse cells among adult human fibroblasts and verified that their trigerminal differentiation and self-renewal abilities were not newly conferred by transduction of the four Yamanaka factors but rather that the Muse cells originally possessed these abilities. In addition, when Muse cells became Muse-iPS cells, they showed the ability to form teratomas and an increased pluripotency-related gene expression level. Further analyses of Muse cells and Muse-iPS cells will deepen the understanding of the mechanisms of iPS generation from human fibroblasts.
Muse cells have the potential to self-renew and give rise to cells of the three germ layers, but, in contrast to popular pluripotent stem cells such as ES cells or iPS cells, they do not form teratomas. On the other hand, epiblast stem cells cultured under certain conditions are considered pluripotent cells, and they also do not form teratomas when transplanted into immunodeficient mouse testes (33), suggesting that even pluripotent stem cells do not always exhibit teratoma-forming activity.
The expression pattern of genes related to pluripotency in Muse cells tended to be similar to that of Muse-iPS cells as evaluated by qPCR, but the expression levels of genes, including Oct3/4, Nanog, and Sox2, was very low in Muse cells. In addition, the promoter regions of Nanog and Oct3/4 were partly methylated in Muse cells. When Muse cells developed into Muse-iPS cells, genes related to cell-cycle progression were mostly up-regulated. Muse cells transplanted into immunodeficient mouse testes did not form teratomas for up to 6 mo, but transplantation of Muse-iPS cells resulted in teratoma formation within 12 wk, indicating that nontumorigenic Muse cells acquired tumorigenic proliferation activity after iPS cell induction. It is noteworthy that Nanog and Oct-4 are reported to accelerate cell-cycle progression in pluripotent stem cells such as ES cells (34, 35). Therefore, it is possible that the generation of iPS cells from Muse cells requires a much higher expression of critical transcription factors, including pluripotency markers that may lead to the activation of genes related to cell-cycle progression. If so, because Muse cells reside in the adult tissues, the expression of pluripotency markers might be regulated at much lower levels in Muse cells than in iPS cells.
In contrast to Muse cells, no iPS cells were generated from the non-Muse cell population among adult human fibroblasts. Even in the naive state, the promoter regions of Nanog and Oct3/4 were more methylated in non-Muse cells than in Muse cells. During iPS cell generation, TRA-1–81+ colonies did not appear in non-Muse cells after the transduction of the four factors. Although some pluripotency-related factors such as TERT and UTF1 were expressed weakly in non-Muse colonies, major factors such as Nanog and endogenous Sox2 were not detected for the entire period during iPS cell generation. These findings were confirmed further by the transduction with a single polycistronic vector encoding the four factors. Furthermore, up-regulation of genes related to pluripotency or demethylation of Nanog and Oct3/4 promoter regions, as observed in Muse-iPS cells, was not observed in non-Muse colonies. It is noteworthy that Rex1, Abcg2, Dnmt3b, and Cdx2 were not expressed in non-Muse colonies. Recently, these factors were reported to be expressed in fully reprogrammed iPS cells but not in incompletely reprogrammed states (31). In addition, incompletely reprogrammed colonies are proposed to be divided into type I and II colonies; type I colonies, which do not express Rex1, Abcg2, Dnmt3b, or Cdx2, remain in the incompletely reprogrammed state and do not develop into iPS cells, whereas type II colonies, which do not express Rex1, Abcg2, or Dnmt3b but are Cdx2+, occasionally spontaneously transit to iPS cells (31). Therefore, non-Muse colonies seem to correspond to the type I colonies. It is unclear whether the colonies derived from four factor-transduced non-Muse cells are still able to become iPS cells by further administration of additional factors. However, given that iPS cells were generated successfully from Muse cells by using the same procedure and time course, the response of non-Muse cells to the four factors appears to be limited, and thus they are unable to generate iPS cells.
In the framework of Muse and non-Muse cells, human fibroblasts can be divided into two populations: cells that primarily contribute to the iPS cell generation and those that do not. Our results demonstrate that the human fibroblast system fits into the previously proposed elite model of iPS cell generation (6). Further studies will clarify the potential of this system to generate iPS cells from other tissues.
Because of the lack of unified criteria for identifying the generation of iPS cells to date, the basis for iPS cell generation efficiency differs among reports; some reports calculate the generation efficiency based only on ALP staining, whereas others are based on the expression of a single pluripotency marker. Therefore, the reported generation efficiencies cannot be compared. In fact, not all colonies positive for ALP staining are iPS cells, and, likewise, colonies that are positive for only a single pluripotency marker may not meet the criteria for iPS cells (29, 30). Therefore, we judged iPS cell colonies by the expression of factors such as Nanog, endogenous Oct3/4, and Sox2 as well as Rex1, Abcg2, Dnmt3b, and Cdx2 by RT-PCR and Tra-1–81, determined by immunocytochemistry. As a result, Muse cells demonstrated 30 times higher efficiency than naive human fibroblasts. Although the overall efficiency of Muse cells still is not so high, the efficiency of generation is expected to increase further if these induction procedures are combined with other approaches, such as the use of valproic acid, the application of ERK, GSK-3β and/or ALK5 inhibitors, or the suppression of p53 (7, 9, 10). Furthermore, some unidentified conditions or factors might affect the efficiency of iPS cell generation. Investigation of optimized systems is expected to improve the efficiency of iPS cell generation from Muse cells.
Materials and Methods
Three strains of cultured human dermal fibroblasts were used; two strains were obtained from Lonza and one from ScienCell. For cell sorting, cells were incubated with anti–SSEA-3 (1:100; Millipore; detected by DyLight649-conjugated anti-rat IgM) and anti-CD105 (1:50; Becton Dickinson; detected by FITC) in the FACS antibody diluents and sorted by Special Order Research Products FACSAriaII (Becton Dickinson) using FACS buffer and the four-way purity-sorting mode to obtain the highest purity of the sorted cells (16). A low stream speed was used to ensure a high level of cell survival.
iPS cell were generated as reported by Takahashi et al. (4). For construction of a single polycistronic virus vector encoding all four factors, 4T2A retroviral vectors containing the ORFs of human Oct3/4, Klf4, Sox2, c-Myc, and EGFP were amplified by RT-PCR and inserted into the pMXs retroviral vector (SI Materials and Methods).
Detailed protocols for cell culture, single-cell suspension culture and cluster formation, ALP staining, immunocytochemistry, telomerase activity, growth rate analysis, karyotyping, transplantation experiments, RT-PCR, immunohistochemistry, in vitro differentiation, iPS cell generation, injection of cells into testes, qPCR, bisulfite sequencing, and statistics are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Drs. Thomas Walz (Harvard Medical School) and Yo-ichi Nabeshima (Kyoto University) for helpful discussions and proofreading the manuscript. We are grateful to the late Keiji Takita, Director General of the Japan New Energy and Industrial Technology Development Organization (NEDO), who died during this study. This work was supported by NEDO.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1100816108/-/DCSupplemental.
References
- 1.Kørbling M, Estrov Z. Adult stem cells for tissue repair - a new therapeutic concept? N Engl J Med. 2003;349:570–582. doi: 10.1056/NEJMra022361. [DOI] [PubMed] [Google Scholar]
- 2.Thomas ED. Landmarks in the development of hematopoietic cell transplantation. World J Surg. 2000;24:815–818. doi: 10.1007/s002680010130. [DOI] [PubMed] [Google Scholar]
- 3.Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan GJ, Bai Y, Fletcher J, Wilmut I. Induced pluripotent stem cells: Epigenetic memories and practical implications. Mol Hum Reprod. 2010;16:880–885. doi: 10.1093/molehr/gaq091. [DOI] [PubMed] [Google Scholar]
- 6.Yamanaka S. Elite and stochastic models for induced pluripotent stem cell generation. Nature. 2009;460:49–52. doi: 10.1038/nature08180. [DOI] [PubMed] [Google Scholar]
- 7.Huangfu D, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26:1269–1275. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 8.Kawamura T, et al. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature. 2009;460:1140–1144. doi: 10.1038/nature08311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li W, et al. Generation of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors. Cell Stem Cell. 2009;4:16–19. doi: 10.1016/j.stem.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Lin T, et al. A chemical platform for improved induction of human iPSCs. Nat Methods. 2009;6:805–808. doi: 10.1038/nmeth.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byrne JA, Nguyen HN, Reijo Pera RA. Enhanced generation of induced pluripotent stem cells from a subpopulation of human fibroblasts. PLoS ONE. 2009;4:e7118. doi: 10.1371/journal.pone.0007118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eminli S, et al. Differentiation stage determines potential of hematopoietic cells for reprogramming into induced pluripotent stem cells. Nat Genet. 2009;41:968–976. doi: 10.1038/ng.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanna J, et al. Direct cell reprogramming is a stochastic process amenable to acceleration. Nature. 2009;462:595–601. doi: 10.1038/nature08592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ou L, Wang X, Zou F. Is iPS cell the panacea? IUBMB Life. 2010;62:170–175. doi: 10.1002/iub.289. [DOI] [PubMed] [Google Scholar]
- 15.Abollo-Jiménez F, Jiménez R, Cobaleda C. Physiological cellular reprogramming and cancer. Semin Cancer Biol. 2010;20:98–106. doi: 10.1016/j.semcancer.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Kuroda Y, et al. Unique multipotent cells in adult human mesenchymal cell populations. Proc Natl Acad Sci USA. 2010;107:8639–8643. doi: 10.1073/pnas.0911647107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishimura EK, et al. Dominant role of the niche in melanocyte stem-cell fate determination. Nature. 2002;416:854–860. doi: 10.1038/416854a. [DOI] [PubMed] [Google Scholar]
- 18.Murga M, Yao L, Tosato G. Derivation of endothelial cells from CD34- umbilical cord blood. Stem Cells. 2004;22:385–395. doi: 10.1634/stemcells.22-3-385. [DOI] [PubMed] [Google Scholar]
- 19.Middleton J, et al. A comparative study of endothelial cell markers expressed in chronically inflamed human tissues: MECA-79, Duffy antigen receptor for chemokines, von Willebrand factor, CD31, CD34, CD105 and CD146. J Pathol. 2005;206:260–268. doi: 10.1002/path.1788. [DOI] [PubMed] [Google Scholar]
- 20.Crisan M, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Nagoshi N, et al. Ontogeny and multipotency of neural crest-derived stem cells in mouse bone marrow, dorsal root ganglia, and whisker pad. Cell Stem Cell. 2008;2:392–403. doi: 10.1016/j.stem.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Fernandes KJ, et al. A dermal niche for multipotent adult skin-derived precursor cells. Nat Cell Biol. 2004;6:1082–1093. doi: 10.1038/ncb1181. [DOI] [PubMed] [Google Scholar]
- 23.Biernaskie J, et al. SKPs derive from hair follicle precursors and exhibit properties of adult dermal stem cells. Cell Stem Cell. 2009;5:610–623. doi: 10.1016/j.stem.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hermann A, et al. Efficient generation of neural stem cell-like cells from adult human bone marrow stromal cells. J Cell Sci. 2004;117:4411–4422. doi: 10.1242/jcs.01307. [DOI] [PubMed] [Google Scholar]
- 26.Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 27.Dezawa M, et al. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest. 2004;113:1701–1710. doi: 10.1172/JCI20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oyagi S, et al. Therapeutic effect of transplanting HGF-treated bone marrow mesenchymal cells into CCl4-injured rats. J Hepatol. 2006;44:742–748. doi: 10.1016/j.jhep.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 29.Lowry WE, et al. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc Natl Acad Sci USA. 2008;105:2883–2888. doi: 10.1073/pnas.0711983105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subramanyam D, Blelloch R. Watching reprogramming in real time. Nat Biotechnol. 2009;27:997–998. doi: 10.1038/nbt1109-997. [DOI] [PubMed] [Google Scholar]
- 31.Chan EM, et al. Live cell imaging distinguishes bona fide human iPS cells from partially reprogrammed cells. Nat Biotechnol. 2009;27:1033–1037. doi: 10.1038/nbt.1580. [DOI] [PubMed] [Google Scholar]
- 32.Sommer CA, et al. Induced pluripotent stem cell generation using a single lentiviral stem cell cassette. Stem Cells. 2009;27:543–549. doi: 10.1634/stemcells.2008-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chou YF, et al. The growth factor environment defines distinct pluripotent ground states in novel blastocyst-derived stem cells. Cell. 2008;135:449–461. doi: 10.1016/j.cell.2008.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang X, et al. A role for NANOG in G1 to S transition in human embryonic stem cells through direct binding of CDK6 and CDC25A. J Cell Biol. 2009;184:67–82. doi: 10.1083/jcb.200801009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee J, Go Y, Kang I, Han YM, Kim J. Oct-4 controls cell-cycle progression of embryonic stem cells. Biochem J. 2010;426:171–181. doi: 10.1042/BJ20091439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.