Abstract
During the past several decades, numerous reports from disparate geographical areas have documented an increased frequency of “bleaching” in reef-forming corals. The phenomenon, triggered by increased sea surface temperatures, occurs when the cnidarian hosts digest and/or expel their intracellular, photosynthetic dinoflagellate symbionts (“zooxanthellae” in the genus Symbiodinium). Although coral bleaching is often followed by the death of the animal hosts, in some cases, the animal survives and can be repopulated with viable zooxanthellae. The physiological factors determining the ability of the coral to survive bleaching events are poorly understood. In this study, we experimentally established that bleaching and death of the host animal involve a caspase-mediated apoptotic cascade induced by reactive oxygen species produced primarily by the algal symbionts. In addition, we demonstrate that, although some corals naturally suppress caspase activity and significantly reduce caspase concentration under high temperatures as a mechanism to prevent colony death from apoptosis, even sensitive corals can be prevented from dying by application of exogenous inhibitors of caspases. Our results indicate that variability in response to thermal stress in corals is determined by a four-element, combinatorial genetic matrix intrinsic to the specific symbiotic association. Based on our experimental data, we present a working model in which the phenotypic expression of this symbiont/host relationship places a selective pressure on the symbiotic association. The model predicts the survival of the host animals in which the caspase-mediated apoptotic cascade is down-regulated.
Bleaching of zooxanthellate corals is a global phenomenon that has dramatically reduced the abundance of corals in tropical reef ecosystems (1, 2). The phenomenon occurs when environmental triggers lead to the expulsion, digestion, or loss of pigmentation of the photosynthetic dinoflagellate symbiotic algae (of the genus Symbiodinium) within the cnidarian host cells (3–5). Although bleaching is highly correlated with increased temperature and high solar irradiance (6–10), it does not always lead to death of the cnidarian host (11–13). However, the physiological and molecular mechanisms that lead to death of the cnidarian are not adequately understood (14–18).
Although apoptosis has previously been suggested as a possible pathway for the demise of symbiotic cnidarians (11, 12, 14, 19), and genes related to apoptosis have been cloned from a subset of these animals (Hydra and sea anemones) (19–24), the pathway has not been experimentally demonstrated in hermatypic corals. Some of the characteristic morphological changes during apoptosis (but not during necrosis) include cytoplasmic shrinkage, plasma membrane blebbing, chromatin condensation, and DNA degradation. Most of these phenomena result from the action of cysteine proteases (i.e., caspases) (25, 26). In vertebrates, caspase activity is up-regulated by reactive oxygen species (ROS) (25, 27–29). ROS can be generated in the coral endodermal cells by photosynthetic activity of the symbiotic algae and by aerobic respiratory activity in the host mitochondria, and are frequently related to coral stress (30–33).
In this study, we experimentally demonstrate that a caspase-induced apoptotic pathway initiated by the production of ROS plays a key role in bleaching and subsequent death in several zooxanthellate coral species. Phenotypic variability leading to regulation of the apoptotic cascade in the coral can potentially prevent death of the coral. We examined the relationship among increased temperature, irradiance, and caspase activity in a variety of zooxanthellate corals maintained under highly controlled conditions in aquaria and under natural environmental conditions in the field. Our results provide a mechanistic framework that combines the response of the algal symbionts and the cnidarian host to thermal stress, leading to the induction of an apoptotic pathway in the animal cells. Based on these results, we propose a phenotypic combinatorial matrix that provides a diagnostic and prognostic profile for assessing whether a cnidarian host is likely to live or die following a bleaching event.
Results
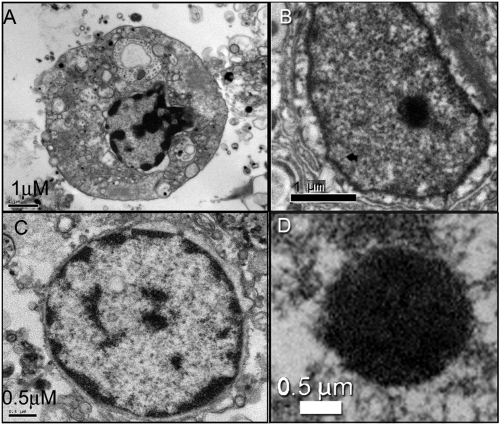
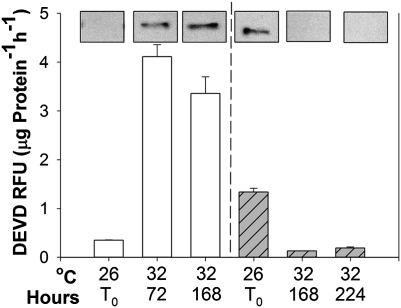
To investigate thermal stress distinct from other environmental triggers, specimens of Seriatopora hystrix and Stylophora pistillata were grown for several months at 26 °C and transferred to 32 °C. Both corals eventually bleached; however, S. hystrix retained morphological integrity after 6 wk of exposure to the elevated temperature, whereas S. pistillata lost all recognizable tissue after 1 wk. Transmission electron micrographs (Fig. 1) revealed ultrastructural changes typical of apoptosis in S. pistillata, including membrane blebbing (Fig. 1A) and the lateral shift in chromatin to the periphery of the nuclei (Fig. 1C). Caspase activity increased more than sixfold within 1 wk in S. pistillata (Fig. 2A), but decreased 10-fold during the same time period in S. hystrix and remained low for at least the following 2 wk (Fig. 2B). Western blot analysis indicated that, in both species, caspase activity was correlated with the abundance of a protein that migrates on a denaturing gel at an approximate molecular weight of 42 kDa and cross-reacts with anti-human caspase 3 antibodies (Fig. 2, Top).
Fig. 1.
Transmission EM images of thin sections of S. pistillata tissue. The coral was kept at 32 °C. Light, provided by 400 W metal halide bulbs, was set at 300 μmol quanta · m−2s−1 on a 12 h/12 h light/dark cycle. These conditions induced an apoptotic response including membrane blebbing (A) and shifts in chromatin from the center to the periphery of the cell nuclei (C). The control cells were at ambient temperature and showed normal features with no evidence of blebbing or chromatin shifts (B and D).
Fig. 2.
Caspase activity measured as DEVD cleavage (in relative fluorescence units, RFU) in coral fragments (n = 3) of S. pistillata (solid bars, A) and S. hystrix (hatched bars, B) grown at 26 °C and 32 °C with 12-h/12-h light/dark cycle at a light intensity of 300 μmol photons m−2s−1. The expression of a caspase-like protein in both coral species was tested by using a Western blot analysis that was performed with recombinant human caspase 3 antibody.
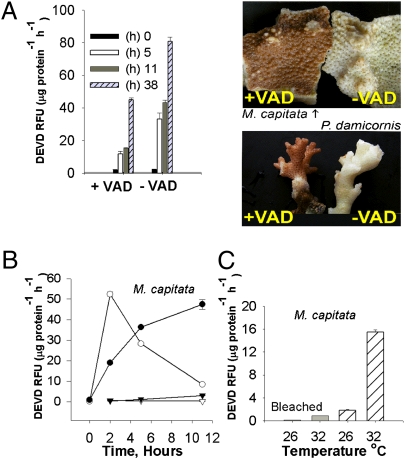
To investigate the factors that signal the caspase cascade, two species of hermatypic corals, Pocillopora damicornis and Montipora capitata, were tested in the field under four experimental conditions: full natural sunlight, low light, ambient temperature, and increased temperature. Whereas elevated temperature (32 °C) led to an increase in caspase activity in both corals under both light regimes, the effect was markedly enhanced under full sunlight (Fig. S1). In P. damicornis, the increase in caspase activity at 32 °C was accompanied by a “laddered” fragmentation of DNA, a characteristic of metazoan apoptosis (Fig. 3). The coral lost all recognizable tissue within 72 h. However, after application of the caspase inhibitor Z-VAD-FMK (VAD), bleaching and apoptosis was completely prevented in both P. damicornis and M. capitata, even after 72 h exposure to the elevated temperature under full sunlight (Fig. 4, photos), and caspase activity was markedly reduced.
Fig. 3.
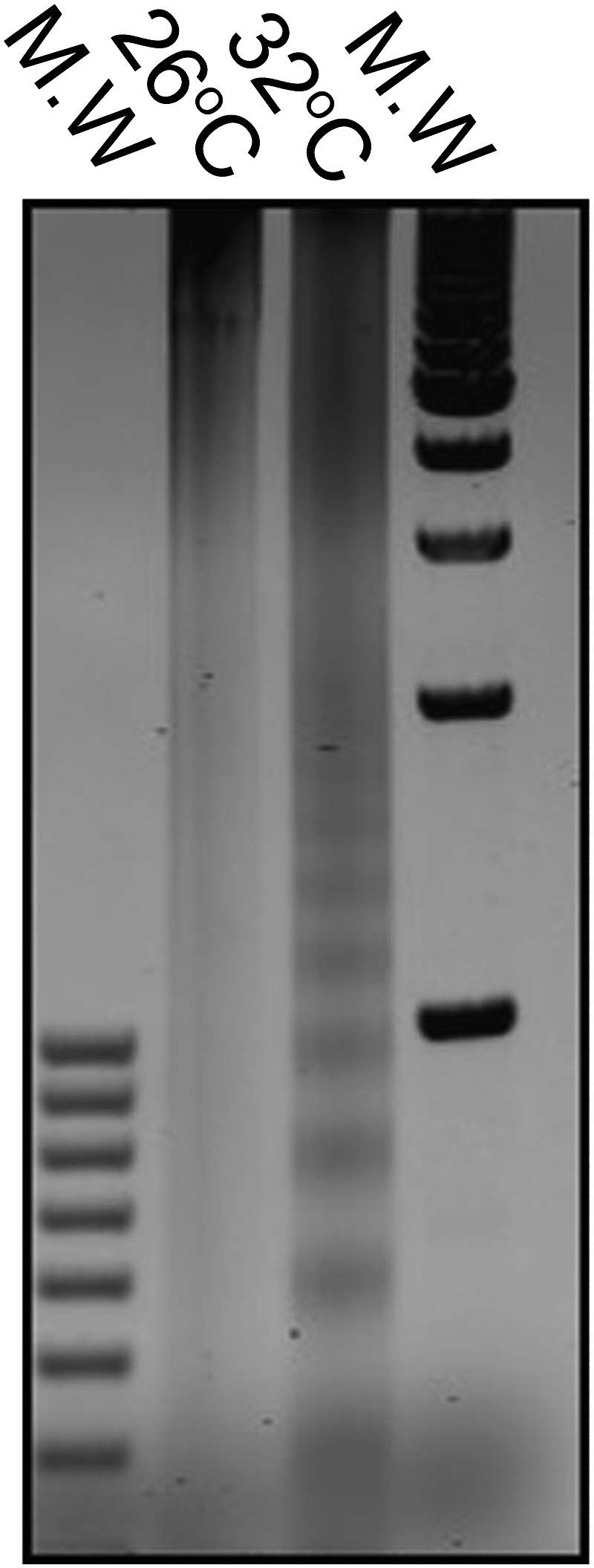
DNA ladder assay indicates apoptotic DNA cleavage in a sample of P. damicornis incubated at 32 °C for 72 h, whereas the control coral at 26 °C shows a normal DNA pattern.
Fig. 4.
(A) Fragments of P. damicornis (n = 3) were incubated for 1 h with a general caspase inhibitor, VAD, and placed under full sunlight in a flow-through aquarium at 32 °C. Samples were tested for DEVD cleavage at 0, 5, 11, and 38 h following addition of the inhibitor. Images are of coral fragments of M. capitata and P. damicornis, incubated for 1 h with VAD (+VAD) or control (−VAD), after 72 h at 32 °C under full sunlight. (B) The induction of DEVD cleavage by ROS using the Fenton reaction in fragments of M. capitata with and without symbionts (n = 3; ▽, bleached without ROS; ▼, control without ROS; ○, bleached with ROS, ●, control with ROS). (C) DEVD cleavage of bleached (gray bar) and control (hatched bars) M. capitata (n = 3). Fragments were incubated for 11 h at 26 and 32 °C in the shade.
To elucidate the potential role and source(s) of ROS generated in the host cells, we measured the steady-state pool of ROS in two colonies of M. capitata, one of which contained zooxanthellae and the other of which had been bleached by long-term incubation in the dark (Fig. 4). In zooxanthellate colonies exposed to 32 °C for 11 h, ROS production was 3.5-fold higher than in control (maintained at 26 °C). Caspase activity followed a similar trend (Fig. S2). To test whether ROS induces caspase activity, we examined the effect of exogenous ROS (hydroxyl anion radicals generated by the Fenton reaction) on corals grown at 26 °C. The addition of hydroxyl anion radicals to zooxanthellate containing colonies of M. capitata in the low light regime led to a 40-fold increase in caspase activity after 11 h (Fig. 4B). In the bleached M. capitata, the addition of exogenous hydroxyl radicals led to a 50-fold increase in caspase activity within 2 h under 26 °C and 50 μmol photons m−2s−1 (Fig. 4B). In both experiments, the host animal subsequently died. In M. capitata (both containing zooxanthellae and in dark-bleached colonies), exposure to 32 °C for 11 h at full sunlight resulted in increased caspase activity (Fig. 4C). However, the activity was much higher in colonies containing zooxanthellae, even at 26 °C.
Discussion
The results of this study provide the framework for the development of a physiological model of how mortality of the host animal is related to the thermal bleaching process.
Broadly, we separate the basic phenomena (bleaching and host death) into two main components, forming a combinatorial matrix: the first is related to factors that trigger the apoptotic response. Depending on the lipid composition in the algal symbionts, high temperature and irradiance can lead to the energetic uncoupling of the thylakoid membranes (34, 35), resulting in an increased rate of production of ROS by the algae (35–37). The thermal sensitivity of thylakoids is genetically dictated by the ratio of saturated to unsaturated fatty acids in the thylakoids and thus causes a chain of events that will effect numerous downstream reactions (ref. 38, but see ref. 39). We hypothesize that, when the net production of ROS by the algal symbiont has exceeded a threshold, these molecules activate a caspase cascade within the host cell (the second component). This second component, which appears to be genetically determined by the cnidarian host, has the potential to result in apoptosis and death of the host. This is supported by the differential response of S. hystrix and S. pistillata to thermal stress (Fig. 2B). Although both corals bleached, the apoptotic response was elevated in S. pistillata that subsequently died, whereas it decreased in S. hystrix that survived. These results suggest that thermally induced bleaching and death of the host are independent, species-specific processes. That the death of the host resulted from apoptosis is further supported by analysis of the ultrastructural changes in the animal cells (Fig. 1), DNA laddering (Fig. 3), and the increase in caspase activity.
The likelihood of apoptosis serving as a key component inducing the mortality of host tissue is further supported by two of our experiments. In the first, the introduction of hydroxyl radicals induced high levels of caspase activity despite low ambient temperature and low irradiance conditions, regardless of whether the corals contained zooxanthellae (Fig. 4B). The results obtained are in agreement with those reported by other investigators (33–36), and further suggest that, although zooxanthellae can potentially generate significant amounts of ROS in high light and thereby induce a caspase cascade, the algae can also scavenge ROS under low light conditions, thereby protecting the coral from chronic damage induced by radicals. Moreover, the addition of the caspase inhibitor VAD rescued the host, even under high ROS production. These results strongly suggest that, if the caspase cascade is interrupted, the coral can be rescued from apoptosis and moderate bleaching, regardless of whether elevated temperatures and/or light are the physiological triggers. The involvement of caspase(s) in the host apoptotic response is further supported by our Western blot analysis (Fig. 2). Caspase activity was correlated with the abundance of an immunoreactive protein, the size of which is in agreement with the predicted amino acid sequence of a caspase cDNA cloned from heat-stressed S. pistillata (Fig. S3). Hence, if the caspase cascade is arrested at an early stage, the apoptotic response does not occur. Moreover, this response may be controlled by the balance between pro- and antiapoptotic molecules, such as the Bcl-2 and Bax gene families (19, 24, 40). The exact biochemical sequence triggering the caspase cascade in the host remains elusive; however, based on studies in metazoan models (41), we suggest that the ROS produced by the algal symbionts compromise the structural integrity of mitochondrial membrane in the host cells, thereby stimulating the release of factors initiating a caspase cascade. In the case of sensitive corals, it is most likely that ROS levels will continue to accumulate after bleaching as a result of mitochondrial dysfunction and further evoke the apoptotic machinery (6, 33, 42).
In addition, it is worthwhile to investigate in the future the impairment of Ca2+ release as a part of the overall suppression of exocytosis and apoptosis in corals (17, 43, 44), as Ca2+ is known signal for apoptosis initiation.
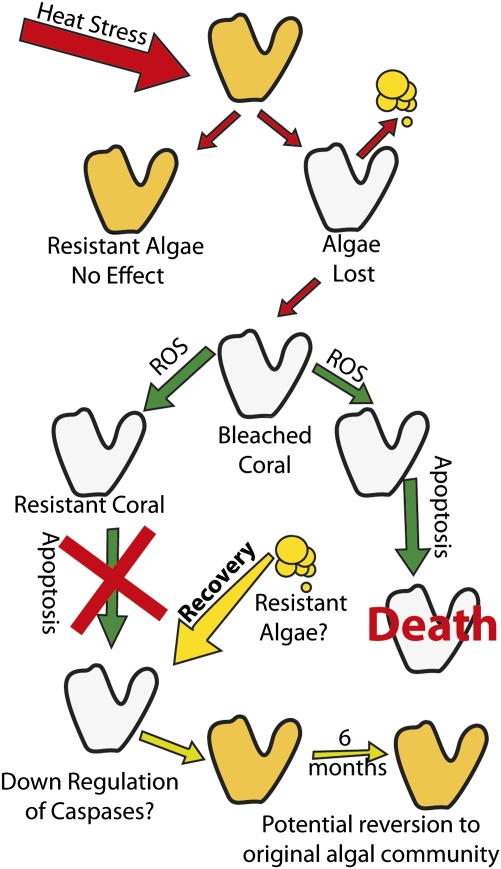
Our model (Fig. 5) predicts that, if the zooxanthellae are sensitive and the host activates a caspase cascade in response to the endogenous production of ROS (30–34, 37), the host will likely both bleach and die. If the zooxanthellae are sensitive but the host does not activate the cascade or down-regulates the activity of these proteases (Fig. 2), the host will lose most of its symbionts (i.e., bleach) but will survive the event. Recovery of algal populations within the bleached colony may then occur, either by the same type of algae (if stressful conditions quickly return to normal) or by resistant algae (if conditions remain stressful). In both cases, the source of these algae may be residual symbionts remaining in the colony at low abundance, or “new” symbionts acquired from the environment (45, 46). If the zooxanthellae are thermally tolerant, the production of ROS will not exceed the rate of enzymatically catalyzed destruction of these molecules. Accordingly, the host will probably not respond to a high temperature event, regardless of whether it has a propensity to activate a caspase cascade.
Fig. 5.
A model explaining the two divergent responses of corals to bleaching, one leading to recovery of the host animal, the other to its death. According to this model, thermal-induced bleaching and apoptosis in zooxanthellate corals are comprised of two major components that form a combinatorial matrix: the first is related to factors that can trigger the apoptotic response. If the zooxanthellae are thermally sensitive (i.e., the physical integrity of the thylakoid membranes in the algal plastid becomes compromised), ROS production from the endosymbiotic algae is accelerated. Second, when the ROS leaks into the host cell, it can initiate a caspase cascade. If the latter occurs, the coral will bleach and die. In the case of sensitive zooxanthellae and a host that does not activate the cascade or down-regulates the activity of caspases, we expect that the bleaching will occur but the host tissue will survive the event. Potential reversion to the original algal community within the bleached colony may occur depending on the environment. If the zooxanthellae are thermally resistant, ROS production will not be accelerated. The host will probably not respond to a high temperature event, i.e., bleach, regardless of whether it has a propensity to activate a caspase cascade.
Our results provide a mechanistic working model for understanding the cellular basis for thermal bleaching in zooxanthellate corals. This model (Fig. 5) explains the spatially heterogeneous patterns of bleaching observed on reefs (47). Some corals do not bleach, others bleach but do not die, and still others bleach and die (13). Clearly, if sea surface temperatures continue to increase during the next century, a strong selection for corals with algal symbionts that generate less ROS or hosts that do not activate a caspase cascade in response to the production of ROS is to be expected. A similar selection pressure almost certainly occurred 55 million years ago, at the end of the Paleocene, when sea surface temperatures increased sharply in response to a sudden, massive increase in greenhouse warming (48). Zooxanthellate corals survived that environmental crisis with apparently little loss of diversity, but during the ensuing 50 million years, a slow depletion in atmospheric CO2 (driven primarily by geochemical weathering reactions), increasingly selected host–algal symbiotic associations that were adapted to lower sea surface temperatures (47). High temperature-resistant symbiotic phenotypes have persisted in some locations (49) and are potentially poised to become increasingly dominant in the Anthropocene.
Materials and Methods
Collection and Maintenance of Corals.
S. hystrix and S. pistillata were grown for 6 mo at the Osborn Laboratories (New York Aquarium) at 26 °C (a temperature that does not cause bleaching) in 800 L aquaria with running artificial seawater (Instant Ocean Sea Salt) (50). Light, at 200 μmol quanta m−2s−1 on a 12-h/12-h light/dark cycle, was provided by 400 W metal halide bulbs (Iwasaki). Nutrients (NO3−, NO2−, NH4+, and PO43−) were kept at submicromolar concentrations by foam fractioning and biological filtration (i.e., “live” sand). For thermal stress experiments, triplicate colonies were transferred to 300 L aquaria, which were then heated to 32 °C and maintained at that temperature for 2 mo or until the colonies died. P. damicornis and M. capitata were collected from Kaneohe Bay, Oahu, Hawaii. These two species were maintained in 300 L aquaria with flowing sea water at the Hawaii Institute of Marine Biology. Triplicate colonies were tested under four conditions: full natural sunlight (maximum irradiance of 2,000 μmol photons m−2s−1), low light (maximum irradiance of 50 μmol photons m−2s−1), ambient temperature (26 °C), and elevated temperature (32 °C). M. capitata was induced to bleach by subjecting the colony to a 9-mo darkness stress (51). The colony was fed with Artemia salina nauplii. For caspase inhibition, coral fragments were incubated for 1 h with 1 μM of caspase inhibitor VAD (Calbiochem). In all experiments, statistical significance of caspase activity levels between the control and treatment groups was tested by using one-way ANOVA and a t test.
EM.
Samples were preserved in Trump EM fixative (4% formaldehyde and 1% glutaraldehyde in phosphate buffer, pH 7.2), rinsed three times (2 × 15 min) in Millonig phosphate buffer, pH 7.3, postfixed for 2 h in 1% buffered OsO4, washed three times, and dehydrated through a graded series of EtOH. After replacement of ethanol with propylene oxide, cells were embedded in Epon–Araldite mixture. Sections were cut by using a LKB 2088 ultramicrotone, collected on 200-mesh copper grids, and stained with uranyl acetate and lead citrate. The stained sections were photographed in a model 100CXII electron microscope (JEM).
Production of Hydroxyl Radical Anions (Fenton Reaction) and ROS Assay.
Corals were treated for 20 min with a reaction mixture containing 0.1 mM FeSO4 and 0.6 mM H2O2 in seawater; this solution was added to the corals to give a final concentration of 100 μM OH−. After 20 min, the water was exchanged with filtered seawater. ROS were assayed by using dihydrorhodamine (DHR 123) fluorescence, which, upon oxidation, is converted to rhodamine 123, which fluoresces at 515 nm.
Caspase Activity Determination.
Caspase activity was determined by incubation of cell lysate with 50 μM Z-DEVD-AFC, a fluorogenic substrate for caspases (Calbiochem), in 100 μL buffer containing 50 mM Hepes, pH 7.3, 100 mM NaCl, 10% sucrose, 0.1% CHAPS, and 10 mM DTT (52). Fluorescence was measured every 5 min for 2 h in a Spectra Max Gemini XS plate reader (exλ 400 nm, emλ 505 nm). Activity was inhibited with 1 μM of the irreversible caspase inhibitor VAD (Z-Val-Ala-Asp-CH2F*; Calbiochem). Cell lysates were preincubated with inhibitor for 1 h before the fluorogenic substrate was added.
Western Blots.
Samples for immunochemical analysis were loaded on an equal protein basis, separated on 12% polyacrylamide gels, and transferred to PVDF membranes. Membranes were probed with polyclonal antibodies for recombinant human caspase 3 (Calbiochem) and detected by using a horseradish peroxidase chemiluminescence system (SuperSignal; Pierce).
Supplementary Material
Acknowledgments
We thank Robert Kinzie III, Paul Jokiel, Kevin Wyman, Lisa Warden, and Ruth Gates for their invaluable help in conducting the research. This research was supported by the Strategic Environmental Research and Development Program under Contract DACA7203C005 (to M.Y.G. and P.G.F.) and by Israeli Science Foundation Grant 981/05.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1106924108/-/DCSupplemental.
References
- 1.Hughes TP, et al. Climate change, human impacts, and the resilience of coral reefs. Science. 2003;301:929–933. doi: 10.1126/science.1085046. [DOI] [PubMed] [Google Scholar]
- 2.Carpenter KE, et al. One-third of reef-building corals face elevated extinction risk from climate change and local impacts. Science. 2008;321:560–563. doi: 10.1126/science.1159196. [DOI] [PubMed] [Google Scholar]
- 3.Brown BE, Dunne RP, Goodson MS, Douglas AE. Experience shapes the susceptibility of a reef coral to bleaching. Coral Reefs. 2002;21:119–126. [Google Scholar]
- 4.Hoegh-Guldberg O. Climate change, coral bleaching and the future of the world's coral reefs. Mar Freshw Res. 1999;50:839–866. [Google Scholar]
- 5.Hoegh-Guldberg O, Furnas M, Williams DM, Wilkinson C, Marshall P. Reef is in danger. New Scientist. 2003;177:24. [Google Scholar]
- 6.Weis VM. Cellular mechanisms of Cnidarian bleaching: Stress causes the collapse of symbiosis. J Exp Biol. 2008;211:3059–3066. doi: 10.1242/jeb.009597. [DOI] [PubMed] [Google Scholar]
- 7.Edmunds PJ, Gates RD. Has coral bleaching delayed our understanding of fundamental aspects of coral-dinoflagellate symbioses? BioSci. 2003;53:976–980. [Google Scholar]
- 8.Gates RD, Baghdasarian G, Muscatine L. Temperature stress causes host cell detachment in symbiotic cnidarians: Implications for coral bleaching. Biol Bull. 1992;182:324–332. doi: 10.2307/1542252. [DOI] [PubMed] [Google Scholar]
- 9.Roberts L. Coral bleaching threatens atlantic reefs: Unexplained changes are occurring in some of the most productive ecosystems on the planet, the Caribbean coral reefs. Science. 1987;238:1228–1229. doi: 10.1126/science.238.4831.1228. [DOI] [PubMed] [Google Scholar]
- 10.Coles SL, Jokiel PL, Lewis CR. Thermal tolerance in tropical versus subtropical Pacific reef corals. Pac Sci. 1976;30:159–166. [Google Scholar]
- 11.Baker AC, Starger CJ, McClanahan TR, Glynn PW. Coral reefs: corals’ adaptive response to climate change. Nature. 2004;430:741. doi: 10.1038/430741a. [DOI] [PubMed] [Google Scholar]
- 12.Buddemeier RW, Kleypas JA, Aronson RB. Coral Reefs and Global Climate Change: Potential Contributions of Climate Change to Stresses on Coral Reef Ecosystems. Arlington, VA: Pew Center for Global Climate Change; 2004. [Google Scholar]
- 13.McClanahan T. The relationship between bleaching and mortality of common corals. Mar Biol. 2004;144:1239–1245. [Google Scholar]
- 14.DeSalvo MK, et al. Differential gene expression during thermal stress and bleaching in the Caribbean coral Montastraea faveolata. Mol Ecol. 2008;17:3952–3971. doi: 10.1111/j.1365-294X.2008.03879.x. [DOI] [PubMed] [Google Scholar]
- 15.Grottoli AG, Rodrigues LJ, Palardy JE. Heterotrophic plasticity and resilience in bleached corals. Nature. 2006;440:1186–1189. doi: 10.1038/nature04565. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez-Lanetty M, Harii S, Hoegh-Guldberg O. Early molecular responses of coral larvae to hyperthermal stress. Mol Ecol. 2009;18:5101–5114. doi: 10.1111/j.1365-294X.2009.04419.x. [DOI] [PubMed] [Google Scholar]
- 17.Voolstra CR, et al. Effects of temperature on gene expression in embryos of the coral Montastraea faveolata. BMC Genomics. 2009;10:627. doi: 10.1186/1471-2164-10-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strychar KB, Sammarco PW. Exaptation in corals to high seawater temperatures: Low concentrations of apoptotic and necrotic cells in host coral tissue under bleaching conditions. J Exp Mar Biol Ecol. 2009;369:31–42. [Google Scholar]
- 19.Ainsworth TD, Hoegh-Guldberg O, Heron SF, Skirving WJ, Leggat W. Early cellular changes are indicators of pre-bleaching thermal stress in the coral host. J Exp Mar Biol Ecol. 2008;364:63–71. [Google Scholar]
- 20.Richier S, et al. Oxidative stress and apoptotic events during thermal stress in the symbiotic sea anemone, Anemonia viridis. FEBS J. 2006;273:4186–4198. doi: 10.1111/j.1742-4658.2006.05414.x. [DOI] [PubMed] [Google Scholar]
- 21.Dunn SR, Phillips WS, Spatafora JW, Green DR, Weis VM. Highly conserved caspase and Bcl-2 homologues from the sea anemone Aiptasia pallida Lower metazoans as models for the study of apoptosis evolution. J Mol Evol. 2006;63:95–107. doi: 10.1007/s00239-005-0236-7. [DOI] [PubMed] [Google Scholar]
- 22.Böttger A, Alexandrova O. Programmed cell death in Hydra. Semin Cancer Biol. 2007;17:134–146. doi: 10.1016/j.semcancer.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Lasi M, David CN, Böttger A. Apoptosis in pre-Bilaterians: Hydra as a model. Apoptosis. 2010;15:269–278. doi: 10.1007/s10495-009-0442-7. [DOI] [PubMed] [Google Scholar]
- 24.Lasi M, et al. The molecular cell death machinery in the simple cnidarian Hydra includes an expanded caspase family and pro- and anti-apoptotic Bcl-2 proteins. Cell Res. 2010;20:812–825. doi: 10.1038/cr.2010.66. [DOI] [PubMed] [Google Scholar]
- 25.Strasser A, O'Connor L, Dixit VM. Apoptosis signaling. Annu Rev Biochem. 2000;69:217–245. doi: 10.1146/annurev.biochem.69.1.217. [DOI] [PubMed] [Google Scholar]
- 26.Fuentes-Prior P, Salvesen GS. The protein structures that shape caspase activity, specificity, activation and inhibition. Biochem J. 2004;384:201–232. doi: 10.1042/BJ20041142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki YJ, Forman HJ, Sevanian A. Oxidants as stimulators of signal transduction. Free Radic Biol Med. 1997;22:269–285. doi: 10.1016/s0891-5849(96)00275-4. [DOI] [PubMed] [Google Scholar]
- 28.Zamzami N, et al. Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J Exp Med. 1995;182:367–377. doi: 10.1084/jem.182.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buttke TM, Sandstrom PA. Redox regulation of programmed cell death in lymphocytes. Free Radic Res. 1995;22:389–397. doi: 10.3109/10715769509147548. [DOI] [PubMed] [Google Scholar]
- 30.Shick JM, Lesser MP, Jokiel PL. Effects of ultraviolet radiation on corals and other coral reef organisms. Glob Change Biol. 1996;2:527–545. [Google Scholar]
- 31.Kuhl M, Cohen Y, Dalsgaard T, Jorgensen BB, Revsbech NP. Microenvironment and photosynthesis of zooxanthellae in scleractinian corals studied with microsensors for O2, pH and light. Mar Ecol Prog Ser. 1995;117:159–172. [Google Scholar]
- 32.Shashar N, Cohen Y, Loya Y. Extreme diel fluctuations of oxygen in diffusive boundary layers surrounding stony corals. Biol Bull. 1993;185:455–461. doi: 10.2307/1542485. [DOI] [PubMed] [Google Scholar]
- 33.Downs CA, et al. Oxidative stress and seasonal coral bleaching. Free Radic Biol Med. 2002;33:533–543. doi: 10.1016/s0891-5849(02)00907-3. [DOI] [PubMed] [Google Scholar]
- 34.Tchernov D, et al. Membrane lipids of symbiotic algae are diagnostic of sensitivity to thermal bleaching in corals. Proc Natl Acad Sci USA. 2004;101:13531–13535. doi: 10.1073/pnas.0402907101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lesser MP. Oxidative stress causes coral bleaching during exposure to elevated temperatures. Coral Reefs. 1997;16:187–192. [Google Scholar]
- 36.Lesser MP. Elevated temperatures and ultraviolet radiation cause oxidative stress and inhibit photosynthesis in symbiotic dinoflagellates. Limnol Oceanogr. 1996;41:271–283. [Google Scholar]
- 37.Saragosti E, Tchernov D, Katsir A, Shaked Y. Extracellular production and degradation of superoxide in the coral Stylophora pistillata and cultured Symbiodinium. Plos ONE. 2010;5:e12508. doi: 10.1371/journal.pone.0012508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warner ME, Fitt WK, Schmidt GW. Damage to photosystem II in symbiotic dinoflagellates: A determinant of coral bleaching. Proc Natl Acad Sci USA. 1999;96:8007–8012. doi: 10.1073/pnas.96.14.8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diaz-Almeyda E, Thome PE, El Hafidi M, Iglesias-Prieto R. Differential stability of photosynthetic membranes and fatty acid composition at elevated temperature in Symbiodinium. Coral Reefs. 2011;30:217–225. [Google Scholar]
- 40.Pernice M, et al. Regulation of apoptotic mediators reveals dynamic responses to thermal stress in the reef building coral Acropora millepora. PLoS ONE. 2011;6:e16095. doi: 10.1371/journal.pone.0016095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 42.Blackstone N. Mitochondria and the redox control of development in cnidarians. Semin Cell Dev Biol. 2009;20:330–336. doi: 10.1016/j.semcdb.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 43.Sandeman I. Fragmentation of the gastrodermis and detachment of zooxanthellae in symbiotic cnidarians: A role for hydrogen peroxide and Ca2+ in coral bleaching and algal density control. Rev Biol Trop. 2006;54:79–96. [Google Scholar]
- 44.Huang SP, Lin KL, Fang LS. The involvement of calcium in heat-induced coral bleaching. Zool Stud. 1998;37:89–94. [Google Scholar]
- 45.Baker AC. Reef corals bleach to survive change. Nature. 2001;411:765–766. doi: 10.1038/35081151. [DOI] [PubMed] [Google Scholar]
- 46.Baker AC. Flexibility and specificity in coral-algal symbiosis: Diversity, ecology, and biogeography of Symbiodinium. Annu Rev Ecol Evol Syst. 2003;34:661–689. [Google Scholar]
- 47.Rowan R, Knowlton N, Baker A, Jara J. Landscape ecology of algal symbionts creates variation in episodes of coral bleaching. Nature. 1997;388:265–269. doi: 10.1038/40843. [DOI] [PubMed] [Google Scholar]
- 48.Katz ME, Pak DK, Dickens GR, Miller KG. The source and fate of massive carbon input during the latest Paleocene thermal maximum. Science. 1999;286:1531–1533. doi: 10.1126/science.286.5444.1531. [DOI] [PubMed] [Google Scholar]
- 49.Silverstein RN, Correa AMS, LaJeunesse TC, Baker AC. Novel algal symbiont (Symbiodinium spp.) diversity in reef corals of Western Australia. Mar Ecol Prog Ser. 2011;422:63–75. [Google Scholar]
- 50.Sprung J, Delbeek JC. The Reef Aquarium—A Comprehensive Guide to the Identification and Care of Tropical Marine Invertebrates. Coconut Grove, FL: Ricordea; 1997. Vol 2. [Google Scholar]
- 51.Koren S, Dubinsky Z, Chomsky O. Induced bleaching of Stylophora pistillata by darkness stress and its subsequent recovery. Proceedings of the 11th International Coral Reef Symposium. 2008 . Available at http://www.reefbase.org/resource_center/publication/icrs.aspx?icrs=ICRS11. [Google Scholar]
- 52.Lauber K, et al. The adapter protein apoptotic protease-activating factor-1 (Apaf-1) is proteolytically processed during apoptosis. J Biol Chem. 2001;276:29772–29781. doi: 10.1074/jbc.M101524200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.