Abstract
The molecular mechanisms regulating noncanonical protein transport across cellular membranes are poorly understood. Cross-presentation of exogenous antigens on MHC I molecules by dendritic cells (DCs) generally requires antigen translocation from the endosomal compartment into the cytosol for proteasomal degradation. In this study, we demonstrate that such translocation is controlled by the endocytic receptor and regulated by ubiquitination. Antigens internalized by the mannose receptor (MR), an endocytic receptor that targets its ligands specifically toward cross-presentation, were translocated into the cytosol only after attachment of a lysin48-linked polyubiquitin chain to the cytosolic region of the MR. Furthermore, we identify TSG101 as a central regulator of MR ubiquitination and antigen translocation. Importantly, we demonstrate that MR polyubiquitination mediates the recruitment of p97, a member of the ER-associated degradation machinery that provides the driving force for antigen translocation, toward the endosomal membrane, proving the central role of the endocytic receptor and its ubiquitination in antigen translocation.
Dendritic cells (DCs) are professional antigen-presenting cells that recognize extracellular antigens in the peripheral tissue. On recognition of microbial substances, DCs migrate toward the draining lymph node, where they can activate antigen-specific T cells (1). For T-cell activation, internalized antigens are degraded, and the resulting peptides are complexed to MHC molecules and transported to the DC surface, where these complexes can be recognized by antigen-specific T cells. Antigen presentation on MHC II molecules can induce activation of CD4+ T helper cells (2), whereas the presentation of extracellular antigens on MHC I molecules, a process termed cross-presentation, activates CD8+ cytotoxic T cells (3, 4).
Efficient cross-presentation requires the entrance of antigen into a specific intracellular pathway. In a previous study, we demonstrated that this entrance is determined by the mechanism of antigen uptake (4). If DCs internalized the model antigen ovalbumin (OVA) by the mannose receptor (MR), then entrance was targeted into a distinct early-endosomal compartment, which did not mature further into lysosomes and from which the antigen was processed exclusively for cross-presentation.
Despite intensive investigation, the molecular mechanisms of cross-presentation remain incompletely resolved. Substantial evidence points to a critical role of the proteasome in generating epitopes for cross-presentation (5–8), which demonstrates the need for transport of the internalized antigen across the endosomal membrane into the cytoplasm. This process has been postulated to be mediated by members of the ER-associated degradation machinery (9, 10), a protein complex required for the dislocation of misfolded proteins from the ER. Within this complex, the p97 AAA ATPase plays a crucial role by providing the driving force for this protein transport (11). During dislocation, p97 is recruited to the ER membrane by binding to polyubiquitinated proteins (12, 13). The recognition of lysin48-linked polyubiquitin chains by p97 strengthens its attachment to the ER membrane (12), which is required for efficient dislocation (12, 14). Importantly, it has been postulated that p97 also is involved in antigen transport across the endosomal membrane for cross-presentation (10); however, the underlying mechanisms regulating p97 recruitment toward the endosomal membrane remain unclear. In this study, we demonstrate that polyubiquitination of the MR enables such recruitment and thus is a key process in regulating cross-presentation.
Results
MR Is Essential for OVA Transport into Cytoplasm.
The MR targets OVA to a distinct early-endosomal compartment, where it colocalizes with the endosomal markers EEA1 and Rab5, with the MR itself, and with Trf. From these endosomes, MR-internalized OVA is processed for cross-presentation (4). We demonstrated previously that proteins also can be targeted specifically toward this compartment in the absence of the MR by covalent coupling to Trf (15); thus, we wondered whether targeting of OVA toward these endosomes by covalent linkage to Trf (OVA-Trf) would result in MR-independent cross-presentation.
To answer this question, we incubated bone marrow-derived DCs (BM-DCs) with OVA-Trf and monitored its uptake. Whereas only marginal amounts of OVA are internalized by MR-deficient DCs by pinocytosis (4), coupling OVA to Trf resulted in efficient internalization in the absence of the MR as well (Fig. 1A; quantification in Fig. S1). Analysis of the intracellular localization of these complexes in MR-deficient DCs revealed that OVA-Trf colocalizes with EEA1 and the transferrin receptor, both markers of MR-targeted endosomes (4), but not with the lysosomal marker LAMP-1 or with Lucifer yellow (Fig. S2A), a pinocytosis marker targeted toward lysosomes (4). To demonstrate that OVA-Trf is indeed targeted toward the same endosomes as MR-internalized antigens, we analyzed uptake of OVA-Trf in WT BM-DCs pretreated with mannan. Because mannan prevents MR-mediated endocytosis (8) (Fig. S2B), uptake of OVA-Trf in these pretreated cells is independent of the MR. Importantly, in these cells, OVA-Trf colocalized with the MR (Fig. S2A), demonstrating that OVA-Trf indeed is targeted into the same endosomal compartment as MR-internalized antigens.
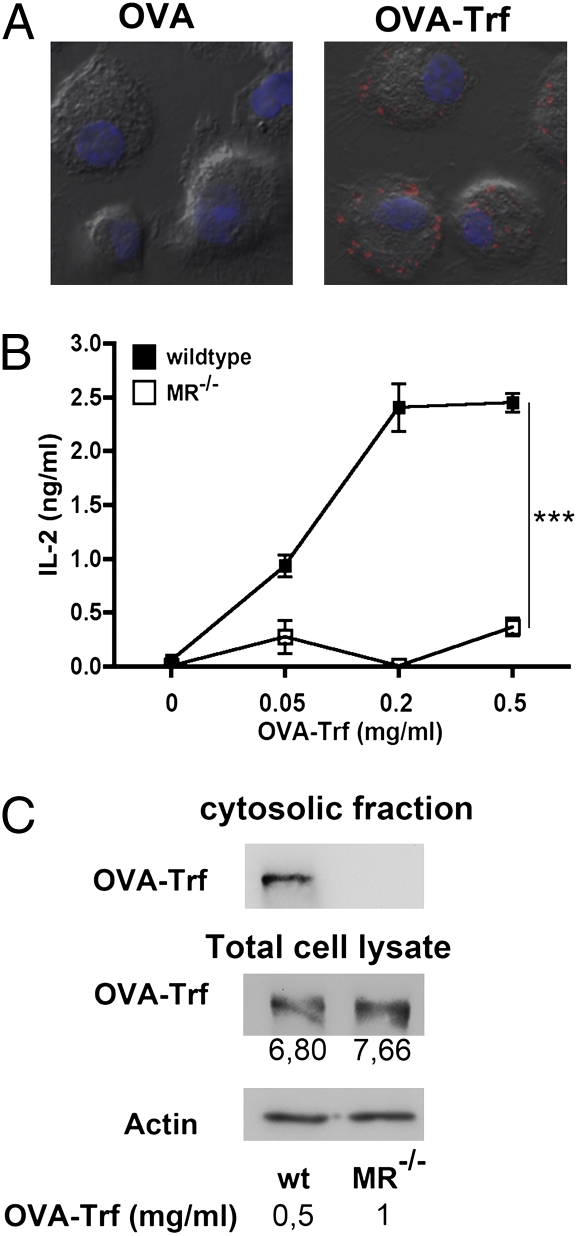
Fig. 1.
Endosomal OVA translocation depends on the MR. (A) BM-DCs from MR-deficient mice were incubated with equimolar amounts (0.2 μM) of fluorescently labeled OVA and OVA-Trf for 15 min and chased with medium for another 30 min. This DIC image depicts OVA and OVA-Trf in red and DAPI staining in blue. (B) WT or MR-deficient BM-DCs were incubated with OVA-Trf for 2 h and cocultured with OT-I T cells. After another 18 h, T-cell activation was determined based on the secretion of IL-2, as measured by ELISA. Data are presented as mean ± SD. (C) To obtain similar amounts of total OVA-Trf uptake, BM-DCs were incubated with 0.5 (WT) or 1 mg/mL (MR−/−) biotinylated OVA-Trf in the presence of the proteasome inhibitor MG132 for 15 min and chased with medium containing MG132 for another 45 min. Then cytoplasmic fractions were isolated, and OVA-Trf was analyzed after streptavidin-based affinity chromatography by Western blot analysis. Numbers indicate densicometric quantification of total OVA-Trf uptake. All graphs depict representative results of at least three independent experiments. ***P < 0.001.
We next analyzed whether targeting OVA into these endosomes by its conjugation to Trf also would lead to cross-presentation in the absence of the MR. Toward this end, we incubated OVA-Trf-treated BM-DCs with OVA-specific CD8+ T cells (OT-I cells). We observed a dose-dependent T-cell activation after coculture with OVA-Trf–treated WT DCs, but not with MR-deficient cells (Fig. 1B), indicating that despite antigen targeting toward the same endosomal subset, no cross-presentation occurred in the absence of the MR.
We then analyzed whether impaired cross-presentation of OVA-Trf in the absence of the MR was due to altered antigen transport into the cytoplasm. To compare such dislocation between WT DCs and MR-deficient DCs, we ensured similar amounts of total OVA-Trf uptake by incubating MR−/− DCs with double the amounts of OVA-Trf compared with that used for WT DCs (Fig. 1C). Importantly, we could detect antigen export into the cytoplasm only in WT cells (Fig. 1C), indicating that the presence of the MR in the antigen-containing endosomes is crucial for antigen export into the cytosol.
Ubiquitination of MR Regulates Antigen Transport into Cytosol.
The foregoing findings pointed to the possibility that covalent modifications on the MR might be involved in the regulation of antigen translocation. One such modification that is known to play a key role in antigen internalization and sorting in endosomes is ubiquitination (16, 17). To investigate the influence of ubiquitination on antigen translocation, we first analyzed whether the MR is ubiquitinated after ligand binding. Toward this end, we overexpressed histidine (His)-tagged ubiquitin in BM-DCs and isolated ubiquitinated proteins by affinity chromatography under denaturing conditions. Subsequent Western blot analysis demonstrated that the MR is indeed polyubiquitinated after addition of OVA (Fig. 2 A and B). Because ubiquitination typically occurs at a lysine residue, and because the cytoplasmic tail of the MR contains only a single lysine (Lys1441), we constructed a MR mutant, in which we replaced this lysine by arginine (MR1441). After expression of this mutant in the DC line DC2.4, which lacks endogenous MR, polyubiquitination of the MR was abolished (Fig. 2 C and D), demonstrating that such ubiquitination of the MR indeed occurred at lysine1441.
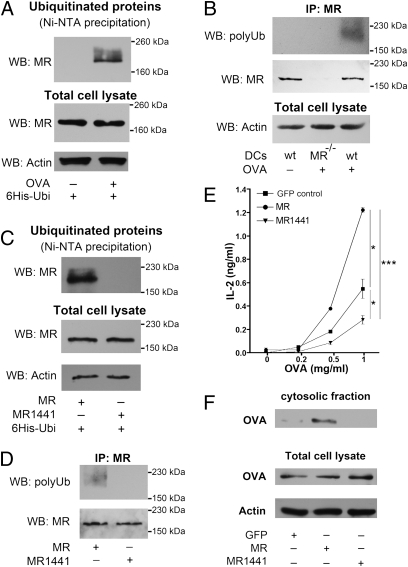
Fig. 2.
MR ubiquitination is essential for OVA translocation into the cytosol. (A) BM-DCs were transfected with mRNA encoding 6× His-tagged ubiquitin. At 1 h after electroporation, OVA was added in the presence of MG132. After another 60 min, cells were lysed and ubiquitinated proteins were isolated by Ni-NTA–based affinity chromatography. Ubiquitination of the MR was analyzed by Western blot analysis using a MR-specific antibody. (B) BM-DCs were treated with OVA and MG132 as in A. Then immune precipitation using a MR-specific antibody was performed, and polyubiquitinated MR was detected by Western blot analysis using the FK1 antibody, which specifically recognizes polyubiquitinated proteins. (C) DC2.4 cells were transfected with expression plasmids encoding 6× His-tagged ubiquitin, MR, or MR1441. At 48 h after electroporation, OVA was added for 1 h, and ubiquitinated proteins were analyzed as in B. (D) DC2.4 cells were transfected as in C. Immune precipitation using a MR-specific antibody was performed, and polyubiquitinated MR was detected by Western blot analysis using the FK1 antibody. (E) DC2.4 cells were transfected with expression plasmids of GFP (control), MR, or MR1441, then incubated with OVA for 2 h and cocultured with OT-I cells. IL-2 secretion in the supernatant was determined after another 18 h by ELISA. Data are presented as mean ± SEM. (F) DC2.4 cells were transfected with plasmids encoding GFP, MR, or MR1441. At 48 h after electroporation, cells were incubated with biotinylated OVA in the presence of the proteasome inhibitor MG132 for 15 min and chased with medium containing MG132 for another 45 min. Then cytoplasmic fractions were isolated, and the amount of OVA was analyzed after streptavidin-based affinity chromatography by Western blot analysis. All graphs depict representative results of at least three independent experiments. *P < 0.05; ***P < 0.001.
To investigate whether MR ubiquitination is important for cross-presentation, we transfected DCs with WT MR or the ubiquitination-deficient mutant MR1441. Importantly, OVA uptake remained unaltered after expression of MR1441 (Fig. S3 A and B), which is in agreement with previous observations that MR internalization is regulated by a dihydrophobic motive in its C terminus (18). Furthermore, no differences in intracellular localization of the internalized OVA were observed after expression of WT MR or MR1441 (Fig. S3C). To test the effect of MR ubiquitination on cross-presentation, we incubated transfected DCs with OVA and cocultured them with OT-I cells. After expression of MR1441, cross-presentation was clearly impaired (Fig. 2E; transfection controls in Fig. S3D), indicating that ubiquitination of the MR indeed is important for cross-presentation. In addition, the moderate cross-presentation efficiency of control cells indicates the existence of alternative pathways in these cells, which has already been observed by others (19, 20). Subsequently, we investigated whether reduced cross-presentation was due to an impaired antigen transport into the cytoplasm. After expression of the ubiquitination-deficient mutant MR1441, no OVA could be detected in the cytoplasmic fraction (Fig. 2F), demonstrating that MR ubiquitination is indeed essential for antigen transport into the cytoplasm.
Polyubiquitination of MR Is Essential for Endosomal Antigen Export and for Cross-Presentation.
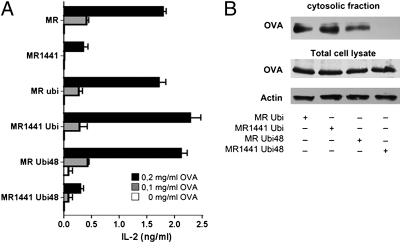
We next investigated whether antigen translocation is mediated by monoubiquitination or polyubiquitination of the MR. For this, we constructed additional MR mutants by generating fusion constructs of MR or MR1441 with ubiquitin (MR Ubi and MR1441 Ubi) (Fig. S4A), which mimics monoubiquitinated proteins (21, 22). MR Ubi and MR1441 Ubi resemble monoubiquitinated MR, but both constructs can be further (poly-) ubiquitinated in a lysin48-linked manner at either lysine1441 (for MR Ubi) or lysine48 of ubiquitin (for both MR Ubi and MR1441 Ubi). In addition, we constructed fusion constructs with a ubiquitin mutant in which lysine48 is replaced by arginine (MR Ubi48 and MR1441 Ubi48) (Fig. S4A). In contrast to MR Ubi48, which still has the capacity to become (poly-)ubiquitinated at lysine1441 of the MR, MR1441 Ubi48 is a monoubiquitinated form of the MR that cannot be polyubiquitinated in a lysin48-linked manner, because both ubiquitin acceptor sites in the MR (lysine1441) and in ubiquitin (lysine48) are replaced.
We found that expression of MR–ubiquitin fusion constructs did not alter intracellular antigen distribution (Fig. S4B), and that expression of all constructs resulted in similar levels of OVA internalization (Fig. S5A and B). However, total uptake was slightly reduced compared with WT MR (Fig. S5A), probably due to reduced stability at the cell membrane of the MR by the attached ubiquitin, which might serve as an additional internalization signal (23, 24).
We next analyzed the influence of these mutants on cross-presentation. Expression of all constructs that bear an acceptor site for lysin48-linked polyubiquitination (MR Ubi, MR1441 Ubi, and MR Ubi48) did not alter cross-presentation compared with WT MR (Fig. 3A). Importantly, after expression of MR1441 Ubi48 (the MR mutant that cannot be polyubiquitinated), cross-presentation was impaired and reached levels similar to that in DCs expressing the ubiquitination-deficient mutant MR1441 (Fig. 3A; transfection controls in Fig. S5C).
Fig. 3.
MR polyubiquitination is essential for OVA translocation and cross-presentation. (A) DC2.4 cells were transfected with plasmids encoding the MR mutants as indicated. At 48 after electroporation, cells were treated with OVA for 2 h and cocultured with OT-I cells. T-cell activation was determined based on IL-2 secretion after 18 h by ELISA. Data are presented as mean ± SEM. (B) DC2.4 cells were transfected with expression plasmids of the MR mutants as indicated. After 48 h, cells were incubated with biotinylated OVA in the presence of the proteasome inhibitor MG132 for 15 min and chased with medium containing MG132 for another 45 min. Then cytoplasmic fractions were isolated, and the amount of OVA was analyzed after streptavidin-based affinity chromatography by Western blot analysis. All graphs depict representative results of at least three independent experiments.
To analyze whether this impaired cross-presentation is due to changes in antigen translocation, we analyzed the cytosolic OVA content. Consistent with the effect on cross-presentation, OVA transport into the cytoplasm was undetectable after expression of the polyubiquitination-deficient mutant MR1441 Ubi48 (Fig. 3B). These results clearly demonstrate a central role of lysin48-linked polyubiquitination of the MR in antigen translocation and exclude the possibility that the lysine1441 mutation exerted its effect by conformational changes distinct from ubiquitination, because the linkage of this mutant to WT ubiquitin, but not to Ubi48, restored its effects.
Regulation of MR Ubiquitination by Tumor Susceptibility Gene 101.
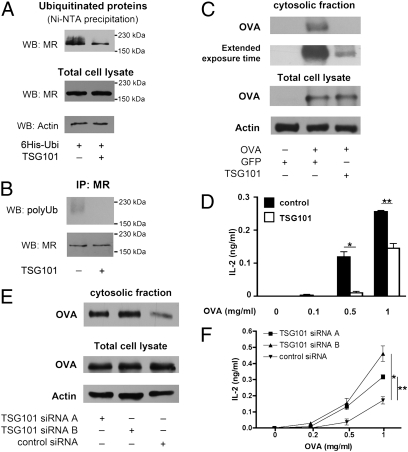
Because polyubiquitination of the MR is essential for antigen transport into the cytoplasm, but ubiquitination at the endosomal membrane is a very tightly regulated process (17, 25), we aspired to elucidate the control mechanisms of such ubiquitination. Of special interest in this context is a protein termed tumor susceptibility gene 101 (TSG101), a dominant negative regulator of polyubiquitination (26, 27) that colocalizes with MR-endocytosed OVA (28). To analyze whether TSG101 influences MR ubiquitination, we cotransfected BM-DCs with His-tagged ubiquitin and TSG101, and found that this did not influence either antigen uptake (Fig. S6A) or intracellular distribution (Fig. S6B). Subsequent Western blot analysis revealed that overexpression of TSG101 clearly decreased the amount of polyubiquitinated MR (Fig. 4 A and B). In addition, we analyzed the effect of TSG101 on antigen translocation and on cross-presentation. Consistent with its role in preventing MR polyubiquitination, overexpression of TSG101 impaired antigen translocation (Fig. 4C) and cross-presentation (Fig. 4D).
Fig. 4.
TSG101 is a regulator of MR ubiquitination and of cross-presentation. (A) BM-DCs were transfected with mRNA encoding 6× His-tagged ubiquitin and TSG101. At 1 h after electroporation, cells were incubated with OVA. After another 60 min, cells were lysed, and ubiquitinated proteins were isolated by Ni-NTA–based affinity chromatography. Ubiquitination of the MR was analyzed by Western blot analysis using a MR-specific antibody. (B) BM-DCs were transfected with mRNA encoding TSG101 and incubated with OVA as in A. Subsequently, immune precipitation using a MR-specific antibody was performed, and polyubiquitinated MR was detected by Western blot analysis using the FK1 antibody. (C) BM-DCs were transfected with mRNA encoding TSG101 or GFP. At 1 h after electroporation, cells were incubated with biotinylated OVA in the presence of the proteasome inhibitor MG132 for 15 min and chased with medium containing MG132 for another 45 min. Then cytoplasmic fractions were isolated, and the amount of OVA was analyzed after streptavidin-based affinity chromatography by Western blot analysis. (D) BM-DCs were transfected with mRNA encoding TSG101 or GFP (control), incubated with OVA for 2 h, and cocultured with OT-I cells. IL-2 concentrations in the supernatant after 18 h were determined by ELISA. Data are presented as mean ± SEM. (E) BM-DCs were treated with TSG101-specific or control siRNAs. After 3 d, cells were treated with biotinylated OVA in the presence of the proteasome inhibitor MG132. The amount of cytoplasmic OVA was analyzed as described above. (F) siRNA-treated BM-DCs were incubated with OVA for 2 h and cocultured with OT-I cells for another 18 h. IL-2 concentrations in the supernatant were determined by ELISA. Data are presented as mean ± SEM. All graphs depict representative results of at least three independent experiments. *P < 0.05; **P < 0.01.
To further support a regulatory function of TSG101 in cross-presentation, we down-regulated its expression by siRNA (Fig. S7A), and found that it did not influence either antigen internalization (Fig. S7B) or its intracellular distribution (Fig. S7C). In agreement with the results of our overexpression experiments, down-regulation of TSG101 increased OVA translocation (Fig. 4E) and cross-presentation (Fig. 4F), supporting its central role in regulating MR polyubiquitination.
Recruitment of p97 to OVA-Containing Endosomes by Polyubiquitinated MR.
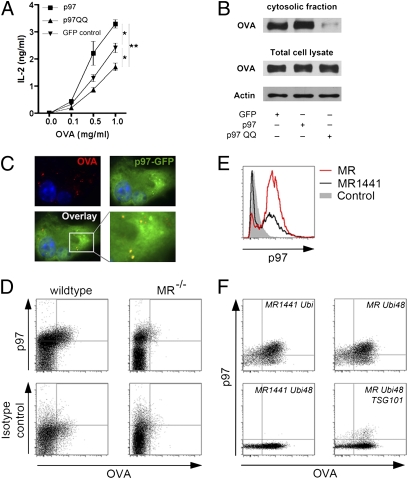
The necessity of MR polyubiquitination for endosomal antigen export suggests a putative role of this process in the recruitment of p97, a member of the ER-associated degradation machinery, which is recruited to the ER membrane by polyubiquitinated proteins during dislocation of ER proteins into the cytosol (12, 13) and also been proposed to play a role in cross-presentation (10, 29). To investigate this hypothesis, we first analyzed the effect of p97 on cross-presentation of MR-endocytosed OVA. We transfected BM-DCs with WT p97 or a dominant negative mutant (p97-QQ), in which the glutamin residues 305 and 578 were replaced by glutamate, resulting in an impaired ATP hydrolysis capacity (30). Cross-presentation was increased after overexpression of WT p97, but impaired after expression of the dominant negative mutant (Fig. 5A), confirming the essential role of p97 in cross-presentation. We determined that this effect of p97 on cross-presentation is indeed due to an altered endosomal antigen export, because expression of p97-QQ clearly decreased the amount of cytosolic OVA (Fig. 5B).
Fig. 5.
Recruitment of p97 to OVA-containing endosomes by polyubiquitinated MR is essential for cross-presentation. (A) BM-DCs were transfected with mRNA encoding p97, p97QQ, or GFP (control). At 1 h after electroporation, cells were incubated with OVA for 2 h and then cocultured with OT-I cells for another 18 h. T-cell activation was analyzed by ELISA. Data are presented as mean ± SEM. (B) BM-DCs were transfected with mRNA encoding p97, p97QQ, or GFP. At 1 h after electroporation, cells were incubated with biotinylated OVA in the presence of the proteasome inhibitor MG132 for 15 min, and then chased with medium containing MG132 for another 45 min. Afterward, cytoplasmic fractions were isolated, and the amount of OVA was analyzed after streptavidin-based affinity chromatography by Western blot analysis. (C) GFP-p97 was expressed in BM-DCs by lentiviral gene transduction. After incubation with fluorochrome-labeled OVA for 15 min, cells were chased with medium for 30 min, and the intracellular localization of p97 was determined by fluorescence microscopy. Nuclear staining by DAPI is depicted in blue. Histograms are depicted in Fig. S8. (D) Endosomal fractions of OVA-treated WT or MR-deficient BM-DCs were isolated, stained using a p97-specific antibody, and analyzed by flow cytometry. (E) p97 staining of isolated endosomes. DC2.4 cells were transfected with expression plasmids of MR or MR1441 and incubated with OVA. After cell lysis, endosomal fractions were isolated, stained with a p97-specific antibody, and analyzed by flow cytometry. (F) p97 staining of isolated endosomes. DC2.4 cells were transfected as indicated and incubated with fluorescent-labeled OVA. Staining of p97 on isolated endosomes was analyzed by flow cytometry. Gray curves depict isotype control stainings. All graphs depict representative results of at least two independent experiments. *P < 0.05; **P < 0.01.
We also analyzed the intracellular distribution of p97 in BM-DCs by immunofluorescence. Apart from the cytosolic distribution of p97 described previously by others (31), we found a clear colocalization of p97 with OVA-containing endosomes (Fig. 5C; histograms in Fig. S8). To monitor the recruitment of p97 to OVA-containing endosomes more accurately, we developed a method to stain isolated endosomes with specific antibodies and analyze their protein content by flow cytometry. After treatment of BM-DCs with fluorescently labeled OVA, we detected OVA-containing endosomes in the endosomal fraction of WT, but not of MR-deficient, BM-DCs (Fig. S9A). Comparison with calibrated beads revealed an endosomal size of ∼500 nm (Fig. S9B), and staining with specific antibodies against the endosomal markers EEA1 and MR demonstrated that these markers were expressed on nearly all OVA-containing organelles (Fig. S9 C and D). We next stained the isolated endosomes using a p97-specific antibody. Importantly, we detected p97 expression on nearly all OVA-containing endosomes (Fig. 5D), confirming the observed colocalization between OVA and p97 in our immunofluorescence experiments (Fig. 5C). To unequivocally investigate the role of MR ubiquitination in the recruitment of p97 toward OVA-containing endosomes, we transfected DC2.4 cells with WT MR or the ubiquitination-deficient mutant MR1441 and analyzed the expression of p97 on isolated endosomes by flow cytometry. Importantly, after expression of MR1441, the recruitment of p97 toward OVA-containing endosomes was severely reduced (Fig. 5E), verifying the need for MR ubiquitination in the recruitment of p97 toward endosomes. To finally prove that this recruitment is mediated by polyubiquitinated MR, we transfected DCs with the aforementioned MR mutants (Fig. S4A). After expression of MR1441 Ubi or MR Ubi48, both of which have the capacity to become polyubiquitinated, p97 recruitment toward the isolated endosomes was still observed (Fig. 5F). However, after expression of the polyubiquitination-deficient mutant MR1441 Ubi48, no endosomal p97 could be detected, confirming the need for MR polyubiquitination in the recruitment of p97 toward endosomes. In agreement with these observations, p97 recruitment was severely impaired after overexpression of TSG101 (Fig. 5F), which prevents MR polyubiquitination.
Discussion
For cross-presentation, internalized antigens must be exported out of endosomal structures to become degraded by the cytosolic proteasome. In this study, we have demonstrated that polyubiquitination of the MR is essential for such export of MR-internalized OVA. Furthermore, we have identified TSG101 as a key regulator of MR ubiquitination and hence of cross-presentation. TSG101 contains a region with high homology to ubiquitin-conjugating enzymes (E2). Importantly, TSG101 lacks the active cysteine residue, which in E2 enzymes is responsible for covalent binding to ubiquitin. Thus, TSG101 cannot function as a proper E2 enzyme, but instead has been postulated to bind ubiquitin residues of monoubiquitinated proteins, thereby protecting the monoubiquitin residue from further ubiquitination (8, 26, 27). In this study, we have demonstrated that TSG101 decreases the amount of polyubiquitinated MR. Whether this decrease is due to the protection of monoubiquitinated MR from further ubiquitination or by other mechanisms, such as the recruitment of deubiquitinating enzymes, remains to be resolved.
Importantly, we have demonstrated that MR ubiquitination is required for the recruitment of p97 toward the endosomal membrane. During dislocation, p97 is recruited toward the ER membrane by polyubiquitinated proteins either by direct interaction with the polyubiquitin tails or by the adaptor molecules Npl4 and Ufd1 (32), which have a high affinity for both polyubiquitinated proteins and p97. In this study, we found that ubiquitination of the MR recruits p97 toward the endosomal membrane. Whether the recruitment of p97 by polyubiquitinated MR is due to a direct interaction of the polyubiquitin tail with p97 or is mediated by adapter proteins like Npl4 and Ufd1 remains to be identified.
We also found p97 recruitment by flow cytometric analysis of endosomal fractions, a technique that allowed us to analyze the protein content of single endosomes, which cannot be achieved by bulk analysis of endosomes isolated by traditional subcellular fractionation techniques. Because such analysis is based on differences in fluorescence, this method also can be used to study differences between endosomal subsets with similar density.
Exploring the mechanisms of antigen transport from endosomal compartments into the cytosol raises questions regarding the translocation pore complex. Sec61 and Derlin-1 have been shown to be involved in the building of such a pore complex in the ER (31, 33). Moreover, Sec61 involvement in antigen translocation for cross-presentation has been proposed (10, 34), and expression of Sec61 in endosomal compartments has been demonstrated (35, 36). Because p97 associates with both Sec61 and Derlin-1 (10, 31), a central role for p97 in cross-presentation would support a potential role of these proteins in cross-presentation. In a previous study, we demonstrated that the recruitment of other members of the MHC I loading machinery from the ER toward endosomes is regulated by the presence of endotoxins (15). It is conceivable that Sec61 and Derlin-1 are recruited toward the endosomes in a similar fashion. More experimental evidence is needed to unequivocally determine the composition of the pore complex and to analyze a putative role of Sec61 and Derlin-1 within this complex.
Materials and Methods
Antibodies.
The primary antibodies used were goat anti-MR (C-20; Santa Cruz Biotechnology) for endosomal stainings, rat anti-MR (MR5D3; AbD Serotec) for Western blot and immunoprecipitation, rabbit anti-actin (20-33; Sigma-Aldrich), mouse anti-p97 and rat anti-LAMP-1 (18/VCP and 1D4B; BD), rabbit anti-EEA1 (PA1-063; Dianova), mouse anti-TSG101 (C-2; Santa Cruz Biotechnology), mouse anti-TrfR (H68.4; Zymed), and mouse anti-ubiquitin (FK1; Biomol). All secondary antibodies were purchased from Invitrogen.
Generation of BM-DCs.
BM-DCs were generated using a GM-CSF–producing cell line as described previously (4, 15).
Cross-Linking of OVA and Trf.
Covalent conjugation using heterobifunctional cross-linkers was performed as described previously (15). After coupling, free OVA was removed by extensive ultrafiltration using vivaspin columns. For the generation of fluorochrome-labeled OVA-Trf, Alexa Fluor 647–coupled OVA was linked to Trf.
Transfection of Dendritic Cells.
All electroporations were performed using a Gene Pulser X Cell electroporator (Biorad). DC2.4 cells were transfected with with 20 μg of DNA using an exponential pulse of 260 V and 960 μF. After incubation for 48 h, cells were used for experiments. BM-DCs were transfected with mRNA generated using the mMessage mMachine Kit (Ambion). For all experiments, BM-DCs were electroporated with 30 μg of mRNA using a square wave pulse of 300 V for 6 ms. Cells were used for experiments at 1 h after electroporation. For down-regulation of TSG101, cells were electroporated with 10 μg of TSG101-specific siRNA (siRNA A: CGUAAACAGUUCCAGCUAAUU; siRNA B: GUACAAUCCCAGUGCGUUAUU) or control siRNA (AAAAACAUGCAGAAAUGCUGU; contains a specific sequence of the luciferase gene) using a double square wave pulse of 1,000 V 0.5 ms and a 5-s pulse interval as described previously (37). Cells were used for experiments at 3 d after electroporation.
Cross-Presentation Assays.
Cross-presentation assays were performed as described previously (4, 15). In brief, cells were plated and incubated for 2 h with increasing amounts of OVA or OVA-Trf. Because cross-presentation in most experiments was analyzed after DC transfection and, especially after transfection with mRNA, expression of foreign proteins is limited to a few hours after transfection, cells were fixed before the addition of T cells. Because such fixation reduces overall T-cell activation, increased OVA concentrations were used. After fixation, DCs were cocultured with OT-I cells, and IL-2 concentrations in the supernatant were detected after 18 h by ELISA.
Isolation and Analysis of Cytoplasmic Fraction of Cell Lysates.
For analysis of the OVA content in the cytosolic fraction, DCs were incubated for 15 min with biotinylated OVA or OVA-Trf (0.5 mg/mL) in the presence of 5 μM MG132. Subsequently, the medium was replaced with medium containing MG132, but not OVA, for another 45 min. Then the cells were harvested, and 10% of the cells were collected in lysis buffer (20 mM NaPO4, 140 mM NaCl, 3 mM MgCl2 and 0.5% Nonidet P-40; pH 8) to build the whole-cell lysate fraction. From the remaining 90% of the cells, the cytoplasmic fraction was isolated as described previously (15). Cytoplasmic OVA was enriched using streptavidin-based affinity chromatography and analyzed by Western blot analysis. Band intensities were quantified using a Gel-Pro Analyzer (MediaCybernetics).
Analysis of the MR Ubiquitination State.
Ubiquitination assays were performed as described previously (15, 28). In brief, cells were transfected with 6× histidine-tagged ubiquitin and incubated with OVA (0.5 mg/mL) and 5 μM MG132. After 1 h, cells were harvested, and 10% of the cells were collected in lysis buffer (20 mM NaPO4, 140 mM NaCl, 3 mM MgCl2, and 0.5% Nonidet P-40; pH 8) to build the whole-cell lysate fraction. The remaining 90% of the cells were lysed under denaturing conditions (8 M urea, 100 mM NaH2PO4, 10 mM Tris-HCl, 20 mM imidazole, 0.5% Nonidet P-40; pH 8) to prevent rapid degradation of ubiquitinated proteins. From these samples, ubiquitinated proteins were isolated by affinity chromatography. For this, Ni-NTA beads were added to the samples. After a 1-h incubation, beads were washed extensively to remove unbound proteins, and ubiquitinated proteins were eluted in 250 mM Tris-HCl and 6.6% SDS (pH 6.8) at 60 °C. Ubiquitinated MR was detected by Western blot analysis using a MR-specific antibody.
For immune precipitation experiments, transfected DCs were incubated with OVA (0.5 mg/mL) and 5 μM MG132. After 1 h, cells were harvested in 10 mM Tris-HCl, 2% Triton X-100, 150 mM NaCl, and 2 mM EDTA (pH 8.2). Then 10% of the lysate was collected to build the whole-cell lysate fraction. The remaining 90% was incubated with a MR-specific antibody for 1 h and with protein G–Sepharose beads (Thermo Scientific) for another 1 h. Then the beads were washed extensively, and bound proteins were eluted in 250 mM Tris-HCl and 6.6% SDS (pH 6.8) at 60 °C. Polyubiquitination of the MR was detected by Western blot analysis using the ubiquitin-specific FK1 antibody.
Immunofluorescence Microscopy.
Cells were pulsed for 15 min with 2 μg/mL of fluorochrome-labeled antigen and chased for another 30 min with medium. Staining experiments were performed as described previously (4). Nuclei were visualized with 1 μg/mL of the DNA-intercalating dye DAPI. Cells were analyzed with an ApoTome microscope (Zeiss). Colocalization was quantified using ImageJ software.
Staining of Endosomal Preparations.
DCs incubated with fluorochrome-labeled OVA were harvested, collected in homogenization buffer [25 mM 2-(N-morpholino)-ethanesulfonic acid, 150 mM NaCl, and 1 mM PMSF; pH 6.5] and disrupted using a stainless Dounce Homogenizator (Grinder). Cellular debris was removed by repetitive centrifugation at 2,000× g. The endosomal population was isolated by centrifugation at 20,000× g, stained with a p97-specific antibody, and analyzed by flow cytometry.
Supplementary Material
Acknowledgments
We thank Professsor Joachim Schultze, Dr. Marc Beyer, the staff of the flow cytometry core facility at the Institute of Molecular Medicine, and the staff of the animal facility at the House for Experimental Therapy for their technical support. This work was supported by German Research Foundation Grant BU2441/1-1.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1102397108/-/DCSupplemental.
References
- 1.Mellman I, Steinman RM. Dendritic cells: Specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 2.Trombetta ES, Ebersold M, Garrett W, Pypaert M, Mellman I. Activation of lysosomal function during dendritic cell maturation. Science. 2003;299:1400–1403. doi: 10.1126/science.1080106. [DOI] [PubMed] [Google Scholar]
- 3.Kurts C, et al. Constitutive class I–restricted exogenous presentation of self antigens in vivo. J Exp Med. 1996;184:923–930. doi: 10.1084/jem.184.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burgdorf S, Kautz A, Böhnert V, Knolle PA, Kurts C. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science. 2007;316:612–616. doi: 10.1126/science.1137971. [DOI] [PubMed] [Google Scholar]
- 5.Norbury CC, Hewlett LJ, Prescott AR, Shastri N, Watts C. Class I MHC presentation of exogenous soluble antigen via macropinocytosis in bone marrow macrophages. Immunity. 1995;3:783–791. doi: 10.1016/1074-7613(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 6.Kovacsovics-Bankowski M, Rock KL. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;267:243–246. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- 7.Ackerman AL, Kyritsis C, Tampé R, Cresswell P. Early phagosomes in dendritic cells form a cellular compartment sufficient for cross-presentation of exogenous antigens. Proc Natl Acad Sci USA. 2003;100:12889–12894. doi: 10.1073/pnas.1735556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burgdorf S, Lukacs-Kornek V, Kurts C. The mannose receptor mediates uptake of soluble but not of cell-associated antigen for cross-presentation. J Immunol. 2006;176:6770–6776. doi: 10.4049/jimmunol.176.11.6770. [DOI] [PubMed] [Google Scholar]
- 9.Imai J, Hasegawa H, Maruya M, Koyasu S, Yahara I. Exogenous antigens are processed through the endoplasmic reticulum–associated degradation (ERAD) in cross-presentation by dendritic cells. Int Immunol. 2005;17:45–53. doi: 10.1093/intimm/dxh184. [DOI] [PubMed] [Google Scholar]
- 10.Ackerman AL, Giodini A, Cresswell P. A role for the endoplasmic reticulum protein retrotranslocation machinery during cross-presentation by dendritic cells. Immunity. 2006;25:607–617. doi: 10.1016/j.immuni.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 11.Jarosch E, Geiss-Friedlander R, Meusser B, Walter J, Sommer T. Protein dislocation from the endoplasmic reticulum—pulling out the suspect. Traffic. 2002;3:530–536. doi: 10.1034/j.1600-0854.2002.30803.x. [DOI] [PubMed] [Google Scholar]
- 12.Ye Y, Meyer HH, Rapoport TA. Function of the p97-Ufd1-Npl4 complex in retrotranslocation from the ER to the cytosol: Dual recognition of nonubiquitinated polypeptide segments and polyubiquitin chains. J Cell Biol. 2003;162:71–84. doi: 10.1083/jcb.200302169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai RM, Li CC. Valosin-containing protein is a multi-ubiquitin chain-targeting factor required in ubiquitin-proteasome degradation. Nat Cell Biol. 2001;3:740–744. doi: 10.1038/35087056. [DOI] [PubMed] [Google Scholar]
- 14.de Virgilio M, Weninger H, Ivessa NE. Ubiquitination is required for the retro-translocation of a short-lived luminal endoplasmic reticulum glycoprotein to the cytosol for degradation by the proteasome. J Biol Chem. 1998;273:9734–9743. doi: 10.1074/jbc.273.16.9734. [DOI] [PubMed] [Google Scholar]
- 15.Burgdorf S, Schölz C, Kautz A, Tampé R, Kurts C. Spatial and mechanistic separation of cross-presentation and endogenous antigen presentation. Nat Immunol. 2008;9:558–566. doi: 10.1038/ni.1601. [DOI] [PubMed] [Google Scholar]
- 16.Strous GJ, Govers R. The ubiquitin-proteasome system and endocytosis. J Cell Sci. 1999;112:1417–1423. doi: 10.1242/jcs.112.10.1417. [DOI] [PubMed] [Google Scholar]
- 17.Dupré S, Volland C, Haguenauer-Tsapis R. Membrane transport: Ubiquitylation in endosomal sorting. Curr Biol. 2001;11:R932–R934. doi: 10.1016/s0960-9822(01)00558-9. [DOI] [PubMed] [Google Scholar]
- 18.East L, Isacke CM. The mannose receptor family. Biochim Biophys Acta. 2002;1572:364–386. doi: 10.1016/s0304-4165(02)00319-7. [DOI] [PubMed] [Google Scholar]
- 19.Lee YR, et al. Cyclosporin A and tacrolimus, but not rapamycin, inhibit MHC-restricted antigen presentation pathways in dendritic cells. Blood. 2005;105:3951–3955. doi: 10.1182/blood-2004-10-3927. [DOI] [PubMed] [Google Scholar]
- 20.Shakushiro K, Yamasaki Y, Nishikawa M, Takakura Y. Efficient scavenger receptor-mediated uptake and cross-presentation of negatively charged soluble antigens by dendritic cells. Immunology. 2004;112:211–218. doi: 10.1111/j.1365-2567.2004.01871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salghetti SE, Caudy AA, Chenoweth JG, Tansey WP. Regulation of transcriptional activation domain function by ubiquitin. Science. 2001;293:1651–1653. doi: 10.1126/science.1062079. [DOI] [PubMed] [Google Scholar]
- 22.Sharma M, et al. Misfolding diverts CFTR from recycling to degradation: Quality control at early endosomes. J Cell Biol. 2004;164:923–933. doi: 10.1083/jcb.200312018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shih SC, Sloper-Mould KE, Hicke L. Monoubiquitin carries a novel internalization signal that is appended to activated receptors. EMBO J. 2000;19:187–198. doi: 10.1093/emboj/19.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hicke L, Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu Rev Cell Dev Biol. 2003;19:141–172. doi: 10.1146/annurev.cellbio.19.110701.154617. [DOI] [PubMed] [Google Scholar]
- 25.Katzmann DJ, Odorizzi G, Emr SD. Receptor down-regulation and multivesicular-body sorting. Nat Rev Mol Cell Biol. 2002;3:893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- 26.Koonin EV, Abagyan RA. TSG101 may be the prototype of a class of dominant-negative ubiquitin regulators. Nat Genet. 1997;16:330–331. doi: 10.1038/ng0897-330. [DOI] [PubMed] [Google Scholar]
- 27.Ponting CP, Cai YD, Bork P. The breast cancer gene product TSG101: A regulator of ubiquitination? J Mol Med. 1997;75:467–469. [PubMed] [Google Scholar]
- 28.Burgdorf S, Leister P, Scheidtmann KH. TSG101 interacts with apoptosis-antagonizing transcription factor and enhances androgen receptor-mediated transcription by promoting its monoubiquitination. J Biol Chem. 2004;279:17524–17534. doi: 10.1074/jbc.M313703200. [DOI] [PubMed] [Google Scholar]
- 29.Giodini A, Rahner C, Cresswell P. Receptor-mediated phagocytosis elicits cross-presentation in nonprofessional antigen-presenting cells. Proc Natl Acad Sci USA. 2009;106:3324–3329. doi: 10.1073/pnas.0813305106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ye Y, Meyer HH, Rapoport TA. The AAA ATPase Cdc48/p97 and its partners transport proteins from the ER into the cytosol. Nature. 2001;414:652–656. doi: 10.1038/414652a. [DOI] [PubMed] [Google Scholar]
- 31.Ye Y, Shibata Y, Yun C, Ron D, Rapoport TA. A membrane protein complex mediates retro-translocation from the ER lumen into the cytosol. Nature. 2004;429:841–847. doi: 10.1038/nature02656. [DOI] [PubMed] [Google Scholar]
- 32.Jarosch E, et al. Protein dislocation from the ER requires polyubiquitination and the AAA-ATPase Cdc48. Nat Cell Biol. 2002;4:134–139. doi: 10.1038/ncb746. [DOI] [PubMed] [Google Scholar]
- 33.Lilley BN, Ploegh HL. A membrane protein required for dislocation of misfolded proteins from the ER. Nature. 2004;429:834–840. doi: 10.1038/nature02592. [DOI] [PubMed] [Google Scholar]
- 34.Giodini A, Cresswell P. Hsp90-mediated cytosolic refolding of exogenous proteins internalized by dendritic cells. EMBO J. 2008;27:201–211. doi: 10.1038/sj.emboj.7601941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Houde M, et al. Phagosomes are competent organelles for antigen cross-presentation. Nature. 2003;425:402–406. doi: 10.1038/nature01912. [DOI] [PubMed] [Google Scholar]
- 36.Guermonprez P, et al. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature. 2003;425:397–402. doi: 10.1038/nature01911. [DOI] [PubMed] [Google Scholar]
- 37.Quast T, et al. Cytohesin-1 controls the activation of RhoA and modulates integrin-dependent adhesion and migration of dendritic cells. Blood. 2009;113:5801–5810. doi: 10.1182/blood-2008-08-176123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.