Abstract
The nucleus accumbens (NAc) serves as a key neural substrate that controls acute and adaptive behavioral responses to cocaine administration. In this circuit, inputs from the NAc are transmitted through two parallel pathways, named the direct and indirect pathways, and converge at the substantia nigra pars reticulata (SNr). Our previous study using reversible neurotransmission blocking (RNB) of each pathway revealed that the dual stimulation of the SNr by both pathways is necessary for the acute response, but that the direct pathway predominantly controls the adaptive response to repeated cocaine administration. This study aimed at exploring the pathway-specific mechanism of cocaine actions at the convergent SNr. We examined a genome-wide expression profile of the SNr of three types of experimental mice: the direct pathway-blocked D-RNB mice, the indirect pathway-blocked I-RNB mice, and wild-type mice. We identified the up-regulation of ephrinA5, EphA4, and EphA5 specific to D-RNB mice during both acute and adaptive responses to cocaine administration. The activation by EphA4 and EphA5 in the SNr of wild-type mice by use of the immunoadhesin technique suppressed the adaptive response to repeated cocaine administration. Furthermore, cocaine exposure stimulated the phosphorylation of Erk1/2 in ephrinA5-expressing SNr cells in a direct pathway-dependent manner. The results have demonstrated that the ephrinA5-EphA4/EphA5 system plays an important role in the direct pathway-dependent regulation of the SNr in both acute and adaptive cocaine responses and would provide valuable therapeutic targets of cocaine addiction.
Keywords: basal ganglia, drug addiction, Eph-ephrin signaling, gene regulation, transmission blocking
The basal ganglia are the key neural substrates that control motor balance and reward-based and aversive learning (1, 2). Dysfunction of the basal ganglia leads to devastating neurological disorders, such as Parkinson disease and drug addiction (3–5). The projection neurons in the striatum and the nucleus accumbens (NAc), the ventral part of the striatum, are GABA-containing medium-sized spiny neurons, which are divided into two subpopulations: striatonigral neurons in the direct pathway and striatopallidal neurons in the indirect pathway (1, 3, 6). The inputs of these two pathways converge at the substantia nigra pars reticulata (SNr) and control the dynamic balance of the basal ganglia-thalamocortical circuitry (1, 7). Cocaine and other psychostimulants massively increase dopamine levels in the NAc and the striatum and induce abnormal behavioral responses both acutely and chronically (8). We previously developed a gene-manipulating technique that allows separate and reversible neurotransmission blocking (RNB) of the direct pathway (D-RNB mice) and the indirect pathway (I-RNB mice) in vivo (9). The use of this technique revealed the distinct regulatory function of the two pathways in acute and chronic responses to cocaine exposure (9). Blockade of the direct pathway abrogates the acute response and then markedly attenuates the chronic response to cocaine administration. In contrast, blockade of the indirect pathway abolishes the acute response as well; but the ability to induce normal levels of the chronic response after repeated cocaine administration is retained. The two pathways are thus necessary for the acute cocaine response but the direct pathway plays a predominant role in the adaptive response to repeated cocaine administration (9). However, the molecular and signaling mechanisms that underlie these different adaptive reactions by the two pathways remain to be clarified.
The SNr is composed mostly of GABAergic projection neurons and serves as a main target nucleus that receives GABAergic inputs from the direct pathway and both GABAergic and glutamatergic inputs from the indirect pathway (1, 7). In this study, we investigated what signaling molecules are involved in the pathway-dependent regulation of the SNr after cocaine administration. To address this question, we examined a genome-wide expression profile of the SNr of the D-RNB, I-RNB, and WT mice by using microarray and quantitative RT-PCR techniques. We identified the specific up-regulation of ephrinA5, EphA4, and EphA5 in the D-RNB mice after cocaine administration. We also revealed the inhibitory role and downstream signaling of the ephrinA5-EphA4/EphA5 system in cocaine-induced behaviors. This study has thus disclosed an important mechanism of the pathway-specific regulation of cocaine actions in the basal ganglia circuitry and would provide valuable therapeutic targets of drug addiction.
Results
Profiling of Gene Expression of the SNr in the D-RNB Mice After Cocaine Administration.
In this study, we used previously developed RNB transgenic mice, in which the tetanus toxin light chain (TN) is restrictedly expressed in cells of either the direct or the indirect pathway (9). TN is a bacterial toxin that cleaves the synaptic vesicle-associated membrane protein-2 and thus blocks transmitter release from the synaptic vesicles. In RNB mice, the expression of TN is controlled by the tetracycline-responsive element (TRE) and thus driven by its interaction with the tetracycline-repressive transcription factor (tTA) in a tetracycline-derivative doxycycline-regulated manner. The restricted expression of tTA in either pathway is achieved by using the adeno-associated virus (AAV)-mediated gene-expression system, in which the expression of tTA is directed by the substance P promoter or the enkephalin promoter. Recombinant AAVs were bilaterally injected into the NAc, and 2 wk after the viral injection, locomotor activity was measured for 10 min immediately after cocaine (10 mg/kg) or saline administration. Both D-RNB and I-RNB mice failed to show acute hyperlocomotion after cocaine administration (9).
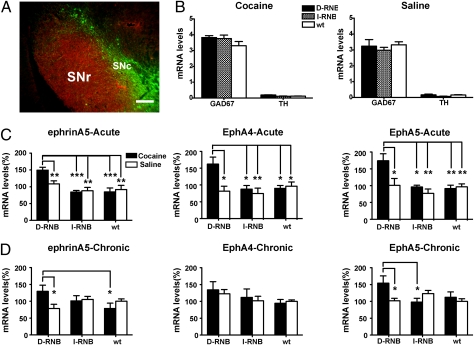
The SNr, which is a main target nucleus of the direct and indirect pathways, is rich in glutamic acid decarboxylase67 (GAD67)-immunoreactive cells and is located adjacent to the substantia nigra pars compacta (SNc), which is characterized by a high density of tyrosine hydroxylase (TH) immunoreactivity (Fig. 1A) (10, 11). The SNr and the SNc could thus be easily separated and dissected by the characteristic architecture and cell shapes of these nuclei. We performed quantitative RT-PCR of dissected SNrs and confirmed that the SNrs used exhibited a high level of the GAD67 mRNA and a minimal contamination of the TH mRNA from the SNc (Fig. 1B). Furthermore, there was no difference in expression levels of the GAD67 mRNA among the D-RNB, I-RNB, and WT mice, regardless of whether the animals were treated or not with cocaine (Fig. 1B).
Fig. 1.
Direct pathway-specific regulation of expression of ephrinA5, EphA4, and EphA5 mRNAs in the SNr by cocaine administration. (A) Double immunostaining of coronal sections of the SNr and SNc of WT mice with the GAD67 antibody and the TH antibody. A merged view showed that the GAD67-immunoreactive SNr (red) is located adjacent to the TH-immunoreactive SNc (green). (Scale bar, 100 μm.) (B) D-RNB, I-RNB, and WT mice were prepared by bilateral injection of the AAVs into the NAc. Two weeks after the viral injection, the animals received a single intraperitoneal injection of either cocaine (10 mg/kg) or saline and were killed 1 h later. The SNr was then isolated, and the levels of the GAD67 and TH mRNAs were quantified by RT-PCR. mRNA levels were normalized by referring to that level of the β-actin mRNA as 1 (n = 6 for D-RNB and I-RNB; n = 12 for WT). (C) The SNr was isolated as in B, and mRNA levels were quantified by PCR (n = 6 for D-RNB and I-RNB; n = 12 for WT). (D) Experiments were performed as in B, except that the animals daily received a single intraperitoneal injection of cocaine (10 mg/kg) or saline for 5 d and the SNr was then isolated 1 h after the last intraperitoneal injection (n = 6 each). In C and D, levels of each mRNA were expressed by referring to those of the corresponding mRNA in saline-injected WT mice. Columns and bars represent the mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001.
After confirmation of the lack of cocaine-induced hyperlocomotion in individual D-RNB and I-RNB mice, SNrs were mechanically isolated from D-RNB, I-RNB, and WT mice 1 h after cocaine or saline administration. Total RNA was extracted from microdissected SNrs and subjected to microarray analysis. As criteria for the selection of candidate genes, we used hybridization signals of >150 at least in one of the three types of the experimental animals and more than 1.4-fold changes between cocaine and saline treatments in either D-RNB or I-RNB mice, but not in the WT mice. Candidate genes thus selected were further confirmed by quantitative RT-PCR analysis. Among a few candidate genes, we focused on and analyzed in detail the ephrinA5, EphA4, and EphA5 mRNAs, all of which were up-regulated in the SNr of only the D-RNB mice (Fig. 1C) (one-way ANOVA analysis for ephrinA5, P < 0.001–0.01; for EphA4, P < 0.01–0.05; for EphA5, P < 0.01–0.05). The up-regulation of these mRNAs was not only specific to D-RNB mice after cocaine administration but in addition, the expression of these mRNAs was not altered in saline-treated RNB or WT mice (Fig. 1 C and D), indicating that the up-regulation of these mRNAs depended on both blockade of the direct pathway and cocaine administration.
Because the previous blockade study indicated a key role of the direct pathway in the adaptive response to repeated cocaine administration (9), we next addressed whether the ephrinA5, EphA4, and EphA5 mRNAs remained up-regulated in the SNr of D-RNB mice after repeated administration of cocaine. The SNr was isolated and microdissected after repeated cocaine administration for 5 d. Quantitative RT-PCR showed that the ephrinA5 and EphA5 mRNAs remained up-regulated in the SNr of only the D-RNB mice (Fig. 1D) (one-way ANOVA analysis for ephrinA5, P < 0.05; for EphA5, P < 0.05). The EphA4 mRNA, although not being statistically significant, tended to be up-regulated in the D-RNB mice (Fig. 1D). These results indicate that ephrin-Eph receptor signaling molecules are specifically up-regulated in the D-RNB mice not only at the acute phase but also at the adaptive phase of cocaine administration.
Localization of EphrinA5, EphA4, and EphA5 in the SNr.
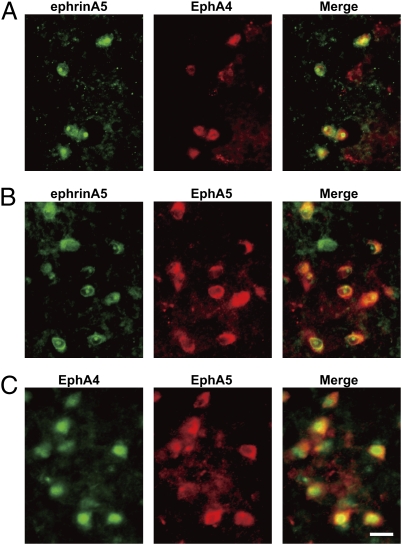
GABAergic neurons represent a major cell population, amounting to more than 90% of the neurons in the SNr (12). We investigated the cellular expression patterns of ephrinA5, EphA4, and EphA5 in the SNr by double immunostaining for each of these molecules and either gephyrin, a postsynaptic marker of GABAergic neurons (13), or glial fibrillary acidic protein (GFAP), a marker of astrocytes (14). This analysis showed that gephyrin-immunoreactive GABAergic neurons expressed both the ephrinA5 ligand and the EphA4 and EphA5 receptors (Fig. 2 A, C, and E). In contrast, the GFAP-positive astrocytes expressed the EphA4 and EphA5 receptors, but not the ephrinA5 ligand (Fig. 2 B, D, and F). In gephyrin-immunoreactive neurons, EphA4 and EphA5 were mostly localized in the soma and proximal dendrites, whereas the ephrinA5 localization extended from the soma to the distal dendrites. Furthermore, double immunostaining among ephrinA5, EphA4, and EphA5 indicated that all three molecules were ubiquitously colocalized in more than 80% of the SNr neurons (Fig. 3).
Fig. 2.
Immunohistological analysis of ephrinA5, EphA4, and EphA5 in the SNr cells. Coronal sections of the SNr of WT mice were double-immunostained with the following antibodies and visualized by confocal microscopic analysis: (A and B) green, ephrinA5; (C and D) green, EphA4; (E and F) green, EphA5; (A, C, and E) red, gephyrin; (B, D, and F) red, GFAP. (Scale bar, 10 μm.)
Fig. 3.
Coexistence of ephrinA5, EphA4, and EphA5 in the SNr neurons. Coronal sections of the SNr were double-immunostained with the following antibodies and visualized by light microscopic analysis: (A) ephrinA5 (green) and EphA4 (red); (B) ephrinA5 (green) and EphA5 (red); (C) EphA4 (green) and EphA5 (red). (Scale bar, 50 μm.)
Effects of EphrinA5, EphA4, and EphA5 on Cocaine Sensitization.
Because the above results indicate that the deficit of the direct-pathway transmission selectively up-regulated the ephrin-Eph signaling molecules in the SNr of the cocaine-treated mice, we next addressed whether the activation of this signaling could suppress the adaptive response induced by repeated cocaine administration. To address this question, we used specific immunoadhesin chimeras, which were the fusion proteins consisting of the Fc domain of human IgG and the respective extracellular binding domain of the ephrin or the Eph receptors (15, 16). These immunoadhesins were the dimerized forms, which activate the corresponding receptors or ligands (17, 18). The immunoadhesin or the control Fc was attached to fluorescent microspheres to prevent diffusion into other brain regions (19).
The immunoadhesin- or the control Fc-attached microspheres were bilaterally injected into the SNr of WT mice, and restricted injection into the SNr was confirmed by visualization of the microsphere fluorescence in brain slices of individual mice killed after behavioral analysis. Four days after injection of the immunoadhesins, cocaine (10 mg/kg) was daily administered for 4 d and locomotor activity was measured immediately after each cocaine administration. Repeated cocaine administration induced a progressive increase in locomotor activity, called locomotor sensitization (9). Both EphA4-Fc and EphA5-Fc significantly suppressed cocaine-induced locomotor sensitization compared with that of the control Fc-injected mice (Fig. 4 B and C) (analyzed by repeated-measure ANOVA: between EphA4-Fc (n = 10) and control Fc (n = 6), for immunoadhesin, P < 0.005; for day, P < 0.005; for interaction immunoadhesin × day, P < 0.01; between EphA5-Fc (n = 8) and control Fc(n = 6), for immunoadhesin, P < 0.005; for day, P < 0.005; for interaction immunoadhesin × day, P < 0.05). EphrinA5-Fc showed no statistically significant suppression of cocaine-induced hyperlocomotion, as analyzed by repeated-measure ANOVA but tended to reduce locomotor sensitization on days 3 and 4 (Fig. 4A). These results indicate that the EphA4 and EphA5 receptors in the SNr play an important role in controlling adaptive responses to repeated administration of cocaine.
Fig. 4.
Suppression of cocaine-induced hyperlocomotion by EphA4 and EphA5 in the SNr. Fluorescent microspheres with attached ephrinA5-Fc (A), EphA4-Fc (B), EphA5-Fc (C), or control Fc (A–C) were bilaterally injected into the SNr of WT mice. One day after immunoadhesin injection, animals received intraperitoneal saline once a day and were habituated for 3 d. Cocaine (10 mg/kg) was then intraperitoneally injected once a day from day 1 to day 4; and immediately after each cocaine injection, locomotor activity was counted for a 10-min period. Symbols and bars represent the mean ± SEM (ephrinA5-Fc, n = 14; EphA4-Fc, n = 10; EphA5-Fc, n = 8; control Fc, n = 6). Statistical significance was analyzed by repeated-measure ANOVA; **P < 0.01, ***P < 0.001 (EphA4-Fc or EphA5-Fc vs. control Fc).
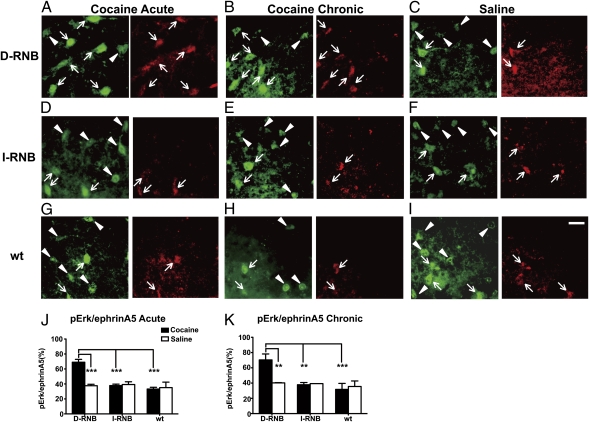
Erk Phosphorylation in EphrinA5-Positive Cells Specific to the Cocaine-Treated D-RNB Mice.
Both EphA4 and EphA5 bind to ephrinA5, and this binding reversely stimulates the phosphorylation of the MAP kinases, Erk1 and Erk2, in ephrinA5-bearing cells (20). Therefore, we addressed whether cocaine could enhance phosphorylation of Erk1/2 in ephrinA5-bearing neurons specific to the SNr of D-RNB mice. The SNr of D-RNB, I-RNB, or WT mice was analyzed by double immunostaining with antibodies against ephrinA5 and phospho-Erk1/2 (pErk1/2) after acute or chronic cocaine administration (Fig. 5 A–I). The numbers of cells positive for ephrinA5 or pErk1/2 were counted, and the ratio of pErk1/2-ephrinA5 double-positive cells to ephrinA5-positive cells was calculated. This ratio markedly increased in D-RNB mice in the acute phase of cocaine administration (D-RNB, 69.0 ± 3.8%; I-RNB, 37.0 ± 1.4%; WT, 33.2 ± 2.3%; P < 0.001, D-RNB vs. I-RNB or WT) (Fig. 5J). Similarly, this ratio significantly increased in D-RNB mice at the chronic phase of cocaine administration (D-RNB, 70.4 ± 7.8%; I-RNB, 37.8 ± 2.9%; WT, 31.8 ± 7.8%; P < 0.001–0.01, D-RNB vs. I-RNB or WT) (Fig. 5K). Importantly, there was no difference in the relative ratio of two types of cells in three groups of saline-treated mice (Fig. 5 J and K). Upon double immunostaining for NeuN, a marker of mature neurons (21), ephrinA5-immunoreactive cells amounted to 94% to 98% of the NeuN-positive cells in all three groups of mice, regardless of treatment or not with cocaine. Thus, there was a good correlation in the pathway specificity between the regulation of the EphA4/EphA5-ephrinA5 system and phosphorylation of Erk1/2 in the SNr neurons. This correlation suggests that the Erk1/2 signaling downstream of ephrin actions in the SNr is important for the cocaine-induced behavioral responses.
Fig. 5.
Activation of Erk1/2 in ephrinA5-expressing SNr neurons specific to D-RNB mice. For Cocaine Acute (A, D, and G) and Saline (C, F, and I), D-RNB, I-RNB, and WT mice received a single intraperitoneal injection of cocaine (10 mg/kg) and saline, respectively, and the SNr was isolated 6 h after cocaine or saline injection. For Cocaine Chronic (B, E, and H), three groups of mice daily received a single intraperitoneal injection of cocaine (10 mg/kg) for 5 d and the SNr was isolated 1 h after cocaine injection. Coronal sections were double-immunostained with antibodies against ephrinA5 (green) and pErk1/2 (red) and visualized by light microscopic analysis. Arrows and arrowheads indicate the pErk1/2-positive and pErk1/2-negative cells, respectively, that were also immunopositive for ephrinA5. (Scale bar, 50 μm.) (J and K) The numbers of Erk1/2-immunopositive and Erk1/2-immunonegative cells among the ephrinA5-immunoreactive cells were counted, and the ratios of pErk1/2-ephrinA5 double-immunopositive cells to ephrinA5-immunoreactive cells are indicated in J and K. Columns and error bars represent the mean ± SEM (n = 4 each). The statistical significance was analyzed by one-way ANOVA. **P < 0.01, ***P < 0.001.
Discussion
The principal striatal neurons receive inputs from the cerebral cortex and thalamus and send their inputs to the SNr through two parallel pathways (1, 7). In the basal ganglia circuit, cocaine inhibits the dopamine transporter and massively increases dopamine levels in the striatum and the NAc (8). This rapid increase in dopamine activates both the low-affinity D1 receptor in the direct pathway and the high-affinity D2 receptor in the indirect pathway (22). The chronic cocaine exposure then persistently activates the D1 and D2 receptors and differentially induces long-term potentiation at striatonigral neurons of the direct pathway and long-term depression at striatopallidal neurons of the indirect pathway (23). The long-term potentiation of the direct pathway is thought to be critical for inducing the adaptive response to chronic cocaine exposure (9, 23). However, the pathway-specific regulatory mechanisms of cocaine actions at the convergent SNr remained to be clarified. This investigation has revealed an important mechanism, in which the ephrinA5 ligand-EphA4/EphA5 receptors are regulated in the SNr via a direct pathway-specific mechanism in both the acute and chronic phases of cocaine responses. These ephrin-Eph molecules were up-regulated specifically by blocking inputs of the direct pathway after cocaine administration. Conversely, the EphA4 and EphA5 receptors in the SNr suppressed adaptive response to repeated cocaine administration. Furthermore, cocaine exposure activated the Erk1/2 signaling cascade in ephrinA5-expressing SNr cells in a direct pathway-dependent manner. These results indicate that the ephrinA5-EphA4/EphA5 signaling molecules are specifically regulated by inputs of the direct pathway and play an important role in the acute and adaptive responses to cocaine exposure.
The ephrin-Eph system consists of the large family of both ephrins and Eph receptors and controls a large variety of cellular responses, including contact-mediated attraction or repulsion, synapse formation, spine morphogenesis, and neural plasticity (24, 25). One of the characteristic features of the ephrin-Eph system is its bidirectional signaling cascade, in which the interaction of ephrin with the Eph receptor induces a forward signaling in the Eph-bearing cells and simultaneously elicits a reverse signaling in the ephrin-bearing cells (20, 26). Although ephrinA5, EphA4, and EphA5 were all up-regulated by blocking the direct pathway at least at the acute phase of cocaine administration, activation by EphA4 and EphA5 was more effective than activation by ephrinA5 in suppressing the cocaine sensitization. This reverse signaling could thus play a predominant role in the transmission regulation of the direct pathway in the SNr. This regulation may occur by interaction of the presynaptic EphA4/EphA5 of striatal cells (27, 28) and the postsynaptic ephrinA5 of the SNr neurons. Astrocytes also highly express EphA4 and EphA5, which may stimulate ephrinA5 in neurons (26). Recently, the ephrin-Eph cis interaction within the same cellular membrane has been shown to transduce a key signaling in the ephrin-Eph system (26, 29, 30). Because both ephrinA5 and EphA4/EphA5 are commonly distributed in most of the SNr neurons, the cis interaction of this system could be involved in the direct pathway-specific regulation of cocaine responses. Whatever the mechanisms of the ephrinA5-EphA4/EphA5 system in the SNr, our finding that the Erk1/2 signaling is pathway-specifically regulated in ephrinA5-expressing cells strongly suggests that the ephrinA5-EphA4/EphA5–expressing SNr neurons play an important role in cocaine-induced input transmission of the direct pathway.
No alteration of the ephrinA5-EphA4/EphA5 system was observed in saline-treated D-RNB mice, indicating that the observed changes in this ephrin-Eph system were linked to the action of cocaine and not a consequence of impaired transmission per se of the direct pathway. Our previous study using the RNB technique revealed that blockade of either the direct or the indirect pathway abolished the acute cocaine response (9). The dual stimulation of the two pathways is thus necessary for the rapid response to cocaine administration (9). In the chronic response to repeated cocaine administration, blockade of the direct pathway—but not that of the indirect pathway—severely impaired cocaine-induced adaptive responses, indicating that the direct pathway plays a predominant role in input transmission for the adaptive response to cocaine (9). However, despite the defectiveness of the acute response by blockade of either of the two pathways (9), up-regulation of the ephrinA5-EphA4/EphA5 system as well as activation of Erk1/2 was observed in a direct pathway-selective manner at both acute and chronic phases of cocaine administration. The ephrinA5-EphA4/EphA5 system is thus most likely to contribute to triggering the acute response and then inducing the adaptive response to cocaine actions. The cellular response to ephrin-Eph engagement is often repulsive between the two cells, although a repulsive or attractive response depends on the cellular context (26). This ephrin-Eph system is also important for cell-cell communication by controlling spine morphogenesis and neural plasticity (18, 24, 26, 31). Present findings of the pathway-selective ephrin-Eph engagement in cocaine-induced responses thus shed light on the action of cocaine and would provide valuable therapeutic targets for the treatment of drug addiction.
Materials and Methods
Animals and Behavioral Analysis.
All animal handling procedures were performed according to the guidelines of the Osaka Bioscience Institute. The RNB mice, in which transmission of either the direct or the indirect pathway was selectively blocked, was generated as described previously (9). Briefly, the expression of TN was driven in the TN mice by the TRE and induced by interaction with the tTA (32). The expression of tTA was restricted to the direct or indirect pathway by injecting one of two types of the recombinant AAVs into the NAc, in which tTA was exclusively expressed in either the direct or the indirect pathway under the control of the substance P promoter or the enkephalin promoter, respectively (9). The recombinant AAV was bilaterally injected into four sites of the NAc by stereotaxic techniques (9). The RNB mice and their WT littermates were used for all experiments.
Locomotor activity was measured with an infrared activity monitor (MED Associates). For measurement of cocaine-induced hyperlocomotion, animals received intraperitoneal saline once a day and were habituated to a novel chamber for 3 d. Cocaine (10 mg/kg) or saline was then intraperitoneally injected once a day from day 1 to day 4, and immediately thereafter the locomotor activity was counted for a 10-min period.
Microdissection of the SNr.
One hour after cocaine or saline administration, mice were killed, and frozen coronal sections (40 μm) were obtained from the brain embedded in OCT compound. Microdissection was performed by using a Micro Dissector PPMD (Eppendorf), consisting of a 1-mm diameter stainless-steel needle (Eppendorf) set at a 45° angle to the surface of the microscope table. A micropipette was mounted on a 3-axis–controlled, motorized micromanipulator (Eppendorf) attached to the microscope. After cryosections were covered with a pool of 15 μL of xylene for visualization, the SNr was dissected as an ultrasonically oscillating needle was moved along a selected tissue area.
Microarray Analysis.
Total RNA of dissected SNrs was extracted with the reagents of an RNeasy Mini Kit (Qiagen) after evaporation of xylene in a vacuum concentrator. Approximately 5 ng of total RNA was labeled by using GeneChip Two-Cycle Target Labeling and Control Reagents (Affymetrix). Hybridization signals were calculated by analyzing raw data with Microarray Suite 5.0 (Affymetrix) and further analyzed with GeneSpring GX 11.0 software (Agilent Technologies) and Ingenuity Pathway Analysis 6.0 software (Ingenuity Systems). The data were normalized to the 75th percentile for per-chip normalization.
Quantitative RT-PCR.
Reverse transcription was carried out by using the SuperScript First-Strand Synthesis System (Affymetrix) with the T7-oligo(dT) primer. cDNAs thus synthesized were amplified by a cycle of T7 amplification by using the MEGAscript High Yield Transcription Kit (Applied Biosystems). Specific primers were designed to generate 60- to 150-bp PCR products corresponding to the 3′ region of each mRNA. All reactions were performed in duplicate, and β-actin mRNA was used as an internal control for mRNA quantification.
Immunohistological Analysis.
Immunohistochemistry of frozen coronal sections (20 μm) of the adult mouse brain was performed as described by Schneider Gasser et al. (33) by using the primary antibodies against ephrinA5 (Abcam), EphA4, EphA5 (for both, Abcam or Santa Cruz), pErk1/2 (Santa Cruz), GAD67, TH (Millipore), gephyrin (Synaptic Systems), and GFAP (Sigma Aldrich). The secondary antibodies used were Alexa488- or Alexa594-conjugated goat IgG (Molecular Probes), and specific immunoreactivity was confirmed by performing immunohistochemical analysis without addition of the primary antibody.
Immunoadhesin Analysis.
Three different immunoadhesins were used; that is, fusion proteins consisting of the binding domain of either ephrinA5, EphA4, or EphA5 attached to the Fc domain of human IgG (R&D Systems). To prevent diffusion of immunoadhesins into other brain regions, we attached fluorescent microspheres (Lumafluor) to each immunoadhesin, as described by Riddle et al. (19). Immunoadhesin was injected stereotaxically at four sites in the SNr of WT mice (3.4-mm and 3.6-mm posterior to the bregma, ± 1.5-mm lateral from the midline, 4.0-mm depth from the dura). Four days after immunoadhesin injection, locomotor activity was measured immediately after daily administration of cocaine (10 mg/kg). After the behavioral analysis, injection sites of immunoadhesins were confirmed by visualization of immunoadhesin-attached fluorescent microspheres in the SNr of brain-slice preparations.
Statistical Analysis.
Statistical analysis was conducted by using Graph Pad PRISM 5.0 (GraphPad Software). Data were analyzed by one-way ANOVA or repeated-measure ANOVA and were presented as the mean ± SEM.
Acknowledgments
This work was supported by Research Grants KAKENHI 22220005 (to S.N.), 22659069, and 23110522 (to T.H.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, Grant on Regulatory Science of Pharmaceuticals and Medical Devices from the Ministry of Health and Labour and Welfare (to T.H.), a grant from the Japan Science and Technology Agency Precursory Research for Embryonic Science and Technology Program (T.H.), and grants from the Takeda Science Foundation (to S.N.) and Daiichi-Sankyo Foundation of Life Science (to T.H.)
Footnotes
The authors declare no conflict of interest.
References
- 1.Graybiel AM. The basal ganglia. Curr Biol. 2000;10:R509–R511. doi: 10.1016/s0960-9822(00)00593-5. [DOI] [PubMed] [Google Scholar]
- 2.Wickens JR, Reynolds JNJ, Hyland BI. Neural mechanisms of reward-related motor learning. Curr Opin Neurobiol. 2003;13:685–690. doi: 10.1016/j.conb.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Israel Z, Bergman H. Pathophysiology of the basal ganglia and movement disorders: From animal models to human clinical applications. Neurosci Biobehav Rev. 2008;32:367–377. doi: 10.1016/j.neubiorev.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Obeso JA, et al. The basal ganglia in Parkinson's disease: Current concepts and unexplained observations. Ann Neurol. 2008;64(Suppl 2):S30–S46. doi: 10.1002/ana.21481. [DOI] [PubMed] [Google Scholar]
- 5.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: The role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 6.Redgrave P, et al. Goal-directed and habitual control in the basal ganglia: Implications for Parkinson's disease. Nat Rev Neurosci. 2010;11:760–772. doi: 10.1038/nrn2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deniau JM, Mailly P, Maurice N, Charpier S. The pars reticulata of the substantia nigra: A window to basal ganglia output. Prog Brain Res. 2007;160:151–172. doi: 10.1016/S0079-6123(06)60009-5. [DOI] [PubMed] [Google Scholar]
- 8.Riddle EL, Fleckenstein AE, Hanson GR. Role of monoamine transporters in mediating psychostimulant effects. AAPS J. 2005;7:E847–E851. doi: 10.1208/aapsj070481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. 2010;66:896–907. doi: 10.1016/j.neuron.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Esclapez M, Tillakaratne NJ, Kaufman DL, Tobin AJ, Houser CR. Comparative localization of two forms of glutamic acid decarboxylase and their mRNAs in rat brain supports the concept of functional differences between the forms. J Neurosci. 1994;14:1834–1855. doi: 10.1523/JNEUROSCI.14-03-01834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oertel WH, Tappaz ML, Berod A, Mugnaini E. Two-color immunohistochemistry for dopamine and GABA neurons in rat substantia nigra and zona incerta. Brain Res Bull. 1982;9:463–474. doi: 10.1016/0361-9230(82)90155-1. [DOI] [PubMed] [Google Scholar]
- 12.Mugnaini E, Oertel WH. In: in Handbook of Chemical Nenroanatomy, Volume 4: GABA and Neuropeptides in the CNS, Part I. Björklund A, Hökfelt T, editors. Amsterdam: Elsevier; 1985. pp. 436–595. [Google Scholar]
- 13.Sassoè-Pognetto M, Fritschy J-M. Mini-review: Gephyrin, a major postsynaptic protein of GABAergic synapses. Eur J Neurosci. 2000;12:2205–2210. doi: 10.1046/j.1460-9568.2000.00106.x. [DOI] [PubMed] [Google Scholar]
- 14.Ghandour MS, Langley OK, Vincendon G, Gombos G. Double labeling immunohistochemical technique provides evidence of the specificity of glial cell markers. J Histochem Cytochem. 1979;27:1634–1637. doi: 10.1177/27.12.118210. [DOI] [PubMed] [Google Scholar]
- 15.Ashkenazi A, Chamow SM. Immunoadhesins as research tools and therapeutic agents. Curr Opin Immunol. 1997;9:195–200. doi: 10.1016/s0952-7915(97)80135-5. [DOI] [PubMed] [Google Scholar]
- 16.Gerlai R, et al. Protein targeting in the analysis of learning and memory: A potential alternative to gene targeting. Exp Brain Res. 1998;123:24–35. doi: 10.1007/s002210050541. [DOI] [PubMed] [Google Scholar]
- 17.Lim BK, Matsuda N, Poo M-M. Ephrin-B reverse signaling promotes structural and functional synaptic maturation in vivo. Nat Neurosci. 2008;11:160–169. doi: 10.1038/nn2033. [DOI] [PubMed] [Google Scholar]
- 18.Fu W-Y, et al. Cdk5 regulates EphA4-mediated dendritic spine retraction through an ephexin1-dependent mechanism. Nat Neurosci. 2007;10:67–76. doi: 10.1038/nn1811. [DOI] [PubMed] [Google Scholar]
- 19.Riddle DR, Katz LC, Lo DC. Focal delivery of neurotrophins into the central nervous system using fluorescent latex microspheres. Biotechniques. 1997;23:928–934. doi: 10.2144/97235rr02. 936–937. [DOI] [PubMed] [Google Scholar]
- 20.Davy A, Robbins SM. Ephrin-A5 modulates cell adhesion and morphology in an integrin-dependent manner. EMBO J. 2000;19:5396–5405. doi: 10.1093/emboj/19.20.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- 22.Hikosaka O. Basal ganglia mechanisms of reward-oriented eye movement. Ann N Y Acad Sci. 2007;1104:229–249. doi: 10.1196/annals.1390.012. [DOI] [PubMed] [Google Scholar]
- 23.Goto Y, Grace AA. Dopamine-dependent interactions between limbic and prefrontal cortical plasticity in the nucleus accumbens: Disruption by cocaine sensitization. Neuron. 2005;47:255–266. doi: 10.1016/j.neuron.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 24.Lai K-O, Ip NY. Synapse development and plasticity: Roles of ephrin/Eph receptor signaling. Curr Opin Neurobiol. 2009;19:275–283. doi: 10.1016/j.conb.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Scicolone G, Ortalli AL, Carri NG. Key roles of Ephs and ephrins in retinotectal topographic map formation. Brain Res Bull. 2009;79:227–247. doi: 10.1016/j.brainresbull.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Egea J, Klein R. Bidirectional Eph-ephrin signaling during axon guidance. Trends Cell Biol. 2007;17:230–238. doi: 10.1016/j.tcb.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Martone ME, Holash JA, Bayardo A, Pasquale EB, Ellisman MH. Immunolocalization of the receptor tyrosine kinase EphA4 in the adult rat central nervous system. Brain Res. 1997;771:238–250. doi: 10.1016/s0006-8993(97)00792-0. [DOI] [PubMed] [Google Scholar]
- 28.Cooper MA, Crockett DP, Nowakowski RS, Gale NW, Zhou R. Distribution of EphA5 receptor protein in the developing and adult mouse nervous system. J Comp Neurol. 2009;514:310–328. doi: 10.1002/cne.22030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carvalho RF, et al. Silencing of EphA3 through a cis interaction with ephrinA5. Nat Neurosci. 2006;9:322–330. doi: 10.1038/nn1655. [DOI] [PubMed] [Google Scholar]
- 30.Marquardt T, et al. Coexpressed EphA receptors and ephrin-A ligands mediate opposing actions on growth cone navigation from distinct membrane domains. Cell. 2005;121:127–139. doi: 10.1016/j.cell.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 31.Fu AKY, et al. APCCdh1 mediates EphA4-dependent downregulation of AMPA receptors in homeostatic plasticity. Nat Neurosci. 2011;14:181–189. doi: 10.1038/nn.2715. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto M, et al. Reversible suppression of glutamatergic neurotransmission of cerebellar granule cells in vivo by genetically manipulated expression of tetanus neurotoxin light chain. J Neurosci. 2003;23:6759–6767. doi: 10.1523/JNEUROSCI.23-17-06759.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneider Gasser EM, et al. Immunofluorescence in brain sections: Simultaneous detection of presynaptic and postsynaptic proteins in identified neurons. Nat Protoc. 2006;1:1887–1897. doi: 10.1038/nprot.2006.265. [DOI] [PubMed] [Google Scholar]