The transition from diploid (2n) to polyploid (3n, 4n, and so forth), a relatively common process in plants and some animal taxa, represents rapid speciation, as only rarely are hybrids able to form genetically balanced gametes (1). In vertebrates, polyploidy is often associated with asexual reproduction: sperm-dependent (gynogenesis, hybridogenesis, and kleptogenesis) in amphibians and fish, and sperm-independent (parthenogenesis) in squamate reptiles (2). For the latter, approximately 0.6% of known species are parthenogenetic, and, with few exceptions, these arose via hybridization between sexually reproducing progenitors (3). Given the relatively recent origin of most parthenogenetic taxa (2), it should then be possible to recreate parthenogenetic lineages of varying ploidy by hybridizing known progenitors in the laboratory, as has been achieved for some diploid hybrid plant species (4) and unisexual fish and frogs (see ref. 5). However, until now, this has not been achieved for parthenogenetic reptiles (e.g., ref. 6). In this context, the report by Lutes et al. (5) in PNAS is highly significant. Following from an earlier observation of a potentially fertile, field-collected 4n female (7), Lutes et al. (5) crossed parthenogenetic 3n Aspidoscelis exsanguis females and a sexual Aspidoscelis inornata male to produce three generations of parthenogenetically reproducing 4n female lizards. Parthenogenetic reproduction was confirmed by cytogenetic analysis of female meiosis and multilocus genotyping across multiple generations of the progeny. That a self-sustaining 4n lineage can be produced in the laboratory, but is not observed in nature, raises the question of what constrains development of cascading polyploid series, as seen in some invertebrates (8). Further, is it possible that 3n asexual lineages can form a bridge in the evolution from 2n to 4n sexually reproducing species (1)?
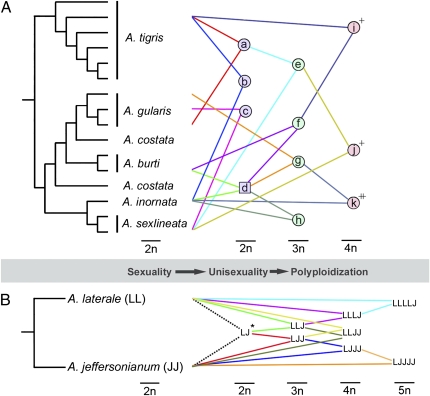
The whiptail lizards of the western deserts of North America are remarkable in having a substantial diversity of both 2n and 3n parthenogenetic lineages, all of which arose via hybridization among sexual species (9) (Fig. 1A). Parthenogenetic lineages are often sympatric with congeneric sexual taxa, resulting in numerous reports of high ploidy hybrids in nature (reviewed in refs. 10 and 11). As Aspidoscelis have XY sex determination with slightly heteromorphic sex chromosomes, both male and female hybrids are observed, although these are sterile or of unknown fertility (10). This is the case for previously reported and cytogenetically confirmed 4n hybrids between 3n parthenogens and 2n sexual males (10, 11). Both these and the hybrids generated by Lutes et al. (5) have unbalanced (i.e., AABC) chromosome sets (Fig. 1A), so why the latter should be fertile but former not (or not known to be) is a mystery.
Fig. 1.
Phylogeny of Aspidoscelis for representative sexuals and unisexuals, based on figure 6 in ref. 9 (A), and a possible route of ploidy elevation of unisexual salamanders in the Ambystoma laterale–jeffersonianum complex (B) (19). Species in A are as follows: a, Aspidoscelis tesselata complex; b, Aspidoscelis neomexicana; c, Aspidoscelis laredoensis complex; d, intermediate ancestor; e, Aspidoscelis neotesselata complex; f, Aspidoscelis flagellicauda and Aspidoscelis sonorae complexes; g, Aspidoscelis exsanguis; h, Aspidoscelis opatae, Aspidoscelis uniparens, and Aspidoscelis velox complexes; i, 4n by A. sonorae (female) × Aspidoscelis tigris (male; references 25, 26, and 28 in ref. 5); j, 4n by A. neotesselata (female) × Aspidoscelis sexlineata (male; reference 27 in ref. 5); and k, 4n by A. exsanguis (female) × A. inornata (male) (5, 7). *LJ is not a “true” hybrid directly derived by A. laterale × A. jeffersonianum (19); +sterile or unknown fertility; ╫fertile (5) or potentially fertile (7).
In other sexual–parthenogenetic complexes of reptiles, higher ploidy hybrids are also typically sterile. For example, Caucasian rock lizards of the genus Darevskia contain several diploid parthenogenetic lineages of hybrid ancestry. Triploids, as commonly found in nature where 2n parthenogens and sexuals coexist, are typically sterile (ref. 10; but see ref. 12). This has been attributed to disruption of sex determination, as these species have moderately to highly heteromorphic ZW sex chromosomes (13). However, this does not explain sterility of such hybrids in Heteronotia and Lepidodactylus geckos, which lack heteromorphic sex chromosomes (14, 15). In contrast, cascading polyploidy elevation readily occurs in some groups of amphibians [e.g., Bufo viridis group (16)] and fish (17, 18). For example, North American unisexual mole salamanders of the genus Ambystoma occasionally incorporate sperm from congeneric sexual males in syntopy to generate natural lineages with increasing ploidy (19) (Fig. 1B).
Why are there no such examples in reptiles? One simple possibility is that successful elevation to higher ploidy is a rare event and is facilitated in unisexual amphibians and fish because of their continuing dependence on sperm to initiate the onset of development. In unisexual fish, for example, this often leads to variable amount of paternal leakage that ranges from microchromosomes to a whole genome set (17). However, in some circumstances, hybrids between parthenogenetic and sexual reptiles are very common [e.g., as high as 50% (12)], suggesting that this alone is not the whole story. Another possibility is that such transitions depend on intense selection for rare balanced gametes and, thus, are more probable in taxa with high female fecundity (1).
A related question is whether unisexual triploidy is a bridge to formation of sexually reproducing tetraploids (1). Such transitions have been observed in fish (18), but how most sexual polyploid vertebrates (e.g., amphibians and fish) arose is poorly understood. In general, 4n sexual reproduction is most likely with matched chromosome sets (AABB), allowing balanced pairing between homologous (A–A and B–B) rather than homeologous (A–B) chromosomes. As yet, all known 4n hybrids of Aspidoscelis, including those generated by Lutes et al. (5), have unmatched chromosome sets (Fig. 1A). Therefore, and notwithstanding previous efforts (6), it would be fascinating to
Lutes et al. demonstrate the potential to generate higher-ploidy parthenogenetic lineages of reptiles in the laboratory.
extend these experiments to generate 4n hybrids with balanced chromosome sets (e.g., A. sonorae × A. inornata, or A. uniparens × Aspidoscelis burti, Fig. 1A).
The results from Lutes et al. (5) demonstrate the potential to generate higher-ploidy parthenogenetic lineages of reptiles in the laboratory. So, given that these particular taxa, A. exsanguis and A. inornata, are known to occur in sympatry (10), why do we not see a self-sustaining 4n lineage in nature? One possibility might be that very specific genomic combinations of particular parental individuals are needed to initiate or to maintain unisexuality (2). For example, populations of unisexual salamanders are characterized by complex ploidy and genome combinations, but all share a single origin, and no recurrent hybridization between known sexual sperm donors has generated new unisexual lineages (19). Lutes et al. (5) find that the laboratory-produced 4n hybrids are perfectly viable with no competitive disadvantage in prey capture when housed with their progenitors. However, as Lutes et al. (5) are aware, this does not ensure that these newly formed 4n parthenogens are capable of persisting under natural conditions, and in the face of competitive pressure from both sexual and unisexual progenitors in a constantly changing environment.
The ability to produce self-sustaining, higher-ploidy hybrids between parthenogenetic and sexual lineages in the laboratory, but their absence in nature, raises many questions and also creates opportunities for further research. The relatively recent ability to develop genomic resources for such systems and, therefore, to examine patterns of gene expression and intergenomic interactions in sterile versus fertile hybrids relative to their parthenogenetic and sexual progenitors offers a new window into often proposed, but still poorly understood, genetic constraints on the evolution of parthenogenesis and polyploidy (20).
Acknowledgments
C.M. is supported by grants from the National Science Foundation. K.B. is supported by a Natural Sciences and Engineering Research Council postdoctoral fellowship.
Footnotes
The authors declare no conflict of interest.
See companion article on page 9910.
References
- 1.Mable BK. ‘Why polyploidy is rarer in animals than in plants’: Myths and mechanisms. Biol J Linn Soc Lond. 2004;82:453–466. [Google Scholar]
- 2.Avise JC. Clonality: The Genetics, Ecology, and Evolution of Sexual Abstinence in Vertebrate Animals. New York: Oxford University Press; 2008. [Google Scholar]
- 3.Kearney M, Fujita MK, Ridenour J. Lost sex in the reptiles: constraints and correlations. In: Schön I, Martens K, van Dijk P, editors. Lost Sex: The Evolutionary Biology of Parthenogenesis. Dordrecht, The Netherlands: Springer; 2009. pp. 447–474. [Google Scholar]
- 4.Rieseberg LH, Sinervo B, Linder CR, Ungerer MC, Arias DM. Role of gene interactions in hybrid speciation: Evidence from ancient and experimental hybrids. Science. 1996;272:741–745. doi: 10.1126/science.272.5262.741. [DOI] [PubMed] [Google Scholar]
- 5.Lutes AA, Baumann DP, Neaves WB, Baumann P. Laboratory synthesis of an independently reproducing vertebrate species. Proc Natl Acad Sci USA. 2011;108:9910–9915. doi: 10.1073/pnas.1102811108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole CJ, Hardy LM, Dessauer HC, Taylor HL, Townsend CR. Laboratory hybridization among North American whiptail lizards, including Aspidoscelis inornata arizonae × A. tigris marmorata (Squamata: Teiidae), ancestors of unisexual clones in nature. Am Mus Novit. 2010;3698:1–43. [Google Scholar]
- 7.Neaves WB. Tetraploidy in a hybrid lizard of the genus Cnemidophorus (Teiidae) Brev Mus Comp Zool. 1971;381:1–25. [Google Scholar]
- 8.Suomalainen E, Saura A, Lokki J. Cytology and Evolution in Parthenogenesis. Boca Raton, FL: CRC Press; 1987. [Google Scholar]
- 9.Reeder TW, Cole CJ, Dessauer HC. Phylogenetic relationships of whiptail lizards of the genus Cnemidophorus (Squamata: Teiidae): A test of monophyly, reevaluation of karyotypic evolution, and review of hybrid origins. Am Mus Novit. 2002;3365:1–61. [Google Scholar]
- 10.Darevsky IS, Kupriyanova LA, Uzell T. Parthenogenesis in reptiles. In: Gans C, Billet F, editors. Biology of the Reptilia. Vol. 15. New York: Wiley; 1985. pp. 411–526. [Google Scholar]
- 11.Cole JC, Painter CW, Dessauer HC, Taylor HL. Hybridization between the endangered unisexual gray-checkered whiptail lizard (Aspidoscelis dixoni) and the bisexual western whiptail lizard (Aspidoscelis tigris) in southwestern New Mexico. Am Mus Novit. 2007;3555:1–31. [Google Scholar]
- 12.Danielyan F, Arakelyan M, Stepanyan I. Hybrids of Darevskia valentini, D. armeniaca and D. unisexualis from a sympatric population in Armenia. Amphibia-Reptilia. 2008;29:487–504. [Google Scholar]
- 13.Kupriyanova L. Cytogenetic and genetic trends in the evolution of unisexual lizards. Cytogenet Genome Res. 2009;127:273–279. doi: 10.1159/000303325. [DOI] [PubMed] [Google Scholar]
- 14.Moritz C. The origin and evolution of parthenogenesis in Heteronotia binoei (Gekkonidae). 1. Chromosome banding studies. Chromosoma. 1984;89:151–162. [Google Scholar]
- 15.Radtke R, Fisher R, Donnellan S, Moritz C, Case T. When species collide: the origin and spread of an asexual species of gecko. Proc Biol Sci. 1995;258:145–152. [Google Scholar]
- 16.Stöck M, et al. A vertebrate reproductive system involving three ploidy levels: hybrid origin of triploids in a contact zone of diploid and tetraploid palearctic green toads (Bufo viridis subgroup) Evolution. 2010;64:944–959. doi: 10.1111/j.1558-5646.2009.00876.x. [DOI] [PubMed] [Google Scholar]
- 17.Lamatsch DK, Stöck M. Sperm-dependent parthenogenesis and hybridogenesis in teleost fish. In: Schön I, Martens K, van Dijk P, editors. Lost Sex: The Evolutionary Biology of Parthenogenesis. Dordrecht, The Netherlands: Springer; 2009. pp. 399–432. [Google Scholar]
- 18.Cunha C, Ignacio D, Coelho MM. Speciation towards tetraploidization after intermediate processes of non-sexual reproduction. Philos Trans R Soc Lond B Biol Sci. 2008;363:2921–2929. doi: 10.1098/rstb.2008.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bogart JP, Bi K, Fu J, Noble DW, Niedzwiecki J. Unisexual salamanders (genus Ambystoma) present a new reproductive mode for eukaryotes. Genome. 2007;50:119–136. doi: 10.1139/g06-152. [DOI] [PubMed] [Google Scholar]
- 20.Fujita MK, Moritz C. Origin and evolution of parthenogenetic genomes in lizards: Current state and future directions. Cytogenet Genome Res. 2009;127:261–272. doi: 10.1159/000295177. [DOI] [PubMed] [Google Scholar]