Abstract
L-selectin mediates homing of lymphocytes to lymph nodes (LN). Transgenic mice that express rat insulin promoter regulated simian virus 40 Tag (RIP-Tag) develop large, local cancers that metastasize to liver but not LN. To test whether this lack of LN metastases reflects their absence from the circulation, transgenic mice were produced that express Tag (T), L-selectin (L), and Escherichia coli LacZ (Z), in pancreatic β cells. LTZ mice developed insulinomas that specifically had LN metastases; metastasis was blocked by an anti L-selectin mAb. LacZ+ tumor cells from these LN homed to secondary LN upon transfer. These results suggest that the highly vascularized islet carcinomas are shedding tumor cells into the bloodstream, which is a necessary but insufficient condition for metastasis to occur; L-selectin can facilitate homing of such tumor cells to LN, resulting in metastasis.
Metastatic disease is a significant parameter of tumor progression and the primary determinant of poor prognosis. Bloodborne metastasis is a major pathway by which tumor cells seed additional sites in the body. The bloodborne metastatic process can, in principle, be divided into four steps: (i) invasion of cancer cells that have detached from the primary tumor into the vascular system; (ii) dissemination of tumor cells via the circulatory system; (iii) extravasation of tumor cells at a distinct site; and (iv) establishment of a new tumor at that location. Metastasis has principally been studied by using two types of assays (1, 2): i.v. injection of tumor cells to assess their ability to survive, adhere to blood vessel endothelium, extravasate, and establish tumors; and injection into local tissue (usually s.c.) to assess their capability to metastasize from a primary tumor mass. Neither of these assays accurately mimics the metastatic process of endogenously arising primary tumors. The establishment of metastatic lesions requires not only tumor cell proliferation but also induction of angiogenesis to sustain tumor growth (3). In general, however, it remains unclear which events are rate limiting for metastasis to occur. Are tumor cells frequently shed from tumors undergoing persistent neovascularization? Or is it dissemination via the bloodstream or the ability to extravasate that is limiting? We have sought to address these questions in a transgenic mouse model of endogenous primary multistage carcinogenesis in which metastasis is infrequent. We have done this by producing transgenic mice that express L-selectin on endogenous primary insulinomas and then asking whether the frequency of metastasis is affected.
RIP-Tag transgenic mice develop islet cell carcinomas as a result of the simian virus 40 Tag oncogene expression in islet β cells (4). Tumor development proceeds through a series of well defined stages, which include: a dysplasia/carcinoma in situ-like stage, an angiogenic islet stage (5, 6), the solid tumor stage, in which tumors vary in regard to their size and apoptotic incidence (7), and an invasive carcinoma stage (6–8). Metastasis of islet cell tumors to sites other than the liver via the portal vein is infrequent in these transgenic mice (4, 8) despite the aggressive appearance of the tumor cells, their high proliferation index, their acquired resistance to apoptosis, and the intense angiogenesis they evoke.
In the studies described here we have asked whether addition of another migratory cell-cell interaction molecule could influence metastasis. To do so, we have targeted the tumor cells to express L-selectin (CD62L) (9). CD62L normally directs the extravasation of bloodborne lymphocytes into peripheral lymph nodes (PLN) and mesenteric lymph nodes (MLN) via recognition of the cognate peripheral node addressins (PNAd) expressed on the luminal face of postcapillary high endothelial venules (HEV), followed by a multistep process of adhesion and transvascular migration (9–13).
Materials and Methods
Constructs.
Two new DNA constructs were used for the generation of the triple transgenic mice. The first construct contained a 1,165-bp fragment of a rat insulin gene 1 promoter (14), a mouse L-selectin cDNA (9), and the untranslated 3′ end of the human insulin gene (15). The 1,165-bp fragment from the rat insulin gene 1 consists of a 986-bp 5′-flanking region, the first exon, the first intron, and 7 bp of the second exon, which all are untranslated. The 1,400 bp of mouse lymphocyte homing receptor cDNA include the translation start and termination codons. The 950 bp of the part of the human insulin gene include 226 bp of the second intron, the third exon, and 520 bp of the 3′ flanking region, which all are untranslated, followed by a 260-bp simian virus 40 poly(A) region. This whole construct can be released for microinjection by digestion with BssHII and EcoRI or by BssHII and SspI. The second construct included the rat insulin gene 2 promoter (4) and the LacZ gene under its control. This construct allowed us to easily identify and trace the tumor cells.
Transgenic Mouse Strains.
To confirm that the construct used for generating transgenic mice was engineered correctly, the construct that contained L-selectin was first tested by transient expression in the two insulinoma cell lines BTC3 and Hit. Cell surface expression of L-selectin was confirmed by immunohistochemistry and FACS (data not shown). L-selectin and LacZ double transgenic mice (LZ) were generated by coinjection of both respective constructs. The purified construct DNAs were injected into (C57BL/Ka × C3H)F1 eggs and implanted into the uterus of C129F A16 mice. The tail DNAs of the offspring were examined by PCR, and the resulting positives were mated to each other to generate homozygotes. Triple transgenic mice (LTZ) were generated by crossing these double transgenic (LZ) homozygotes with the RIP1-Tag2 strain (4), which expresses Tag (T) in its pancreatic β cells (by virtue of the rat insulin 2 gene promoter); 100% of the RIP-Tag2 mice develop β cell adenomas and carcinomas in a multistage pathway. The tumors in the LTZ mice resembled those previously reported, with two variations. First, in many of the tumors, large regions of the tumor were acellular, perhaps indicating cell death. Second, very few of the tumor-bearing mice had abnormal blood sugar levels, in contrast to previously described hyperinsulinemia and hypoglycemia in RIP-Tag mice (4). RIP-LacZ single transgenic mice also were generated by injection of the LacZ construct alone (unfortunately, this line was recently lost); crossing the homozygous LacZ mice (ZZ) with the Rat2 strain (TT) gave rise to another group of double transgenic mice (ZT), which expressed both the LacZ and the Tag genes.
Immunohistochemical and 5-Bromo-4-chloro-3-indolyl β-d-galactoside (X-Gal) Staining.
Frozen sections (5 μm) of the mouse organs including pancreas, PLN, MLN, Peyer's patch (PP), spleen and thymus were quickly dipped in acetone (16), and then stained with either a primary antibody against Tag (4) and a secondary anti-rabbit IgG- horseradish peroxidase antibody, or Mel-14 (a mAb to L-selectin) as the primary antibody (17) followed by an anti-rat IgG-horseradish peroxidase as the secondary antibody. Staining was detected with the ABC kit (Vector Laboratories). To detect β-galactosidase (β-gal) expression, either the whole organ or the frozen section were fixed in fresh 4% paraformaldehyde for 1 h at 4°C, rinsed, and stained with 0.1% X-gal, 5 mM K3Fe (CN)6, and 5 mM K4Fe(CN)6 for 24 h at 37°C.
Homing to LN.
Primary culture cells isolated from a LTZ insulinoma were fluorescently labeled with DiI C18 (1,1′-dioctadecyl-3,3,3′,3′-tetramathyl-indocarbocyanine perchlorate; Molecular Probes), which gives the cells an orange appearance. Primary culture cells isolated from a ZT mouse insulinoma were fluorescently labeled with DiO C18(3′,3′-dioctadecyloxacarbocyanine perchlorate, Molecular Probes) as described (18). This label gives the cells a green appearance under the fluorescent microscope. An equal number of labeled cells from each group were mixed. The mixture was preincubated with either PBS or a blocking antibody to L-selectin (Mel-14; 10 μg/ml) before injection into the tail veins of wild-type mice at 2 × 105 cells/mouse. Half an hour after the injections, the mice were killed and their organs were collected, sectioned, and observed under a fluorescent microscope.
Implantation of the PLN from the LTZ Mice.
PLN collected from LTZ mice were minced and implanted s.c. in the axillary region of normal, 6-week-old mice that had been previously irradiated with 500 rad of x-rays. After 40 days, the mice were killed and their tumor growth was examined.
Results
We generated triple transgenic mice (LTZ) that expressed simian virus 40 large T antigen (Tag), mouse L-selectin, and β-gal in their pancreatic cells. As a result, these mice developed highly angiogenic islet cell carcinomas that expressed L-selectin on the cell surface and β-gal in the cell cytoplasm. The blue color of the β-gal marker allowed for the easy tracing and identification of the tumor cells. Double transgenic mice carrying each pairwise combination of the three genes also were produced and found to express the cognate gene products (not shown). Expression of all three genes appeared as early as embryonic day 12 (data not shown). Adult LTZ mice developed β-cell tumors with high expression of L-selectin on the tumor cell surface and β-gal in the tumor cell cytoplasm (Fig. 1). Twenty percent of the LTZ mice had enlarged PLN when compared with PLN from wild-type mice (Fig. 2). To trace tumor cell metastasis, sections of different organs including PLN, MLN, PP, spleen, and thymus were prepared and incubated with the X-gal solution. In the LTZ mice, blue-colored tumor cells were detected frequently within the cortical regions in both PLN and MLN, infrequently in PP and spleen, and never in the thymus. The LacZ+ cells had not remained closely associated with HEV, but rather had extravasated into the LN parenchyma. In double transgenic ZT mice lacking L-selectin, the blue-colored tumor cells were rarely seen in MLN and never seen in PLN and were undetected in any other organ (Figs. 3 and 4). In double transgenic LZ mice lacking the oncogene, blue cells were never found in PLN, MLN, or any of the other organs (data not shown).
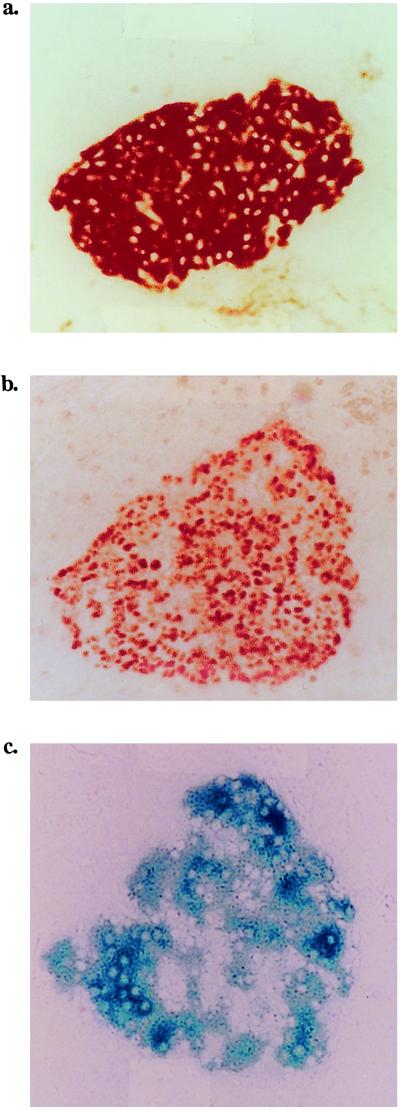
Figure 1.
Expression of L-selectin, Tag, and β-gal by the insulin-producing cells in LTZ triple transgenic mice. Expression of the three transgenes in a hyperplastic islet in a 7-week-old mouse. An angiogenic islet in a 9-week-old mouse is shown. Frozen sections of the pancreas were stained first with Ab to L-selectin (a) or to Tag (b) followed by horseradish peroxidase–conjugated secondary antibodies, or incubated with X-gal (c). (Magnification: ×160.)
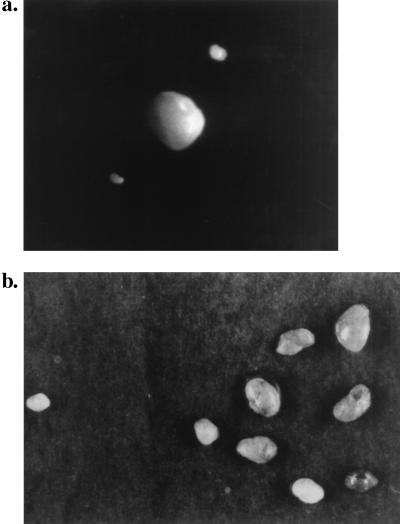
Figure 2.
Comparison of the PLN of wild-type and LTZ mice. (a) An enlarged PLN from the neck region of a triple-transgenic mouse (Middle) is compared with two normal-size LN from a wild-type mouse. (b) A group of the enlarged PLN from a triple transgenic mouse (Right) is compared with a normal-size LN from a wild-type mouse (Left). (Magnification: ×2)
Figure 3.
Increased frequency of metastatic tumor cells to PLN and MLN in LTZ mice. Different organs were sectioned and incubated with X-gal and examined under the microscope for detection of β-gal+ cells. A total of 70 LTZ mice, 30 ZT mice, and 30 LZ mice were examined. The percent metastases represents the number of mice in which blue-colored β-gal+ tumor cells were found in the indicated organ divided by the total number of mice examined.
Figure 4.
Tumor cells detected in the PLN of triple transgenic mice. The PLN were isolated from 14-week-old triple transgenic mice. The nodes were sectioned, incubated in X-gal, and examined under the microscope for blue-colored tumor cells. The sections were taken from the PLN of four LTZ mice and photographed at the following magnifications: (a) ×40, (b) ×80, (c) ×320, and (d) ×320.
To confirm that the tumor cells expressing L-selectin could home selectively to the LN, tumor cells from the pancreata of the LTZ as well as the ZT mice were isolated and grown briefly in culture. The primary culture cells were then fluorescently labeled with either DiI (for the LTZ mice) or DiO (3′,3′-dioctadecyloxacarbocyanine perchlorate) (for the ZT mice). A mixture containing an equal number of labeled cells from each strain was injected into the tail vein of wild-type mice. Half an hour after the injection, the mice were killed, and their PLN, MLN, PP, spleen, and thymus were sectioned and examined under a fluorescent microscope. Only the DiI-labeled cells from LTZ mice were found in the PLN (Fig. 5a) and MLN (Fig. 5b) but not in the PP (Fig. 5c), spleen, or thymus (data not shown). Most of the DiI+ tumor cells already had transmigrated into the LN beyond the HEV. In addition, a neutralizing antibody (Mel-14) to L-selectin blocked the homing to PLN almost completely (Fig. 5d).
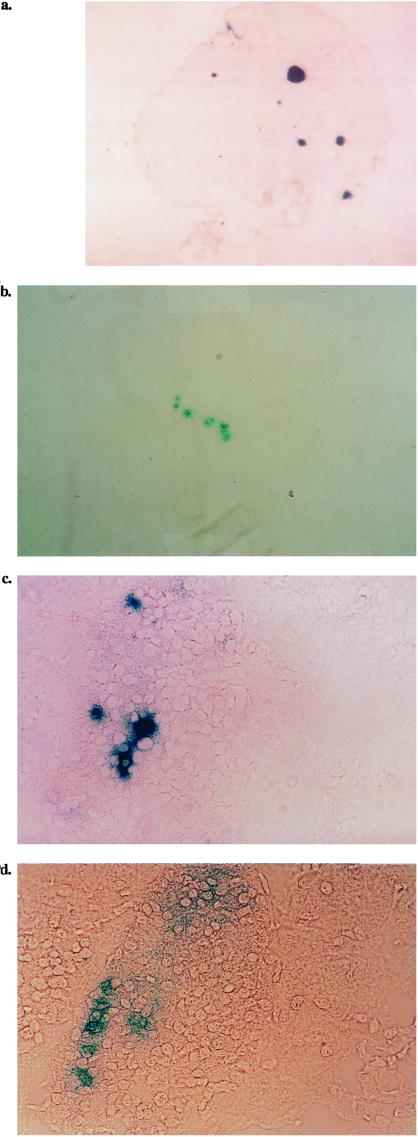
Figure 5.
Specific homing of LTZ tumor cells to PLN and MLN. A primary culture of insulinoma cells was fluorescently labeled with either DiI (for the triple transgenic mice) or DiO (3′,3′-dioctadecyloxacarbocyanine perchlorate for the double transgenic mice lacking L-selectin). An equal number of the two labeled cell populations was preincubated with either PBS or MEL-14 (17) before injection into the tail veins of wild-type mice. Half an hour after the i.v. injection, the mice were killed; PLN, MLN, and PP tissues were collected, sectioned, and examined with a fluorescent microscope. (a) PLN from a mouse injected with the mixture that was preincubated with PBS. (b) MLN from a mouse injected with the mixture preincubated in PBS. (c) PP from a mouse injected with the mixture preincubated in PBS. (d) PLN from a mouse injected with the mixture preincubated with Mel-14, the anti-L-selectin mAb. (Magnification: ×80.)
Because the insulinoma-bearing mice had an average lifespan of 12–14 weeks, it was impossible to see large metastatic tumors in their LN. To confirm that the LacZ+ cells that had seeded in the PLN were actually tumorigenic, the LN from the triple transgenic mice were minced and s.c.-injected into the axillary region of irradiated wild-type mice. After 40 days, insulinomas were found in the implanted region. The tumors appeared bright red (Fig. 6). The immunohistochemical staining of frozen sections of these tumors indicated that in fact the three genes, Tag, Mel-14, and β-gal, were expressed (data not shown).
Figure 6.
Micrometastasis in the PLN of LTZ mice is tumorigenic. PLN collected from triple transgenic mice were minced and s.c.-injected into the axillary region of irradiated wild-type mice. After 40 days, the mice were killed and examined for tumor growth. Shown are dissections of the axillary regions of two different mice that reveal tumor growth. (Magnification: ×2.)
Discussion
L-selectin is a lymphocyte homing receptor (10) for PNAd-positive HEV (14). L-selectin (9, 19–21) has homology to C-type lectins and belongs to a family of selectin homing receptors (22, 23). Recirculating lymphocytes can home to PNAd sites on LN HEV via L-selectin and to MadCAM sites on PP HEV (24, 25) via integrin α4β7 (25–27). L-selectin plays an essential role in the initial steps of lymphocyte homing to lymphoid tissues (10, 17). Lymphocyte extravasation into LNs involves rolling of marginated cells via lymphocyte L-selectin interactions with PNAd ligands on HEV (28, 29). Within seconds, the rolling lymphocytes, responding to the secondary lymphoid-tissue chemokine (SLC), activate cell-surface integrins such as lymphocyte function-associated antigen, type-1 (FLA-1), become firmly adhered, and transmigrate from the blood vessels into the LN tissue (12, 13, 28, 29). In L-selectin-deficient mice, lymphocytes do not bind to PLN HEV; these mice have a severe reduction in the number of lymphocytes in PLN (11). The movement of inflammatory leukocytes to inflamed blood vessels also is initiated by L-selectin (30) rolling on endothelial addressins such as vascular CD34 (13, 28). Leukocytes undergo the same three (or more) steps to transmigrate (12, 13, 28).
It has been shown that L-selectin can play a role in lymphoma metastases to LN in mice (31, 32). In humans, non-Hodgkin's lymphomas localized at PLN sites were L-selectin-positive (33). These findings support the concept that normal lymphocyte homing mechanisms are important for the dissemination of leukemias and lymphomas.
Based on what is known about the role of L-selectin in lymphocyte extravasation and lymphoma dissemination, we sought to ascertain whether expression of L-selectin on the nonlymphoma tumor cells would facilitate their localization to LN HEV. Indeed, we found that insulinoma cells carrying L-selectin on their cell surface showed an increased frequency of tumor cell metastases to PLN and MLN. The LacZ+ Tag+ cells detected in the LN were tumorigenic upon s.c. injection. The LN homing ability also was confirmed by directly injecting the L-selectin expressing tumor cells, labeled with fluorescent dye, into tail veins. In these mice, only the tumor cells carrying L-selectin were detected in the PLN. Surprisingly, both in the LTZ mice and in LN from LTZ tumor-transferred mice, the tumor cells did not simply bind to LN HEV, but in fact rapidly transmigrated to the LN cortex. Factors such as HEV chemokines (34), adhesion molecules, and interlymphoidal chemokines (35) are known to facilitate the binding and extravasation of lymphocytes. This binding suggests that the LTZ insulinoma cells completed the three (or more) steps of extravasation. This result also suggests that RIP-Tag insulinomas only lack L-selectin to enable homing to and through LN HEV. It shall be important to test whether these insulinoma cells possess and use known chemokine receptors and also integrins that allow their transmigration into LN, or if some other processes are involved.
Metastasis is a complex process. For tumor cells to metastasize, they have to detach from the primary tumor mass, invade extracellular matrices, intravasate into blood vessels, survive in and disseminate via the blood stream, localize to distant endothelium, extravasate from the blood stream, and, finally, grow at a new distant location. The expression of cell-surface homing and adhesion molecules also can inhibit metastases. Some high metastatic sublines of the mouse B16 melanoma fail to express the homophilic adhesion and homing heterodimer, integrin α4β1, whereas poorly metastatic lines were integrin α4+β1+ (18). The α4β1 positive cells attached to each other in vitro, whereas the α4β1 negative variants did not (18). The loss of the α4β1 receptor presumably allowed in situ tumors to detach as a prelude to invasion and metastasis. Reintroduction of the α4 transgene into these highly invasive and metastatic cells led to a concomitant loss of invasiveness in vivo and a gain of homotypic cell–cell interactions in vitro (18). Christofori and colleagues (36–38) also have documented the involvement of cell-cell interaction molecules in suppressing the metastatic phenotype in the RIP-Tag model of islet carcinogenesis. Interference with E-cadherin (36, 37) or neural cell adhesion molecule (38) expression in the islet β cells raises the frequency of metastasis from <5% to 25% and 39% of mice, respectively. The predominant metastatic site in each case was the draining (mesenteric) LN for the pancreas. Forced expression of E-cadherin or neural cell adhesion molecule with transgenes blocked the metastasis.
In the experiments described here not all of the triple transgenic mice were found to have LN metastases. Perhaps few cells were shed from the primary tumors in situ, or migrated from the first (hepatic) metastatic site, so that only a few LTZ tumor cells might enter the bloodstream after passage through the liver. Because of the massive and rapid growth of the insulinomas in situ, the average lifespan of these LTZ mice was only 12–14 weeks, and it may very well be that this period was not long enough for all of the mice to disseminate the tumors. Tumor cell metastases were observed in the PP and spleen in ≈1.5% of the LTZ mice, perhaps via PNAd determinants on MadCAM-1 molecules found in both organs (39, 40). Tumor cell metastases in MLN were observed in 3% of the ZT mice. These metastases may be via lymphatic drainage from the pancreas, liver, or invaded gut. The dramatic increase in the frequency of tumor metastases to PLN and MLN in the LTZ mice compared with the ZT mice suggests that tumor formation is necessary but not sufficient for tumor cell metastasis to distant lymphoid organs. No LacZ+ cells were observed in any organ of the LZ mice, indicating that primary tumor formation is necessary for their distribution.
PNAd determinants also are expressed in inflamed blood vessels and are critical for L-selectin-dependent extravasation of inflammatory leucocytes (30, 41). It shall be interesting to test whether LTZ insulinomas also can spread to the sites of inflammation. Finally, it is conceivable that any tumor cell could randomly activate expression of L-selectin during tumor progression, and so it is worth exploring a potential role of L-selectin in LN metastases of nonlymphoid tumors.
Acknowledgments
We thank Dr. Gerhard Christofori for his intellectual discussion and help in this work; and Dr. Dennise Dalma-Weiszhausz and Dr. Annette Schlageter for their assistance in editing the manuscript. This work was supported by U.S. Public Health Service Grant CA 42551 and a grant from SyStemix, Inc. to I.L.W., a Cancer Research Institute Fellowship to F.Q., and grants from the National Cancer Institute to D.H.
Abbreviations
- LN
lymph nodes
- PLN
peripheral LN
- MLN
mesenteric LN
- HEV
high endothelial venules
- X-gal
5-bromo-4-chloro-3-indolyl β-d-galactoside
- β-gal
β-galactosidase
- PP
Peyer's patch
- DiI
1,1′-dioctadecyl-3,3,3′,3′-tetramathyl-indocarbocyanine perchlorate
- PNAd
peripheral node addressin
References
- 1.Chambers A, Matrisian L M. J Natl Cancer Inst. 1997;89:1260–1270. doi: 10.1093/jnci/89.17.1260. [DOI] [PubMed] [Google Scholar]
- 2.Woodhouse E, Chuaqui R, Liotta L. Cancer. 1997;80:1529–1537. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1529::aid-cncr2>3.3.co;2-#. [DOI] [PubMed] [Google Scholar]
- 3.Holmgren L, O'Reilly M S, Folkman J. Nat Med. 1995;1:149–153. doi: 10.1038/nm0295-149. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D. Nature (London) 1985;315:115–122. doi: 10.1038/315115a0. [DOI] [PubMed] [Google Scholar]
- 5.Folkman J, Watson K, Ingber D, Hanahan D. Nature (London) 1989;339:58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- 6.Hanahan D, Christofori G, Naik P, Arbeit J. Eur J Cancer. 1996;32:386–393. doi: 10.1016/s0959-8049(96)00401-7. [DOI] [PubMed] [Google Scholar]
- 7.Naik P, Karrim J, Hanahan D. Genes Dev. 1996;10:2105–2116. doi: 10.1101/gad.10.17.2105. [DOI] [PubMed] [Google Scholar]
- 8.Grant S G, Seidman I, Hanahan D, Bautch V L. Cancer Res. 1991;51:4917–4923. [PubMed] [Google Scholar]
- 9.Siegelman M H, van de Rijn M, Weissman I L. Science. 1989;243:1165–1172. doi: 10.1126/science.2646713. [DOI] [PubMed] [Google Scholar]
- 10.Gallatin M, St. John T P, Siegelman M, Reichert R, Butcher E C, Weissman I L. Cell. 1986;44:673–680. doi: 10.1016/0092-8674(86)90832-9. [DOI] [PubMed] [Google Scholar]
- 11.Arbones M L, Ord D C, Ley K, Ratech H, Maynard-Curry C, Otten G, Capon D J, Tedder T F. Immunity. 1994;1:247–260. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 12.Girard J P, Springer T A. Immunol Today. 1995;16:449–457. doi: 10.1016/0167-5699(95)80023-9. [DOI] [PubMed] [Google Scholar]
- 13.Butcher E C. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 14.Cordell B, Bell G, Tischer E, DeNoto F M, Ullrich A, Pictet R, Rutter W J, Goodman H M. Cell. 1979;18:533–543. doi: 10.1016/0092-8674(79)90070-9. [DOI] [PubMed] [Google Scholar]
- 15.Bell G I, Pictet R L, Rutter W J, Cordell B, Tischer E, Goodman H M. Nature (London) 1980;284:26–32. doi: 10.1038/284026a0. [DOI] [PubMed] [Google Scholar]
- 16.Gutman G A, Weissman I L. Immunology. 1972;23:465–479. [PMC free article] [PubMed] [Google Scholar]
- 17.Gallatin W M, Weissman I L, Butcher E C. Nature (London) 1983;304:30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- 18.Qian F, Vaux D L, Weissman I L. Cell. 1994;77:335–347. doi: 10.1016/0092-8674(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 19.Lasky L A, Singer M S, Yednock T A, Dowbenko D, Fennie C, Rodriguez H, Nguyen T, Stachel S, Rosen S D. Cell. 1989;56:1045–1055. doi: 10.1016/0092-8674(89)90637-5. [DOI] [PubMed] [Google Scholar]
- 20.Siegelman M H, Cheng I C, Weissman I L, Wakeland E K. Cell. 1990;61:611–622. doi: 10.1016/0092-8674(90)90473-r. [DOI] [PubMed] [Google Scholar]
- 21.Tedder T F, Isaacs C M, Ernst T J, Demetri G D, Adler DA, Disteche C M. J Exp Med. 1989;170:123–133. doi: 10.1084/jem.170.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosen S D, Bertozzi C R. Curr Opin Cell Biol. 1994;6:663–673. doi: 10.1016/0955-0674(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 23.Bevilacqua M, Butcher E, Furie B, Furie B, Gallatin M, Gimbrone M, Harlan J, Kishimoto K, Lasky L, McEver R, et al. Cell. 1991;67:233. doi: 10.1016/0092-8674(91)90174-w. [DOI] [PubMed] [Google Scholar]
- 24.Streeter P R, Rouse B T, Butcher E C. J Cell Biol. 1988;107:1853–1862. doi: 10.1083/jcb.107.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berlin C, Berg E L, Briskin M J, Andrew D P, Kilshaw P J, Holzmann B, Weissman I L, Hamann A, Butcher E C. Cell. 1993;74:185–185. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 26.Holzmann B, McIntyre B W, Weissman I L. Cell. 1989;56:37–46. doi: 10.1016/0092-8674(89)90981-1. [DOI] [PubMed] [Google Scholar]
- 27.Hu MC-T, Crowe D, Holzmann B, Weissman I L. Proc Natl Acad Sci USA. 1992;89:8254–8258. doi: 10.1073/pnas.89.17.8254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butcher E C, Picker L J. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 29.von Andrian U H, M'Rini C. Cell Adhes Commun. 1998;6:85–96. doi: 10.3109/15419069809004463. [DOI] [PubMed] [Google Scholar]
- 30.Lewinsohn D, Bargatze R, Butcher E C. J Immunol. 1987;138:4313–4321. [PubMed] [Google Scholar]
- 31.Bargatze R F, Wu N W, Weissman I L, Butcher E C. J Exp Med. 1987;166:1125–1131. doi: 10.1084/jem.166.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sher B T, Bargatze R, Holzmann B, Gallatin W M, Matthews D, Wu N, Picker L, Butcher E C, Weissman I L. Adv Cancer Res. 1988;51:361–390. doi: 10.1016/s0065-230x(08)60226-2. [DOI] [PubMed] [Google Scholar]
- 33.Pals S T, Drillenburg P, Radaszkiewicz T, Manten-Horst E. Acta Haematol. 1997;97:73–80. doi: 10.1159/000203662. [DOI] [PubMed] [Google Scholar]
- 34.Gale L, McColl S R. BioEssays. 1999;21:17–28. doi: 10.1002/(SICI)1521-1878(199901)21:1<17::AID-BIES3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 35.Pachynski R, Wu S W, Gunn M D, Erle D J. J Immunol. 1998;161:952–956. [PubMed] [Google Scholar]
- 36.Christofori G, Semb H. Trends Biochem Sci. 1999;24:73–76. doi: 10.1016/s0968-0004(98)01343-7. [DOI] [PubMed] [Google Scholar]
- 37.Perl A K, Wilgenbus P, Dahl U, Semb H, Christofori G. Nature (London) 1998;392:190–193. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- 38.Perl A K, Dahl U, Wilgenbus P, Cremer H, Semb H, Christofori G. Nat Med. 1999;5:286–291. doi: 10.1038/6502. [DOI] [PubMed] [Google Scholar]
- 39.Steeber D, Tang M L, Zhang X O, Muller W, Wagner N, Tedder T F. J Immunol. 1998;161:6638–6647. [PubMed] [Google Scholar]
- 40.Kraal G, Schornagel K, Streeter P R, Holzmann B, Butcher E C. Am J Pathol. 1995;147:763–771. [PMC free article] [PubMed] [Google Scholar]
- 41.Michie S, Streeter P R, Bolt P A, Butcher E C, Picker L J. Am J Pathol. 1993;143:1688–1698. [PMC free article] [PubMed] [Google Scholar]