A report in PNAS suggests that mild hyperthermia can inhibit the process of homologous recombination (HR) and degrade the BRCA2 protein (1). Cellular hyperthermia, up to temperatures of 41 °C to 43 °C, has long been known to be a potent radiosensitizer of proliferating cells, providing the opportunity to enhance the tumoricidal effects of therapeutical radiation, because most nonmalignant cells in an irradiated field are nonproliferating or slowly proliferating (2). The precise mechanism of action has remained obscure, but hyperthermia appears to be toxic, particularly in the S phase of the cell cycle (3, 4). In the 1980s, radiation oncology departments around the world all constructed hyperthermia units, but they were never fully developed for the treatment of deep-seated tumors, because maintaining hyperthermia was a major technical challenge secondary to tumor blood flow being an effective cooling mechanism (5–7). If the technological issues could be overcome, this therapeutical approach would still have substantial merit. An alternative approach is to understand the mechanism of sensitization by heat in detail and then to develop “thermomimetic” drugs to produce the same selective toxicity (8).
Hyperthermia is a potent inducer of heat shock proteins (HSPs), for which there is an increasingly complex array of stress responses (9, 10). Many of them are chaperone proteins (some constitutive and some induced by heat stress), and they can protect against protein unfolding and degradation (11). Some of the HSPs may offer protective mechanisms to proteins involved in the DNA damage response (DDR), thereby exaggerating or modifying the normal DDR process, in which posttranslational modifications are added and removed in a complex control pathway (12). For example, the recruitment of 53BP1 is delayed after hyperthermia and ionizing radiation (IR), but there is no effect on the recruitment of γ-H2AX or MDC1 (13). The report by Krawczyk et al. (1) in PNAS describes the effect of heating cells or tumors to over 41 °C, which leads to the inhibition of HR much further downstream in the DDR. The potential therapeutical implication of this new finding is that heated cells should become vulnerable to poly-ADP-ribose-polymerase (PARP) inhibitors and, perhaps, HSP90 inhibitors.
The specific target of mild hyperthermia is now reported to be the BRCA2 protein. BRCA2 is the major mediator of RAD51 function in human cells in response to double-strand breaks (DSBs) (14). BRCA2 is downstream of BRCA1 and PALB2 in its engagement at sites of damage (15) but upstream of RAD51. Previous reports describing the effects of hyperthermia on DNA repair had suggested that sensitization could be seen in cells deficient in either HR or nonhomologous end-joining, suggesting that heat was acting more upstream in the DDR (16). However, the long-known observation that hyperthermia has specificity for S phase is consistent with an effect on HR (17, 18).
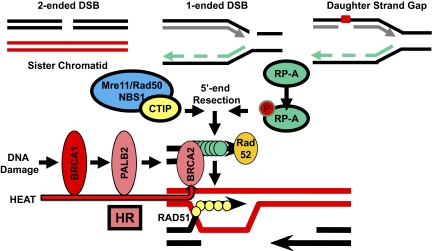
So how was BRCA2 found to be the target of hyperthermia? The initial specificity to HR was suggested by the lack of hyperthermic sensitization in HR-deficient cells, such as Rad54−/− ES cells or XRCC3-depleted cells. Then, the use of single α-particle tracks passing through the nuclei of cells, with or without hyperthermia, revealed normal recruitment of MRE11 and replication protein A (RP-A) (both required for responding to DNA damage) but defective recruitment of BRCA2 and RAD51 only after hyperthermia. The use of single α-particle tracks is a much better way to produce IR damage to subnuclear regions than the use of near-UV lasers (19), because the latter produces complex damage to chromatin and aberrant recruitment of proteins not seen at true IR-induced DSBs. Although these data are fascinating, they do not pinpoint the site of hyperthermia effect exactly at the level of BRCA2. MRE11 and RP-A could be recruited normally, but the defect produced by heat may be somewhere upstream of BRCA2 (Fig. 1). There could also be multiple effects of hyperthermia at many levels in the BRCA2 pathway of HR.
Fig. 1.
DSBs in S and G2 phases of the cell cycle, collapsed replication forks, and daughter-strand gaps all require HR for their repair. The effect of mild hyperthermia does not affect the ability of first responders, such as recruiting the Mre11 complex at DSB or RP-A binding to single-strand DNA. However, the recruitment of BRCA2 and RAD51 is affected, suggesting that somewhere in the HR pathway, there is susceptibility to heat-induced protein degradation. Although Krawczyk et al. (1) suggest that the target is BRCA2, it could be at multiple points along the BRCA2 pathway of HR, including BRCA1 and PALB2. In the absence of the key RP-A-to-RAD51 mediator, BRCA2, there is failure to carry out HR as observed by reduced RAD51 foci and reduced sister chromatid exchanges.
The consequence of hyperthermia is to make cells more sensitive to PARP inhibitors, such as NU1025 and PJ34, plus the use of siRNA depletion of PARP1 recapitulates the effect of PARP inhibitors, at least in one cell line. The possibility is that HSP inhibitors, such as geldanamycin derivatives (e.g., 17-DMAG), could further enhance the effect of heat and PARP inhibition, given our knowledge that HSPs contribute to the heat shock response and heat tolerance (20). The tumor growth delay and animal survival are consistent with at least an additive effect of HSP inhibitors on top of heat and PARP inhibition. There is no doubt that there is a therapeutical opportunity from exploiting a tumor's dependence on HR for its repair of DNA damaging agents, and revisiting hyperthermia, despite its technical difficulties, is justified, but not in tumors with a preexisting HR defect.
Why would a global cellular stress just pick out one target protein for degradation? Heat shock responses stimulate the production of a number of chaperone proteins, the consequence of which is to stimulate the unfolded protein responses, and hence protein degradation, in many targets (21). The ideal experiment would be to reexpress BRCA2 protein in heat-treated cells to see if the effect of heat could be specifically reversed by BRCA2. However, the expression of BRCA2 is challenging, because it is an ∼380-kD protein requiring almost 10 kb of cDNAfor plasmid-based expression. A feature
Heated cells should become vulnerable to PARP inhibitors and, perhaps, HSP90 inhibitors.
seen in DNA repair pathways, and implied for the BRCA1-BRCA2 pathway of HR, is that the level of expression of a downstream protein may depend on the intact function of upstream members of the pathway. The effect of hyperthermia is observed after 75 min of heating plus the postirradiation time. The BRCA2 protein has a half-life in normal cells of about 4 h and siRNA depletion is close to maximum at 24 h (22). Some of the observed effects of hyperthermia could be transmitted down the pathway by increased protein turnover at multiple steps in the HR pathway.
Previous studies of the effect of hyperthermia on DNA repair concluded that the impact was more likely mediated through base-excision repair (BER) (16), at the single-strand break religation step. As we know from the proposed mechanism of PARP inhibitors, unresolved single-strand breaks in S phase can produce DSBs. The conclusion from these earlier studies would be to predict that BRCA2-deficient cells should be sensitive to hyperthermia, which is the opposite of the prediction of the current studies. Whether the primary target effect of hyperthermia is on BER proteins or on HR proteins will need more work with well-defined genetic systems, preferably with complementation. Whatever the final mechanistic explanation of the molecular effects of hyperthermia, these new results will make us rethink the potential use of hyperthermia in the sensitization of tumors to cytotoxic or biologically targeted therapies.
Acknowledgments
This paper was supported by grants from the National Cancer Institute and Susan G. Komen for the Cure.
Footnotes
The authors declare no conflict of interest.
See companion article on page 9851.
References
- 1.Krawczyk PM, et al. Mild hyperthermia inhibits homologous recombination, induces BRCA2 degradation, and sensitizes cancer cells to poly(ADP-ribose) polymerase-1 inhibition. Proc Natl Acad Sci USA. 2011;108:9851–9856. doi: 10.1073/pnas.1101053108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wanner RA, Edwards MJ, Wright RG. The effect of hyperthermia on the neuroepithelium of the 21-day guinea-pig foetus: Histologic and ultrastructural study. J Pathol. 1976;118:235–244. doi: 10.1002/path.1711180406. [DOI] [PubMed] [Google Scholar]
- 3.Dewey WC, Hopwood LE, Sapareto SA, Gerweck LE. Cellular responses to combinations of hyperthermia and radiation. Radiology. 1977;123:463–474. doi: 10.1148/123.2.463. [DOI] [PubMed] [Google Scholar]
- 4.VanderWaal RP, Griffith CL, Wright WD, Borrelli MJ, Roti JL. Delaying S-phase progression rescues cells from heat-induced S-phase hypertoxicity. J Cell Physiol. 2001;187:236–243. doi: 10.1002/jcp.1073. [DOI] [PubMed] [Google Scholar]
- 5.Arora D, Skliar M, Roemer RB. Model-predictive control of hyperthermia treatments. IEEE Trans Biomed Eng. 2002;49:629–639. doi: 10.1109/TBME.2002.1010846. [DOI] [PubMed] [Google Scholar]
- 6.DeFord JA, et al. Design and evaluation of closed-loop feedback control of minimum temperatures in human intracranial tumours treated with interstitial hyperthermia. Med Biol Eng Comput. 1991;29:197–206. doi: 10.1007/BF02447108. [DOI] [PubMed] [Google Scholar]
- 7.Dickson JA. Hyperthermia in the treatment of cancer. Lancet. 1979;1:202–205. doi: 10.1016/s0140-6736(79)90594-4. [DOI] [PubMed] [Google Scholar]
- 8.Roti Roti JL. Cellular responses to hyperthermia (40-46 degrees C): Cell killing and molecular events. Int J Hyperthermia. 2008;24:3–15. doi: 10.1080/02656730701769841. [DOI] [PubMed] [Google Scholar]
- 9.Calderwood SK, Ciocca DR. Heat shock proteins: Stress proteins with Janus-like properties in cancer. Int J Hyperthermia. 2008;24:31–39. doi: 10.1080/02656730701858305. [DOI] [PubMed] [Google Scholar]
- 10.Calderwood SK, Asea A. Targeting HSP70-induced thermotolerance for design of thermal sensitizers. Int J Hyperthermia. 2002;18:597–608. doi: 10.1080/0265673021000019666. [DOI] [PubMed] [Google Scholar]
- 11.Liberek K, Lewandowska A, Zietkiewicz S. Chaperones in control of protein disaggregation. EMBO J. 2008;27:328–335. doi: 10.1038/sj.emboj.7601970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciccia A, Elledge SJ. The DNA damage response: Making it safe to play with knives. Mol Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laszlo A, Fleischer I. Heat-induced perturbations of DNA damage signaling pathways are modulated by molecular chaperones. Cancer Res. 2009;69:2042–2049. doi: 10.1158/0008-5472.CAN-08-1639. [DOI] [PubMed] [Google Scholar]
- 14.Jensen RB, Carreira A, Kowalczykowski SC. Purified human BRCA2 stimulates RAD51-mediated recombination. Nature. 2010;467:678–683. doi: 10.1038/nature09399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia B, et al. Control of BRCA2 cellular and clinical functions by a nuclear partner, PALB2. Mol Cell. 2006;22:719–729. doi: 10.1016/j.molcel.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 16.Kampinga HH, Dikomey E. Hyperthermic radiosensitization: Mode of action and clinical relevance. Int J Radiat Biol. 2001;77:399–408. doi: 10.1080/09553000010024687. [DOI] [PubMed] [Google Scholar]
- 17.Hinz JM, Yamada NA, Salazar EP, Tebbs RS, Thompson LH. Influence of double-strand-break repair pathways on radiosensitivity throughout the cell cycle in CHO cells. DNA Repair (Amst) 2005;4:782–792. doi: 10.1016/j.dnarep.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Rothkamm K, Krüger I, Thompson LH, Löbrich M. Pathways of DNA double-strand break repair during the mammalian cell cycle. Mol Cell Biol. 2003;23:5706–5715. doi: 10.1128/MCB.23.16.5706-5715.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams ES, et al. DNA double-strand breaks are not sufficient to initiate recruitment of TRF2. Nat Genet. 2007;39:696–698. doi: 10.1038/ng0607-696. author reply 698–699. [DOI] [PubMed] [Google Scholar]
- 20.Burdon RH. Thermotolerance and the heat shock proteins. Symp Soc Exp Biol. 1987;41:269–283. [PubMed] [Google Scholar]
- 21.Davenport EL, Morgan GJ, Davies FE. Untangling the unfolded protein response. Cell Cycle. 2008;7:865–869. doi: 10.4161/cc.7.7.5615. [DOI] [PubMed] [Google Scholar]
- 22.Feng Z, et al. Rad52 inactivation is synthetically lethal with BRCA2 deficiency. Proc Natl Acad Sci USA. 2011;11:686–691. doi: 10.1073/pnas.1010959107. [DOI] [PMC free article] [PubMed] [Google Scholar]