Abstract
The crucial process of aminoacyl-tRNA delivery to the ribosome is energized by the GTPase reaction of the elongation factor Tu (EF-Tu). Advances in the elucidation of the structure of the EF-Tu/ribosome complex provide the rare opportunity of gaining a detailed understanding of the activation process of this system. Here, we use quantitative simulation approaches and reproduce the energetics of the GTPase reaction of EF-Tu with and without the ribosome and with several key mutants. Our study provides a novel insight into the activation process. It is found that the critical H84 residue is not likely to behave as a general base but rather contributes to an allosteric effect, which includes a major transition state stabilization by the electrostatic effect of the P loop and other regions of the protein. Our findings have general relevance to GTPase activation, including the processes that control signal transduction.
Keywords: enzymatic catalysis, preorganization, allostery
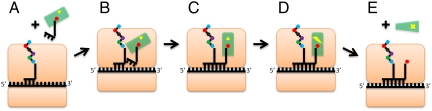
The elongation cycle of protein synthesis uses the elongation factor Tu (EF-Tu) with GTP to deliver aminoacyl-tRNA (aa-tRNA) to the mRNA-programmed ribosome. More specifically, the GTP-bound state of EF-Tu forms a high-affinity ternary complex with the aa-tRNA. Upon binding of the ternary complex to the ribosome, the aa-tRNA occupies the A site and, when the codon–anticodon interaction is cognate, the GTPase activity of the EF-Tu is increased significantly. The conformational change following GTP hydrolysis to GDP and a leaving phosphate group (Pi) leads to dissociation of EF-Tu from the ribosome and accommodation of the aa-tRNA on the A site for peptidyl transfer (see Fig. 1 for a schematic description; for additional details, see refs. 1–3).
Fig. 1.
Schematic representation of the role of EF-Tu during the elongation process. The prokaryotic ribosome is shown (in orange) reading a messenger RNA (mRNA) sequence while in the early stage of elongating a polypeptide with an amino acid bound to an incoming ternary complex comprised of a GTP-bound EF-Tu and aa-tRNA. (A) EF-Tu:GTP:aa-tRNA (ternary complex) and the ribosome with mRNA. (B) Initial binding of ternary complex to the ribosome with mRNA. (C) Codon–anticodon recognition and conformational change of ternary complex on the ribosome with mRNA. (D) Hydrolysis of GTP to GDP on the ribosome with mRNA. (E) EF-Tu:GDP dssociation and aa-tRNA accommodation on the ribosome with mRNA. The small circles represent amino acids, the yellow triangle represents GTP, the yellow lightning bolt represents a chemical transformation (i.e., GTP hydrolysis to GDP), and the yellow cross represents GDP.
Breakthrough in the elucidation of the ribosome structure (2–13) and careful biochemical studies (1–3, 14–25) allow one to begin thinking about the nature of the mechanism of the elongation process. In particular, the elucidation of structure of the EF-Tu/ribosome complex (our study refers to the structure of EF-Tu in the complex as EF-Tu′) (9) opens the way for the exploration of the activation process at the molecular level. However, despite major biochemical and structural breakthroughs, a detailed explanation of how the codon recognition in the 30S subunit leads to GTP hydrolysis remains elusive. That is, although it is known that the precise positioning of H84 is critical for efficient catalysis (1, 9, 11–13, 23–26), the energetics of this positioning and its ultimate role in stabilizing the transition state (TS) are unclear. More precisely, it is frequently assumed that the conserved H84 is moved to a catalytic configuration and then serves as a general base, but this assumption is problematic (see ref. 1 and Reproducing the Overall Catalytic and Mutational Effects below) and has similar pitfalls as in the highly related case of the Ras-RasGAP (RasGAP) system. That is, where it was originally assumed that Q61 in the RasGAP system serves as a general base, but this assumption has been shown to be incorrect (see the review in ref. 27). In fact, it has been argued, and, in some respect demonstrated by simulations (27), that the activation process is due to allosteric changes in the P loop, which are disrupted by mutations of Q61. However, it has been difficult to characterize experimentally the structural changes associated with the Q61 mutation in the RasGAP complex or even to clearly identify structural changes in the P loop upon formation of the complex. Fortunately, there are direct structural evidences (9) in the EF-Tu′/ribosome system, where the P-loop structure changes in the ribosome-bound structure. This evidence suggests allosteric structural changes as much more likely candidates for the activation process than the effect of positioning the H84 side chain in an orientation that will presumably make it a good general base.
It is important to note that the theoretical attempts to study the activation process of EF-Tu at the molecular level have been rather limited, ranging from the study of the isolated EF-Tu (28) to a generation of the structure of the EF-Tu′/ribosome complex through simulation (10).
The present work advances the understanding of the molecular origin of the activation process by addressing its energetics and focusing on the catalytic effect of the transition to the EF-Tu′/ribosome complex. Our study reproduces quantitatively the catalytic effect of EF-Tu (relative to the uncatalyzed reaction) and the increase of this effect in the activated complex and then explores the critical role of H84. It is found that H84 does not act in a direct way but occupies a pivotal position in an exquisitely preorganized catalytic configuration generated by the binding of EF-Tu and aa-tRNA to the ribosome. Selected mutations of H84 destroy this preorganization by indirectly disturbing a particularly sensitive region of the protein and thus change the nature of interaction between the active site residues and the TS. The general implications of this mechanism are also discussed.
Results and Discussion
Reproducing the Overall Catalytic and Mutational Effects.
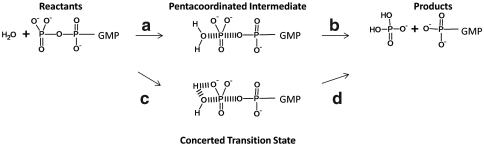
Before exploring the role of H84, it is essential to establish the reliability of our computational approach (see Methods and SI Text) by calculating the acceleration of the GTP hydrolysis by both EF-Tu and its activated complex with the ribosome and the aa-tRNA (i.e., the complex following codon recognition). The GTP hydrolysis mechanism can be described by considering the mechanisms outlined in Fig. 2. It should be noted that, although we demonstrated in earlier studies (29) that the protein catalytic effect is similar for associative and concerted mechanisms, we examined here both options by using the corresponding empirical valence bond (EVB) models (see SI Text and Methods for a discussion of the calibration process). Overall, we obtained very similar results from both models. The calculated EVB free-energy profiles for the GTPase reaction (with the stepwise model) in water, in the isolated EF-Tu, and in the EF-Tu′/ribosome complex are presented in Fig. 3. The results for the concerted model are given in SI Text. The corresponding free-energy barriers with the stepwise model are also summarized in Table 1. As seen from Fig. 3 and Table 1, the experimental trend is reproduced. It is interesting to see that the rate-limiting step for the stepwise model is associated with the second barrier (i.e., except for the H84Q mutation). The height of this activation barrier is reduced in EF-Tu and reduced to a much greater extent in the EF-Tu′/ribosome complex upon stabilization of the TS. It is useful to note that the energetics reported for GTP hydrolysis (see Fig. 3) pertain to the chemical step only, and the removal of the leaving group is not included. After the chemical step is completed, conformational steps are likely to further decrease the free energy of the product state (PS) by moving EF-Tu from a GTP-bound conformation to a more stable GDP-bound conformation. The origin of the total effect of the EF-Tu → EF-Tu′/ribosome transition is discussed in The Ribosome Changes the Preorganization of EF-Tu and Exploring the Storage of the Allosteric Elongation Free Energy.
Fig. 2.
Schematic representation of the GTPase reaction mechanism. The reaction is considered as a two-step (steps a and b) or concerted process (steps c and d). Step a of the two-step process involves an attack of a water molecule on GTP and a formation of a pentacoordinated intermediate, whereas step b of the two-step process involves a cleavage of the Pβ-O bond and the generation of a leaving phosphate group and the GDP.
Fig. 3.
Energetics of the GTPase reaction (with the stepwise mechanism). The average free-energy profiles (kcal mol-1), calculated using the EVB FEP/umbrella sampling procedure, for the GTPase reaction in (A) water, EF-Tu, and EF-Tu’/ribosome complex, and (B) H84A, H84Q, and H84HNP mutants. The free energies are given relative to the free energies of the reactants. The notation NP (nonpolar) indicates that all the residual charges of the given residue are set to zero. The number of points in each profile does not correspond to the total number of frames employed in the FEP mapping of the respective GTPase reaction.
Table 1.
Energetics of the GTPase reaction in different systems
 |
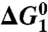 |
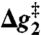 |
 (calc) (calc) |
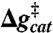 (exp) (exp) |
|
| Water | 20 | 17 | 29 | 29 | 27* |
| EF-Tu | 20 | 12 | 26 | 26 | 23† |
| EF-Tu′/ribosome | 13 | 13 | 18 | 18 | ≤ 14‡,§ |
| H84HNP (EF-Tu′) ¶ | 15 | 15 | 19 | 19 | ∥ |
| H84Q (EF-Tu′) | 17 | 12 | 15 | 17 | ** |
| H84A (EF-Tu′) | 22 | 22 | 26 | 26 | 22†† |
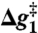 ,
, 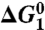 , and
, and 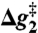 correspond to the relative free energies (kcal mol-1) of the first TS, pentacoordinated intermediate, and the second TS, respectively (see Fig. 2). The exponent of a relative free energy does not correspond to a footnote. The free energies are given relative to the free energies of the reactants. The values of the experimental activation free energy
correspond to the relative free energies (kcal mol-1) of the first TS, pentacoordinated intermediate, and the second TS, respectively (see Fig. 2). The exponent of a relative free energy does not correspond to a footnote. The free energies are given relative to the free energies of the reactants. The values of the experimental activation free energy 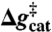 (exp) were obtained from the corresponding rate constants (kcat) using transition state theory (see ref. 40) and a preexponential factor of 6 × 1012 s-1.
(exp) were obtained from the corresponding rate constants (kcat) using transition state theory (see ref. 40) and a preexponential factor of 6 × 1012 s-1.
The calculated results were obtained by averaging a minimum of five simulations with different initial configurations. The effects of different mutations on kcat are not always available due to experimental difficulties. Thus, we provide the experimental results for the H84A mutant only.
*Evaluated from kcat = 5.5 × 10-8 s-1 at 25 ºC, which was estimated from the linear free-energy relationship (LFER) proposed in ref. 50 between log kcat and pKa. A pKa value of 6.7 was estimated from ref. 51, based upon experimental results for the terminal phosphate group of ATP at 0.2 ionic strength.
†Evaluated from kcat = 5 × 10-5 s-1 at 20 ºC, which was taken from ref. 1.
‡Evaluated from kcat = 500 s-1 at 20 °C, which was taken from ref. 1.
§The inequality reflects the fact that the GTPase reaction might not be the rate-limiting step during A-site binding of the ternary complex to the ribosome.
¶NP indicates that the corresponding residue was “mutated” to its hypothetical nonpolar form.
∥The hypothetical H84HNP mutant does not correspond to a real mutation (see Reproducing the Overall Catalytic and Mutational Effects of the text for details).
**See Reproducing the Overall Catalytic and Mutational Effects of the text for experimental details.
††Evaluated from kcat = 3 × 10-4 s-1 at 20 ºC, which was taken from ref. 1.
Another significant point was our finding that H84 has a pKa above 10 and is very likely to be protonated in the complex due to its interaction with the phosphate group of residue A2662 of the 23S rRNA (see SI Text). This finding indicates that H84 is unlikely to serve as a general base. Furthermore, the finding that the rate constant does not change when the pH changes from 6 to 9 (see ref. 1) also provides a strong evidence against the general base idea. Nevertheless, a detailed calculation of the mechanism where H84 is a general base, as performed in our work on Q61 in the RasGAP system (see ref. 27), will help to determine the energetics of the general base mechanism.
Next, we explored our ability to reproduce the effect of the H84A mutation. The results (see Figs. 3b and 4 and Table 1) demonstrate that we can capture the important fact that the H84A mutation leads to loss of the catalytic effect. After demonstrating our ability to reproduce the observed effect of the H84A mutation, we can begin exploring the origin of the GTPase activity and its relationship to the structural orientation of H84. As a first step, we mutated H84 to its nonpolar analogue (H84HNP) by forcing all residual charges on the side chain to be zero (i.e., this “mutation” was done by moving from a model where H84 was explicitly ionized to a model where H84 was completely nonpolar). Interestingly, this mutation did not reproduce the observed large anticatalytic effect of the H84A mutation (see Table 1). That is, the activation barrier increased approximately 1 kcal mol-1 instead of the 5–9 kcal mol-1 observed effect for the H84A mutation. Apparently, H84 interacts strongly with the reacting system (i.e., substrate plus water) in both the reactant state (RS) and the TS. However, because the strong interaction of H84 with the reacting system is similar in the RS and the TS, its overall effect is not large. It might be important to clarify that, although the H84HNP mutation cannot be performed experimentally, it is perhaps the most effective theoretical way for examining the direct catalytic role of H84. That is, if H84 catalyzes the GTPase reaction by its electrostatic effect, then a complete removal of the residual charges would destroy the corresponding catalytic effect. But, if the catalytic effect of H84 is due to an indirect effect (i.e., by helping other residues to remain in a correct catalytic arrangement), the H84HNP mutation will probably not have a large anticatalytic effect. Our results reveal that, indeed, the H84HNP mutation has a small anticatalytic effect (assuming, however, that the protein stays in the EF-Tu′ conformation). Again, because a fully nonpolar mutant does not exist, there is no direct experiment that can reproduce the above finding. Thus, it is essential to demonstrate that we can reproduce the anticatalytic effect of an experimentally investigated mutation, such as the H84A mutation. As seen from Figs. 3B and 4 and Table 1, we were able to reproduce the observed effect of the H84A mutation and thus encouraged to try to predict the effect of the H84Q mutation. Here, we found that the calculated effect of this mutation is much smaller than that of the H84A mutant (see Figs. 3b and 4 and Table 1).
Fig. 4.
The average activation barriers (kcal mol-1) for the reacting systems in this study. For water, EF-Tu, EF-Tu′/rib, and the H84A mutant (EF-Tu′), the experimental data was taken from Table 1. Calculated activation barriers for the Ras and RasGAP systems are provided for the sake of comparison. kcat = 4.7 × 10-4 s-1 at 37 °C for Ras and kcat = 19.1 s-1 at 25 ºC for RasGAP were taken from ref. 52, and the experimental activation barriers were calculated analogously to the method described in Table 1. The notation NP indicates that all the residual charges of the given residue or set of residues are set to zero. The notation K = 0.2 indicates that the simulation was performed with a force constraint using a force constant of K = 0.2 kcal mol-1 Å-2 on the EF-Tu residues. The notation “rib” indicates the presence of the ribosome in the simulations.
It is important to consider the significance of our prediction that the catalytic effect of H84 is indirect and that the H84Q mutation has a small anticatalytic effect (i.e., 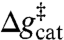 is about 1 kcal mol-1 lower than in the wild-type EF-Tu′/ribosome). This prediction (which assumes, however, that the complex stays in the EF-Tu′ conformation) is similar to our earlier predictions regarding the catalytic effect of residue Q61 in the RasGap complex (27). However, in the present case, we have a hint from direct experiments. That is, experimental studies of the H84Q mutation in Thermus thermophilus (23, 25) revealed that this mutation leads only to a small reduction in the GTP hydrolysis rate (i.e., in the presence of a ribosome and the aa-tRNA) relative to the native enzyme. Unfortunately, in the T. thermophilus system studied in refs. 23 and 25, the absolute values of the GTP hydrolysis rates at 37 °C are small, and the system has not been as well-characterized as the corresponding Escherichia coli system, where an enormous effect of the H84A mutant has been observed (1). Thus, a further validation of our prediction in the E. coli system would be extremely instructive. At any rate, because our model reproduces the large observed anticatalytic effect of the H84A mutation, we can go one step further and explore the origin of these calculated effects. This analysis is presented in The Ribosome Changes the Preorganization of EF-Tu and Exploring the Storage of the Allosteric Elongation Free Energy.
is about 1 kcal mol-1 lower than in the wild-type EF-Tu′/ribosome). This prediction (which assumes, however, that the complex stays in the EF-Tu′ conformation) is similar to our earlier predictions regarding the catalytic effect of residue Q61 in the RasGap complex (27). However, in the present case, we have a hint from direct experiments. That is, experimental studies of the H84Q mutation in Thermus thermophilus (23, 25) revealed that this mutation leads only to a small reduction in the GTP hydrolysis rate (i.e., in the presence of a ribosome and the aa-tRNA) relative to the native enzyme. Unfortunately, in the T. thermophilus system studied in refs. 23 and 25, the absolute values of the GTP hydrolysis rates at 37 °C are small, and the system has not been as well-characterized as the corresponding Escherichia coli system, where an enormous effect of the H84A mutant has been observed (1). Thus, a further validation of our prediction in the E. coli system would be extremely instructive. At any rate, because our model reproduces the large observed anticatalytic effect of the H84A mutation, we can go one step further and explore the origin of these calculated effects. This analysis is presented in The Ribosome Changes the Preorganization of EF-Tu and Exploring the Storage of the Allosteric Elongation Free Energy.
The Ribosome Changes the Preorganization of EF-Tu.
After validating our calculations by reproducing the overall catalytic effect and the effects of different mutations, we can examine the origin of these effects. We start by exploring the overall catalytic effect of the ribosome by focusing on its indirect structural effect through its interaction with EF-Tu. Comparison of the X-ray structure of the isolated EF-Tu to the structure of EF-Tu in the EF-Tu′/ribosome complex reveals (see Fig. 5) some of the structural changes induced by the binding of the ribosome to EF-Tu [e.g., H84 moves closer to the γ-phosphate of GTP (9)]. A graphical display of the structure of the entire catalytic complex structure of EF-Tu is provided in Fig. S1. However, although this comparison is instructive, it cannot explain the actual functional implications of the corresponding structural changes; similarly, TS analogues (TSAs) require extensive theoretical analysis to establish the exact relationship between TSAs and the corresponding TSs (30).
Fig. 5.
Comparing the structures of the active site in both the EF-Tu (gray, from PDB ID code 1EFT) and EF-Tu′/ribosome (yellow, from PDB ID code 2XQD) that were used as starting points of our simulations (PyMOL software was used for the structural alignment). Critical regions (P loop, switch I, and switch II) are labeled. GTP and water are included in the RS configuration of EF-Tu′/ribosome. The Mg2+, aa-tRNA, and ribosome are not shown for the sake of clarity in this diagram. Note that, although the superposition can be subjective, the actual calculated group contributions (see SI Text) are independent of the relative initial orientation on the structures.
To gain a deeper understanding of the nature of the catalytic effect in the EF-Tu′/ribosome complex, it is important to clarify the structural role of the ribosome during the activation process. Here, it is essential to consider the effect of the sarcin–ricin loop (SRL) of the 23S rRNA, and in particular residue A2662 (see Fig. S2), whose critical role has been pointed out before (14–16). Significantly, our simulations of the EF-Tu′/ribosome complex found that a proper H84 positioning, without any additional artificial force constraints, required the presence of the SRL loop with its A2662 residue. The remaining ribosome components that compose the GTPase binding site, which play lesser but nonetheless important roles in the preorganization of the active site required for GTP hydrolysis, are the rRNA residues and the L11 and L12 proteins (17). Interestingly, mutation of the SRL residues near A2662 (16) or cleavage of the SRL between residues A2662 and G2661 with the toxin α-sarcin (18) leads to significantly reduced levels of GTP hydrolysis (9). Our computational studies confirm that the SRL is necessary for moving the system to the EF-Tu′ conformation as the presence of residue A2662 or a related external constraint on H84 (that indirectly pushes EF-Tu to the EF-Tu′ structure) is required to reproduce the experimentally observed catalytic effect (see Fig. 4). A part of this effect might be associated with a strong electrostatic interaction with a protonated H84. More information on the relaxation effects is given in SI Text.
In order to explore the effect of the above-mentioned structural changes, we must employ a systematic computational analysis. First, we explored the activation barriers in the following systems: (i) EF-Tu and EF-Tu′, both without the ribosome and with constraints on the corresponding initial structure, and (ii) EF-Tu′ with the ribosome. Analysis of the corresponding results reported in Fig. 4 indicates that the effect of the ribosome is mainly in moving the EF-Tu to the EF-Tu′ structure, where the active site is preorganized for catalysis.
Another type of analysis was performed by calculating electrostatic group contributions of the protein residues to the TS stabilization (see SI Text). This approach allows us to estimate the functional contributions of different structural changes and to determine which changes have a significant effect along the pathway from the RS to the TS. Thus, we are able to determine the allosteric changes in the individual electrostatic contributions to TS stabilization upon transformation from EF-Tu and EF-Tu′. This analysis is independent of the way one superimposes different structures because it looks upon the internal energy of each system. As discussed in SI Text, these contributions are not equal to the effect of mutating the corresponding residues but give general qualitative hints about mutation effects. Fig. S3 presents the changes in the electrostatic contributions of the EF-Tu residues due to formation of the EF-Tu′/ribosome complex. Figs. S3 a and b describe the contributions in the RS and in the TS [or the intermediate state (IS)], respectively. A similar trend is observed in the comparison of the native and the H84A mutant (generated from a structure that was obtained by starting from the EF-Tu′ structure), but the contributions in the RS and in the TS (or the IS) are significantly less pronounced. Because the IS and the TS have similar properties and the calculations of the intermediate are more stable, we consider here the results for the IS as descriptors of the corresponding TS results. Apparently, significant changes occur in the P loop and switch II (see Fig. 5 for a graphical display of the corresponding structural changes). The reorganization between free EF-Tu and EF-Tu′ is likely of functional importance.
Exploring the Storage of the Allosteric Elongation Free Energy.
At this point, we can move to the long-standing issue of the storage of the free energy that leads to the conformational change from EF-Tu to EF-Tu′. More specifically, upon codon–anticodon recognition, significant conformational changes occur in the ribosome, tRNA, and EF-Tu and thus leads to the activation of the GTPase center (9, 11). The free-energy cost for these conformational changes is drawn from the successful binding of a cognate tRNA and the associated interactions of the 16S rRNA residues with the codon–anticodon helix (5).
As a first step in the attempt to quantify the above effects, we used our linear response approximation (LRA) strategy in the way exploited in previous studies of F1-ATPase (31) and other systems (32, 33). That is, using the LRA strategy we estimated the free-energy change of the entire protein system, upon moving between two configurations in the reactant state. This was done by evaluating the EVB (or related) energy gaps in these two configurations (e.g., see ref. 32):
 |
[1] |
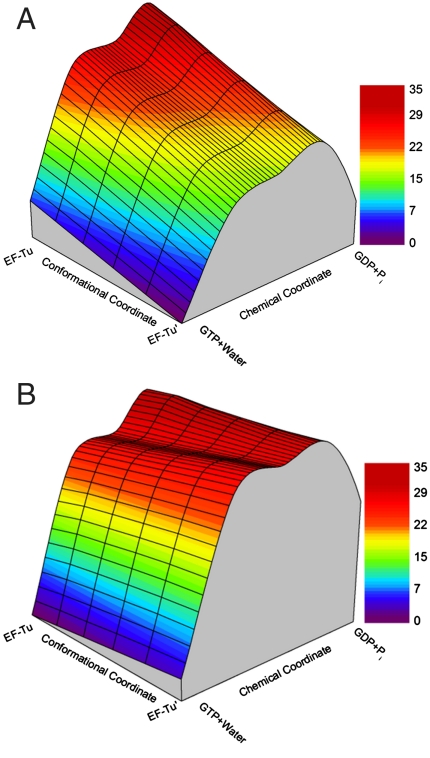
where ϵ1 and ϵ2 are the potential energy surfaces of the actual reactant (polar ligand) and a hypothetical nonpolar state. The notation 〈 〉i designates an average over trajectories that are propagated on ϵ1, where the system is held by a weak constraint near the coordinates of the indicated conformations (ri). Evaluating the LRA terms leads to the results presented in Table 2. The calculated results probably provide an overestimate due to convergence difficulties and more realistic results can be obtained by using an effective dielectric constant (34). These difficulties reflect the long time needed for water penetration and for establishing other compensating electrostatic effects. In the present system, which is highly charged, key relaxation events, such as water penetration, might be missed. In fact, to improve our results, we have future plans to use our renormalization approach (35). However, even the current calculations indicate (see Table 2) that the H84A mutant destabilizes the EF-Tu′ configuration relative to the EF-Tu configuration. Next, we extended our study and simulated the catalytic free-energy landscape in the space defined by the chemical and conformational coordinates. This challenging exploration has been performed by first using targeted molecular dynamics (TMD) (36) along the conformational coordinate to generate representative configurations, and then applying the linear response approximation for configurations along the conformational coordinate, while performing EVB calculations to evaluate the chemical barriers at these configurations (see SI Text). The corresponding results are depicted in Fig. 6 for the native and H84A mutant. As seen from Fig. 6, the mutation shifts the landscape in a way that reduces the population of the EF-Tu′ configuration. This change is a clear allosteric consequence of the binding of EF-Tu (or the ternary complex) to the ribosome. The allosteric effect is driven by a key interaction (i.e., the interactions between H84 and the surrounding residues including the SRL loop). Ultimately, these interactions are coupled to a conformational change that moves the system from the EF-Tu conformation to the EF-Tu′ conformation. In other words, the H84A mutation disrupts the preorganization of the active site groups of the EF-Tu′ system. It should be noted that a part of the effect of the H84A mutation already occurs at the EF-Tu′ configuration and reflects some local structural effects.
Table 2.
LRA free-energy contributions to the conformational change between EF-Tu and EF-Tu′ in the native and H84A systems in the presence of the ribosome and aa-tRNA
| EF-Tu (r1) | EF-Tu′ (r2) | ||
| System |
〈ε2 - ε1〉ε1,r1 |
〈ε2 - ε1〉ε1,r2 |
ΔG(ε1(r1) → ε1(r2)) |
| Native | 1,955.0 | 1,992.0 | −18.5* (−6.2†) |
| H84A mutant | 1,971.0 | 1,956.0 | +7.5* (+2.5†) |
ΔG corresponds to the free energy (kcal mol-1) of the conformational change from r1 (EF-Tu) to r2 (EF-Tu′). The free energies were evaluated using Eq. 1, where ϵ1 and ϵ2 are the potential energy surfaces of the actual reactant (polar ligand) and a hypothetical nonpolar state, and 〈 〉i designates an average over trajectories that are propagated on ϵ1, where the system is held by a weak constraint near the coordinates of the indicated conformations (ri).
*These results most likely provide an overestimate due to convergence difficulties, and more realistic results can be obtained by using an effective dielectric constant (34) (see Exploring the Storage of the Allosteric Elongation Free Energy of the text for details).
†Scaled free-energy contributions to the conformational change using an effective dielectric constant of 3 as a rough estimate to correct convergence difficulties (this should not be confused with the inconsistent use of an effective dielectric constant of 4 in some macroscopic studies).
Fig. 6.
An approximate catalytic free-energy landscape (kcal mol-1) for the coupling between the chemical coordinate (i.e., the movement from the RS to the PS) and the conformational coordinate that connects the EF-Tu and EF-Tu′ conformations in the (A) native and (B) H84A mutant systems.
Concluding Remarks
The present work examined the elusive origin of the activation of EF-Tu by the ribosome through the combination of previously undescribed structural information (9) and quantitative simulation approaches. The simulations reproduced the observed catalytic effects and their change upon activation by the ribosome, as well as the effect of key mutations. These accomplishments indicated that the EVB model employed (see SI Text) can help in resolving the major outstanding question regarding the origin of the long-distance activation of EF-Tu by the ribosome.
In exploring the origin of the catalytic effect we found that the activation is not directly due to the repositioning of H84, but to an overall allosteric transition that forces EF-Tu, and in particularly the P loop, to a preorganized catalytic configuration (EF-Tu′). Moreover, part of the effects of different mutants (e.g., H84A) also reflects local direct perturbations and further exploration of the partition between the direct and indirect effects of a given mutation is clearly needed, preferably with more experimental information on the effect of different mutants. At any rate, in view of the present findings, we consider the indirect effect of the ribosome as the major factor in the activation of the GTPase hydrolysis. Specifically, relatively small structural rearrangements in switch I, switch II, and the P loop (due to the formation of the EF-Tu′/ribosome complex) alter in a drastic way the stabilization of the TS. Overall, the system can be considered metastable with regard to its native sequence, where mutations of some residues change the free-energy landscape and lead to destruction (or enhancement) of the catalytic effect. Mutations of H84 are particularly effective in destroying the catalytic configuration, leaving the system in its inactivated state and thus preventing protein synthesis.
It may be useful to comment here on other factors, such as the “hydrophobic gate” effect (37). Upon initial binding of the ternary complex and codon–anticodon recognition, there is a disruption of the interaction between the tRNA and switch I of EF-Tu, which has been assumed to transmit a signal to the GTPase center by opening the hydrophobic gate (i.e., V20 and I61). This interesting structural element was suggested to block the interaction of G83 and H84 of switch II with either the γ-phosphate of GTP or the catalytic water molecule, thus disrupting the hydrolysis (1, 19, 37). Although this proposal is interesting, the resulting effect on catalysis is unlikely to be significant. That is, the hydrophobic residues can lead to a barrier on the way to the correctly preorganized configuration, but this barrier is unlikely to be a rate-limiting barrier and thus is unlikely to influence the catalysis. In fact, mutations of residues in the hydrophobic gate region (i.e., V20G and I61A) have not caused an increase in the intrinsic or ribosome-induced GTP hydrolysis and thus are unlikely to be involved in a major catalytic effect (20, 21).
One of the obvious questions about our finding may be related to the fact that H84 is universally conserved in all translational GTPases from bacteria to humans. Here, our finding that the H84 group is probably protonated may have special significance, as it might help in establishing the allosteric effect through the electrostatic interaction between H84 and the phosphate group of A2662.
One may wonder about the overall predictions made in this work. One prediction is that the H84Q mutant will have a significant catalytic effect, unless it will not be able to keep the system in the EF-Tu′ conformation (the structural effect of the mutant can be checked by X-ray structural analysis). Another prediction is that removing the negatively charge phosphate of the A2662 residue might lead to pH dependence of the GTP hydrolysis. Predictions of the effect of other mutations are left to subsequent studies. Finally, in our view, being able to reproduce known mutational effect without any adjustable parameters is a way to support the relevance of the corresponding analysis even without predictions.
The current analysis of the nature of the EF-Tu activation by the ribosome provides a significant support to our previous proposal regarding the activation of Ras. That is, even in the RasGAP complex (where it is harder to obtain structural information about Q61 mutants), a similar mode of activation was deduced from a careful simulation study (27). Consequently, these consistent results for the EF-Tu and Ras systems shed light on the general activation mechanism of GTPases, where the binding to another protein or a subunit greatly accelerates the intrinsic reaction. Here we provide further evidence that this activation process involves major allosteric effects.
Methods
The free-energy surface of the reference solution reaction was estimated considering our more recent quantum chemical calculations (38), and the resulting surface reproducing all of the relevant details regarding the solution reaction is shown in Fig. S4. The effective charges obtained at the MPW1PW91/6-311G** level of theory for the RS, IS, and PS were employed in our EVB model (see Table S1). The complex nature of the reaction was accounted for by exploring concerted and stepwise mechanisms. This approach required two and three diabatic states for the concerted and stepwise mechanisms, respectively (see Fig. S5). The EVB calculations were carried out by the MOLARIS simulation program using the ENZYMIX force field (39). The EVB activation barriers were calculated by the same free-energy perturbation umbrella sampling (FEP/US) approach, which has been described in detail elsewhere (40). The atomic coordinates used as a starting point in the simulations were taken from 5P21 (41), 1WQ1 (42), 1EFT (43), 1TTT (44), and 2XQD and 2XQE (9), which correspond to the Research Collaboratory for Structural Bioinformatics Protein Data Bank (PDB) identification codes for Ras, Ras-RasGAP (RasGAP) complex, EF-Tu, EF-Tu ternary complex, and EF-Tu′/ribosome complex, respectively. The simulation systems were solvated by the surface-constrained all-atom solvent (SCAAS) model (45) using a water sphere of 18-Å radius centered on the substrate and surrounded by 2-Å grid of Langevin dipoles and then by a bulk solvent, whereas long-range electrostatic effects were treated by the local reaction field method (46). The EVB region consisted of the substrate and a water molecule that serves as a nucleophile (see Fig. S5). The FEP mapping was evaluated by 25 frames of 10 ps each for the movement along the reaction coordinate using SCAAS model after the respective system underwent a 100-ps relaxation run. All the simulations were performed at 300 K with a time step of 1 fs. To obtain reliable sampling (47), the simulations were repeated at least five times with different initial conditions (obtained from arbitrary points in the relaxation trajectory after the initial 100-ps relaxation run) for each reacting system. The mutant systems were generated from the systems listed in the SI Text via 100-ps relaxation runs. The energetics of the conformational coordinate were explored using TMD simulations (36) and the linear response approximation (LRA) treatment (31–33). The contributions of different residues to the activation barrier were calculated by evaluating respective electrostatic group contributions to the differential binding energy between the RS and TS (48). The ionization states were evaluated by using our Monte Carlo approach (49), and the effects of ionized residues on the activation free energy have been incorporated. For histidine residues, the tautomers were determined automatically by our standard procedure (included in the MOLARIS software package), which selects automatically the configuration with lower electrostatic energy. SI Text provides additional details regarding the specific methods employed in this work.
Supplementary Material
Acknowledgments.
We kindly thank Måns Ehrenberg, Anders Liljas, Marina Rodnina, Adrian Mulholland, and Johan Åqvist for stimulating discussions. We also thank the University of Southern California High Performance Computing and Communication Center for computational resources. This work was supported by National Science Foundation Grant MCB0342276.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105714108/-/DCSupplemental.
References
- 1.Daviter T, Wieden HJ, Rodnina MV. Essential role of histidine 84 in elongation factor Tu for the chemical step of GTP hydrolysis on the ribosome. J Mol Biol. 2003;332:689–699. doi: 10.1016/s0022-2836(03)00947-1. [DOI] [PubMed] [Google Scholar]
- 2.Liljas A. Structural Aspects of Protein Synthesis. Singapore: World Scientific; 2004. [Google Scholar]
- 3.Agirrezabala X, Frank J. Elongation in translation as a dynamic interaction among the ribosome, tRNA, and elongation factors EF-G and EF-Tu. Q Rev Biophys. 2009;42:159–200. doi: 10.1017/S0033583509990060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yonath A. Polar bears, antibiotics, and the evolving ribosome (Nobel lecture) Angew Chem Int Ed Engl. 2010;49:4340–4354. doi: 10.1002/anie.201001297. [DOI] [PubMed] [Google Scholar]
- 5.Ogle JM, et al. Recognition of cognate transfer RNA by the 30S ribosomal subunit. Science. 2001;292:897–902. doi: 10.1126/science.1060612. [DOI] [PubMed] [Google Scholar]
- 6.Steitz TA. From the structure and function of the ribosome to new antibiotics (Nobel lecture) Angew Chem Int Ed Engl. 2010;49:4381–4398. doi: 10.1002/anie.201000708. [DOI] [PubMed] [Google Scholar]
- 7.Ramakrishnan V. Unraveling the structure of the ribosome (Nobel lecture) Angew Chem Int Ed Engl. 2010;49:4355–4380. doi: 10.1002/anie.201001436. [DOI] [PubMed] [Google Scholar]
- 8.Ehrenberg M. The nobel prize in chemistry 2009—scientific background. 2009. http://nobelprize.Org/nobel_prizes/chemistry/laureates/2009/sci.html.
- 9.Voorhees RM, Schmeing TM, Kelley AC, Ramakrishnan V. The mechanism for activation of GTP hydrolysis on the ribosome. Science. 2010;330:835–838. doi: 10.1126/science.1194460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villa E, et al. Ribosome-induced changes in elongation factor Tu conformation control GTP hydrolysis. Proc Natl Acad Sci USA. 2009;106:1063–1068. doi: 10.1073/pnas.0811370106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmeing TM, et al. The crystal structure of the ribosome bound to EF-Tu and aminoacyl-tRNA. Science. 2009;326:688–694. doi: 10.1126/science.1179700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmeing TM, Ramakrishnan V. What recent ribosome structures have revealed about the mechanism of translation. Nature. 2009;461:1234–1242. doi: 10.1038/nature08403. [DOI] [PubMed] [Google Scholar]
- 13.Schuette JC, et al. GTPase activation of elongation factor EF-Tu by the ribosome during decoding. EMBO J. 2009;28:755–765. doi: 10.1038/emboj.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lancaster L, Lambert NJ, Maklan EJ, Horan LH, Noller HF. The sarcin-ricin loop of 23S rRNA is essential for assembly of the functional core of the 50S ribosomal subunit. RNA. 2008;14:1999–2012. doi: 10.1261/rna.1202108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moazed D, Robertson JM, Noller HF. Interaction of elongation-factors EF-G and EF-Tu with a conserved loop in 23S RNA. Nature. 1988;334:362–364. doi: 10.1038/334362a0. [DOI] [PubMed] [Google Scholar]
- 16.Hausner TP, Atmadja J, Nierhaus KH. Evidence that the G2661 region of 23S ribosomal-RNA is located at the ribosomal-binding sites of both elongation-factors. Biochimie. 1987;69:911–923. doi: 10.1016/0300-9084(87)90225-2. [DOI] [PubMed] [Google Scholar]
- 17.Mohr D, Wintermeyer W, Rodnina MV. GTPase activation of elongation factors Tu and G on the ribosome. Biochemistry. 2002;41:12520–12528. doi: 10.1021/bi026301y. [DOI] [PubMed] [Google Scholar]
- 18.Bilgin N, Ehrenberg M. Mutations in 23-S ribosomal-RNA perturb transfer-RNA selection and can lead to streptomycin dependence. J Mol Biol. 1994;235:813–824. doi: 10.1006/jmbi.1994.1041. [DOI] [PubMed] [Google Scholar]
- 19.Knudsen C, Wieden HJ, Rodnina MV. The importance of structural transitions of the switch II region for the functions of elongation factor Tu on the ribosome. J Biol Chem. 2001;276:22183–22190. doi: 10.1074/jbc.M102186200. [DOI] [PubMed] [Google Scholar]
- 20.Jacquet E, Parmeggiani A. Structure-function relationships in the GTP binding domain of EF-Tu—mutation of Val20, the residue homologous to position 12 in p21. EMBO J. 1988;7:2861–2867. doi: 10.1002/j.1460-2075.1988.tb03142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krab IM, Parmeggiani A. Mutagenesis of three residues, isoleucine-60, threonine-61, and aspartic acid-80, implicated in the GTPase activity of Escherichia coli elongation factor Tu. Biochemistry. 1999;38:13035–13041. doi: 10.1021/bi9909748. [DOI] [PubMed] [Google Scholar]
- 22.Tinoco I, Wen JD. Simulation and analysis of single-ribosome translation. Phys Biol. 2009;6:025006. doi: 10.1088/1478-3975/6/2/025006. [DOI] [PubMed] [Google Scholar]
- 23.Zeidler W, et al. Site-directed mutagenesis of Thermus thermophilus elongation factor Tu—replacement of His85, Asp81 and Arg300. Eur J Biochem. 1995;229:596–604. doi: 10.1111/j.1432-1033.1995.tb20503.x. [DOI] [PubMed] [Google Scholar]
- 24.Cool RH, Parmeggiani A. Substitution of histidine-84 and the GTPase mechanism of elongation factor-Tu. Biochemistry. 1991;30:362–366. doi: 10.1021/bi00216a008. [DOI] [PubMed] [Google Scholar]
- 25.Scarano G, Krab IM, Bocchini V, Parmeggiani A. Relevance of histidine-84 in the elongation-factor Tu GTPase activity and in poly(phe) synthesis—Its substitution by glutamine and alanine. FEBS Lett. 1995;365:214–218. doi: 10.1016/0014-5793(95)00469-p. [DOI] [PubMed] [Google Scholar]
- 26.Ramakrishnan V. What we have learned from ribosome structures. Biochem Soc Trans. 2008;36:567–574. doi: 10.1042/BST0360567. [DOI] [PubMed] [Google Scholar]
- 27.Shurki A, Warshel A. Why does the Ras switch “break” by oncogenic mutations? Proteins. 2004;55:1–10. doi: 10.1002/prot.20004. [DOI] [PubMed] [Google Scholar]
- 28.Grigorenko BL, Shadrina MS, Topol IA, Collins JR, Nemukhin AV. Mechanism of the chemical step for the guanosine triphosphate (GTP) hydrolysis catalyzed by elongation factor Tu. Biochim Biophys Acta. 2008;1784:1908–1917. doi: 10.1016/j.bbapap.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glennon TM, Villa J, Warshel A. How does GAP catalyze the GTPase reaction of Ras? A computer simulation study. Biochemistry. 2000;39:9641–9651. doi: 10.1021/bi000640e. [DOI] [PubMed] [Google Scholar]
- 30.Barbany M, Gutierrez-de-Teran H, Sanz F, Villà-Freixa J, Warshel A. On the generation of catalytic antibodies by transition state analogues. Chembiochem. 2003;4:277–285. doi: 10.1002/cbic.200390048. [DOI] [PubMed] [Google Scholar]
- 31.Strajbl M, Shurki A, Warshel A. Converting conformational changes to electrostatic energy in molecular motors: The energetics of ATP synthase. Proc Natl Acad Sci USA. 2003;100:14834–14839. doi: 10.1073/pnas.2436328100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braun-Sand S, Sharma PK, Chu ZT, Pisliakov AV, Warshel A. The energetics of the primary proton transfer in bacteriorhodopsin revisited: It is a sequential light induced charge separation after all. Biochim Biophys Acta. 2008;1777:441–452. doi: 10.1016/j.bbabio.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frushicheva MP, Cao J, Chu ZT, Warshel A. Exploring challenges in rational enzyme design by simulating the catalysis in artificial Kemp eliminase. Proc Natl Acad Sci USA. 2010;107:16869–16874. doi: 10.1073/pnas.1010381107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiang Y, Oelschlaeger P, Florian J, Goodman MF, Warshel A. Simulating the effect of DNA polymerase mutations on transition-state energetics and fidelity: Evaluating amino acid group contribution and allosteric coupling for ionized residues in human pol β. Biochemistry. 2006;45:7036–7048. doi: 10.1021/bi060147o. [DOI] [PubMed] [Google Scholar]
- 35.Dryga A, Warshel A. Renormalizing SMD: The renormalization approach and its use in long time simulations and accelerated PMF calculations of macromolecules. J Phys Chem B. 2010;114:12720–12728. doi: 10.1021/jp1056122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schlitter J, Engels M, Kruger P, Jacoby E, Wollmer A. Targeted molecular-dynamics simulation of conformational change—Application to the T↔R transition in insulin. Mol Simul. 1993;10:291–308. [Google Scholar]
- 37.Berchtold H, et al. Crystal structure of active elongation factor Tu reveals major domain rearrangements. Nature. 1993;365:126–132. doi: 10.1038/365126a0. [DOI] [PubMed] [Google Scholar]
- 38.Klahn M, Rosta E, Warshel A. On the mechanism of hydrolysis of phosphate monoesters dianions in solutions and proteins. J Am Chem Soc. 2006;128:15310–15323. doi: 10.1021/ja065470t. [DOI] [PubMed] [Google Scholar]
- 39.Lee FS, Chu ZT, Warshel A. Microscopic and semimicroscopic calculations of electrostatic energies in proteins by the POLARIS and ENZYMIX programs. J Comput Chem. 1993;14:161–185. [Google Scholar]
- 40.Warshel A. Computer Modeling of Chemical Reactions in Enzymes and Solutions. New York: John Wiley; 1991. [Google Scholar]
- 41.Pai EF, et al. Refined crystal structure of the triphosphate conformation of h-Ras p21 at 1.35 Å resolution: Implications for the mechanism of GTP hydrolysis. EMBO J. 1990;9:2351–2359. doi: 10.1002/j.1460-2075.1990.tb07409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scheffzek K, et al. The Ras-RasGAP complex—structural basis for GTPase activation and its loss in oncogenic Ras mutants. Science. 1997;277:333–338. doi: 10.1126/science.277.5324.333. [DOI] [PubMed] [Google Scholar]
- 43.Kjeldgaard M, Nissen P, Thirup S, Nyborg J. The crystal-structure of elongation-factor EF-Tu from Thermus-aquaticus in the GTP conformation. Structure. 1993;1:35–50. doi: 10.1016/0969-2126(93)90007-4. [DOI] [PubMed] [Google Scholar]
- 44.Nissen P, et al. Crystal structure of the ternary complex of Phe-tRNAPhe, EF-Tu, and a GTP analog. Science. 1995;270:1464–1472. doi: 10.1126/science.270.5241.1464. [DOI] [PubMed] [Google Scholar]
- 45.King G, Warshel A. A surface constrained all-atom solvent model for effective simulations of polar solutions. J Chem Phys. 1989;91:3647–3661. [Google Scholar]
- 46.Lee FS, Warshel A. A local reaction field method for fast evaluation of long-range electrostatic interactions in molecular simulations. J Chem Phys. 1992;97:3100–3107. [Google Scholar]
- 47.Roca M, Vardi-Kilshtain A, Warshel A. Toward accurate screening in computer-aided enzyme design. Biochemistry. 2009;48:3046–3056. doi: 10.1021/bi802191b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muegge I, Tao H, Warshel A. A fast estimate of electrostatic group contributions to the free energy of protein-inhibitor binding. Protein Eng Des Sel. 1997;10:1363–1372. doi: 10.1093/protein/10.12.1363. [DOI] [PubMed] [Google Scholar]
- 49.Messer BM, et al. Multiscale simulations of protein landscapes: Using coarse-grained models as reference potentials to full explicit models. Proteins. 2010;78:1212–1227. doi: 10.1002/prot.22640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kirby AJ, Varvoglis AG. The reactivity of phosphate esters. Monoester hydrolysis. J Am Chem Soc. 1967;89:415–423. [Google Scholar]
- 51.Smith RM, Alberty RA. The apparent stability constants of ionic complexes of various adenosine phosphates with monovalent cations. J Phys Chem. 1956;60:180–184. [Google Scholar]
- 52.Schweins T, et al. Substrate-assisted catalysis as a mechanism for GTP hydrolysis of p21 Ras and other GTP-binding proteins. Nat Struct Biol. 1995;2:36–44. doi: 10.1038/nsb0195-36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.