Abstract
In primates, age, sex, and social status can strongly influence access to food resources. In Pan, these criteria are assumed to influence access to vertebrate meat. However, the significance of meat in terms of its role in the nutrition of Pan is still debated. Here we present a study using stable carbon and nitrogen isotope ratios in hair samples from habituated, wild bonobos (Pan paniscus) to explore these issues. Over a period of 5 mo hair samples were collected from fresh bonobo nests at LuiKotale, Democratic Republic of Congo. Hair samples were assigned to known individuals and were of sufficient length to allow the evaluation of isotopic variation over several months. Samples of plant foods and sympatric fauna were also analyzed. The δ13C and δ15N results of the bonobo hair were remarkably homogeneous over time and for the group as a whole. There are no differences in diet between the sexes. Within the group of males, however, there was a positive correlation between dominance status and δ15N. The isotopic data indicate that the contribution of fauna to bonobo diet is marginal and that plant food is the dietary protein source. In only some cases did elevated δ15N hair values correlate with observed faunivory and not correspond to the δ15N measured in the dominant plant foods. Given the large variation in hunting and meat eating of Pan across the African continent, the detection of seasonal changes in faunivory by elevated δ15N values in sectioned ape hair is a promising approach.
Keywords: feeding ecology, great apes
Reconstructing hominin diets and possible meat-eating behavior is a key research area in the field of human evolution (1, 2) because adaptations for the consumption of animal protein and fat have been related to brain developmental trends in the evolution of the human lineage (3). As our closest living relatives, bonobos (Pan paniscus) and chimpanzees (Pan troglodytes) are often used as referential models in the context of diet composition, feeding behavior, and food processing (4, 5). The diet of both Pan species is dominated by plant foods: fruits account for more than 50% of the daily dietary intake and are complemented by leaves, herbs, and, at least in some populations, underground storage organs. Both species supplement their plant diet with insects and meat from vertebrates acquired by hunting (6). Behavioral observations from meat-eating events suggest that both Pan species consider meat a highly attractive food; meat is generally considered to be a high-quality food resource that offers nutrients that are difficult to obtain from other foods (7). Conflicts among those who compete for access to meat are frequent, and dominant individuals may take carcasses from subordinates. Longitudinal data from one population suggest that meat consumption by female chimpanzees can shorten interbirth intervals (8). Given the close link between the nutritional status of females and reproductive success, the inferred nutritional function of meat eating seems plausible, but requires further exploration. Although existing evidence demonstrates that meat is part of the diet of most or all Pan populations, how meat consumption relates to the overall diet remains unknown (7). Hunting and meat eating is less frequently observed in bonobos (Pan paniscus) compared with some chimpanzee populations (9). Observational data from two sites show that females eat meat more often and in larger amounts than males (10). In Salonga, bonobos especially target immature primates and mature forest antelopes (Cephalophinae) that have an estimated body mass of 4–12 kg (11). After hunts, females are observed to be more successful in monopolizing meat, and meat is rarely shared with males (10).
Biochemical analysis of tissue provides important information on diet composition and allows a better understanding of what is actually incorporated into the body (12). By analyzing the stable isotope ratios of carbon (δ13C) and nitrogen (δ15N) in animal tissue, a stepwise enrichment between trophic levels is observed, which can reveal the main source of protein (13, 14). The analysis of carbon and nitrogen isotope ratios has been widely applied in nutritional animal ecology (15), as well as in archaeology to reconstruct human and hominin paleodiets (12, 16, 17). The method relies on the fractionation of isotopes due to the different kinetic and thermodynamic properties of the light versus heavy isotopes during chemical and physical processes in the biosphere (18). The fractionation of carbon isotopes in terrestrial ecosystems is related mainly to photosynthetic pathways of plants. Consumers in environments dominated by C3 plants show lower δ13C values than in ecosystems dominated by C4 plants (19, 20). In dense tropical forest habitats, plants are 13C-depleted due to low light intensity and photosynthetic recycling of ground-respired CO2. This “canopy effect” causes 13C-depleted values in primary forests with the most negative values near the forest floor and the least negative values in the canopy (21, 22). Although the trophic level effects cause only minor fractionation in δ13C (∼1–2%), δ15N values show a significant stepwise enrichment of 2–5% for each trophic level in the food chain (13). By analyzing tissue such as collagen or keratin, the protein fraction of the diet can be identified to derive mainly from plant or animal protein. However, in terrestrial ecosystems, nitrogen fractionation can also relate to temperature, salinity, and precipitation (23, 24). Therefore, to understand the variability in a species’ isotope signature, it is imperative to analyze samples from different food-web levels of the same ecosystem. A variety of material, such as hair, feathers, and plant fibers, provide reliable isotope information to reconstruct the isotopic ecology of a certain habitat (25, 26).
Numerous studies have applied stable isotope ratio analysis to explore the contribution of different plant sources (e.g., C3 and C4 plants) and niche separation, as well as intraspecific and interspecific variation of the composition of the diets of nonhuman primates (26–30). Other studies on African forest and savanna isotope ecology provide some information on primates but do not have corresponding diet data (20, 25). To date, isotopic information on hominoid primates is restricted to samples from chimpanzees. A study by Schoeninger et al. (28) involved hair samples from two populations living in open savanna habitats at Ugalla (Tanzania) and Ishasha (Democratic Republic of Congo). It showed that chimpanzees focused on plant foods from forest patches and woodland, and not on C4-dominated grassland foods. This finding was supported by a more recent study by Sponheimer et al. (29) of savanna chimpanzees at Fongoli in Senegal. Using samples of bone collagen from various chimpanzees from the Kibale forest, Uganda, Carter (31) found that the chimpanzees had a wide range of δ13C and δ15N values, suggesting more variation in terms of diet composition, including C4 plants originating from domesticated plant species such as sugarcane or corn. A more recent study of a collection of historic chimpanzee crania from northern Liberia found no significant sex differences in diet, but could not reconstruct the chimpanzee's food sources as no environmental background isotopic data were included (32). Compared with the nutritional value of various types of food plants, knowledge about the nutritional contribution of animal food remains elusive. We conducted stable isotope ratio analyses on hair samples from individually known bonobos from LuiKotale, a long-term study site in the Democratic Republic of Congo. The major goal of this study was to explore bonobo isotope ecology and identify their major dietary protein sources. We examined if field observations of meat and plant food consumption could be correlated to isotopic changes in the bonobo hair and evaluated the utility of this technique for future isotopic studies on extant African apes. In addition, the data were used to examine isotopic variations on a temporal scale and in relation to time, age, sex, and social status of subjects. To understand the isotopic variability of different food sources in the natural habitat, a variety of plant food items and sympatric animal species from different ecological niches were also analyzed.
Results
The isotopic results of the floral (n = 33), faunal (n = 32), and bonobo hair (n = 143) samples from Salonga are shown in Tables S1–S3. All plant samples represent fruits, leaves, or piths of species that are known to be eaten or at least partly ingested (e.g., bark) by the LuiKotale bonobos. From observational data we could additionally classify the five most important food plants for each month during the study period (Table S4).The plants show a wide range of δ13C values, −36.2% to −24.7% (mean = −29.0 ± 2.7% 1σ), and of δ15N values, 2.6% to 9.3% (mean = 5.6 ± 1.3% 1σ). Similar heterogeneity is found in the faunal samples. Animals from Salonga forest display δ13C values ranging from −33.6% for a diurnal butterfly to −20.2% measured in an unidentified bird species. The mean δ13C for the faunal samples is −24.9 ± 2.0% (1σ). The δ15N values range between 3.8% in termites and 17.6% in the feather of an unidentified bird species. The mean δ15N for the faunal samples is 10.7 ± 2.7% (1σ).
Compared with the ecological data from plants and animals, the variability of isotopes in the hair samples from bonobos was low (Fig. 1 and Fig. 2). The δ13C values range from −26.7% to −25.2% (mean = −25.8 ± 0.3% 1σ), and the δ15N values range from 7.7% to 9.1% with a mean of 8.4 ± 0.3% (1σ). There were no significant differences in the mean isotope values between females and males (exact Mann–Whitney U test: for δ13C—U = 19, Nfemales = 11, Nmales = 9, P = 0.8357; for δ15N—U = 22, P = 0.9452). The males had a mean δ13C value of −25.7 ± 0.3% (1σ) and a mean δ15N value of 8.4 ± 0.3% (1σ) whereas the females had a mean δ13C value of −25.8 ± 0.3% (1σ) and a mean δ15N value of 8.5 ± 0.2% (1σ). δ15N values correlated significantly with male rank (Spearman correlation: ρ = −0.717, n = 9, P = 0.037) as well as with the relative age of subjects (U = 8, Nadult = 5, Nadolescent = 4, P = 0.032). No differences were found when relating δ13C values with rank (ρ = 0.333, n = 9, P = 0.948) or when comparing δ13C values of adult and adolescent males (U = 8, Nadult = 5, Nadolescent = 4, P = 0.73).
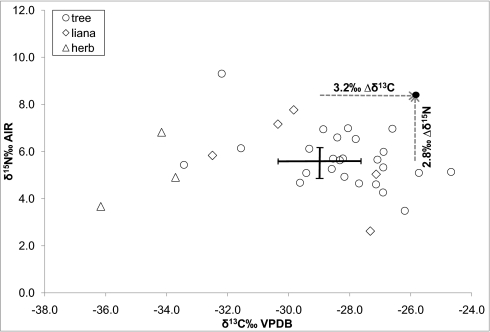
Fig. 1.
Isotope ratios of plant samples from Salonga sorted by plant type, mean value for all floral samples (SD 1σ), and the mean value (black dot) of all bonobo hair samples (n = 143). The dashed arrows display the fractionation (Δ) between mean plant diet and the mean bonobo hair keratin values. The analytical error for δ13C and δ15N is <0.2%.
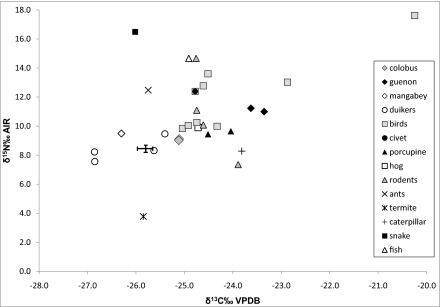
Fig. 2.
Isotope ratios of the sampled faunal species from Salonga and the mean value (1σ SD) for all bonobo hair. The data point of a diurnal butterfly was excluded from this graph due to its extremely depleted 13C value (−33.6%). Analytical error is <0.2%.
Discussion
Floral Samples.
The isotopic results for the plant samples are presented in Fig. 1 in relation to the mean bonobo value. The food plants collected at Salonga revealed a high variability in isotope ratios (Table S1 and Fig. 1) and, in this respect, resemble what has been described for other tropical forests (22, 25, 33). A study on the isotope ecology of the Ituri Forest, also located in Democratic Republic of Congo, provides the best dataset for comparison. Cerling et al. (25) measured δ13C and δ15N values in foliage, fruits, and seeds from different canopy levels (n = 71) and reported isotope values remarkably similar to what was found at Salonga. The mean δ13C value (−31.2 ± 2.8% 1σ) in Ituri was only 1.5% more negative than the floral samples from Salonga (−29.0 ± 2.7% 1σ). This is most likely due to the intentional sampling of lower canopy levels in the Ituri study whereas in Salonga most plant food samples derive from higher canopy levels. Similar 13C-depleted values (−31.4 ± 0.5% 1σ) have been reported from closed canopy forests in Kenya (34). Salonga and Ituri are part of the same evergreen lowland forest of the Congo basin and are strongly affected by the canopy effect and thus 13C-depleted (19, 22). Conversely, Kibale National Park in Uganda (31) comprises a mosaic of habitats that include evergreen forests, open woodlands, and savanna. Correspondingly, the botanical samples from this site are more variable and enriched in terms of 13C (−26.4 ± 6.1% 1σ) in relation to the Democratic Republic of Congo. Nitrogen isotope ratios are known to be elevated in rainforests compared with open woodlands and forests in temperate climates, due to more complex pathways of nitrogen assimilation (33, 35, 36). Not surprisingly, the mean δ15N values of the plants from Ituri (5.4 ± 1.8% 1σ) and Salonga (5.6 ± 1.3% 1σ) are nearly identical. However, the Kibale plants were less 15N-enriched (mean of 3.8 ± 1.6%) compared with Salonga, presumably resulting in a lower baseline δ15N value for consumers in this ecosystem.
Faunal Samples.
The variation of isotope results observed in the animal species at Salonga is similar to those reported from other African forests (12, 25). Depletion of 13C due to the canopy effect was mostly reflected in ground-dwelling species, such as the duiker (Cephalophus), Congo peacock (Afropavo congensis), and guinea fowl (Guttera) as well as in ground-living insects (e.g., army ants and termites). The highest δ13C values were measured in species living and feeding in the higher levels of the forest canopy, represented by the guenon (Cercopithecus), squirrel (Protoxerus stangeri), and two unidentified birds (Fig. 2). Some primates such as bonobos, red colobus (Procolobus thollori), and the mangabey (Cercocebus aterrimus) had relatively low δ13C values, although they can be considered as arboreal-feeding species. Their isotope values were very similar to those of the duikers, which feed mainly on fruits dropped by arboreal primates and some additional herbs (11). We suggest that bonobos, colobus, and mangabeys feed in the intermediate forest levels, which are not as strongly enriched in 13C, and also may occasionally feed on subcanopy food sources. The guenon seems to exclusively feed in the highest canopy levels and therefore occupies a different ecological niche than the other primates in our dataset.
The complexity of the nitrogen assimilation pathways in the tropical forest results in high δ15N values, even in herbivorous species. The lowest δ15N values were found in folivore species such as termites (3.8%), caterpillars (8.3%), colobus monkeys (∼9.0%), and ungulates (8.4 ± 0.8% 1σ). In the duikers, we observed the same pattern as in δ13C: duikers reveal δ15N values very similar to the bonobos, mangabey, and colobus monkeys due to their dietary adaptation of largely consuming fruits dropped by arboreal primates. It should be noted that the values of fruigvorous and folivorous mammals exceed those of forest-living primates reported elsewhere (27, 30, 37). This difference may result from the relatively high floral baseline values of Salonga (Fig. 1). As expected, the nitrogen isotope ratios were found to be more enriched in the higher levels of the food chain. Omnivorous and insectivorous mammals such as guenons, civets, river hog, and insectivorous birds had δ15N values between 9.5% and 13%. Carnivore isotope signatures were found in ants (12.5%), a snake (16.5%), one unidentified rodent (14.7%), and in the feathers of an unidentified bird (17.6%). On the basis of the δ13C result (−20.2%) and the high δ15N value of the unidentified bird, it is possible to say that it was a raptor-hunting arboreal species (Fig. 2).
Bonobo Hair Samples.
We measured isotope ratios of 143 hair samples from serially sectioned hair deriving from at least 21 mature individuals (Figs. 1–4 and Table S3). If the growth rate of bonobo hair is similar to that of humans, the isotope values should provide information on the diet consumed over a period of about 9 mo (December 2008–August 2009). The mean δ13C value of −25.8 ± 0.3% (1σ) in Salonga bonobo hair is the most negative value reported for African apes and for groups with sympatric duikers (see above). Although bonobos of this population occasionally move into grassland, there was no evidence for the consumption of C4 plants. These results strongly contrast with the data from chimpanzee populations living in open woodlands or even in savanna landscapes. Mean isotope values of plants from Kibale (−26.4 ± 6.1% 1σ) indicate that this environment could be less depleted in 13C than the forest of Salonga (−29.0 ± 2.7% 1σ). The higher mean δ13C value in samples of Kibale chimpanzees (−22.9 ± 0.7% 1σ) can also be explained by the chimpanzee's opportunistic feeding strategies. Carter (31) suggested that chimpanzees were 13C-enriched due to regular raids on C4 field crops such as maize and sugarcane in the villages. However, the comparison of δ13C values in samples from Salonga bonobos and Kibale chimpanzees is complicated by the fact that Kibale chimpanzee data were obtained from bone collagen with questionable C/N ratios (31, 38). Moreover, various values for hair-collagen offsets are found in the literature, making a direct comparison of collagen and hair keratin values difficult (39, 40). The same applies to the dataset from the Liberian Ganta chimpanzees; here, direct comparison is further hindered by the need to consider fossil fuel effects, as the Ganta bone collection derives from a historical context (41).
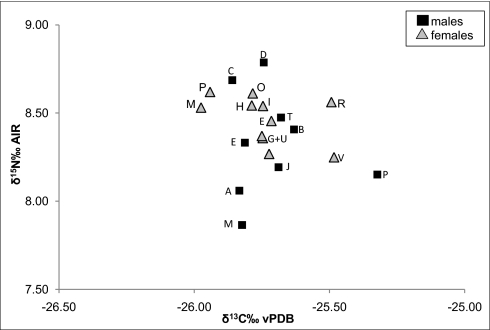
Fig. 4.
Mean isotope values (1σ SD) of hair samples for each individual bonobo of the study community. The initial next to each data point refers to the individual name of each male (square) and female (triangle). See Table S3 for further details. Analytical error is <0.2%.
The Salonga bonobo hair samples had a mean δ15N value of 8.4 ± 0.3% (1σ), and this result represents the highest measured δ15N value from adult African apes to date. This indicates that, compared with other African ape habitats, food sources at Salonga are 15N-enriched. The diet-tissue fractionation (Δplant diet-keratin) between the plant foods (mainly fruits) and bonobo hair is 3.2% for Δδ15Nplant diet-keratin and 2.8% for Δδ13Cplant diet-keratin, representing one trophic level (16, 17). Looking at the isotope values of sympatric animal and plant species, it is obvious that plants are the dominant source of dietary protein for bonobos. However, the overall variation in hair keratin δ15N values is strikingly low (Table S3). This may reflect low temporal fluctuation of nutrient supply in the bonobo diet. The impression of low isotopic variability in bonobo hair remains when comparing different sections of an individual hair sample, representing different points in time (Fig. 3 A and B). It is remarkable that the large isotopic variation in food plants known to be consumed by the Salonga bonobos (42) is not reflected in the hair isotope values. This can be explained by the low range in δ15N in dominant food plants, which were identified in feeding observations (Table S4). Despite the wide range of potential food plants (7–20 species) per month, the main plant foods are commonly restricted to less than five species. These, with a mean δ15N value of 5.5% ± 0.9% (1σ), appear to be quite stable in terms of isotopic variation throughout the study period. From November to March, Dialium fruits (5.1% δ15N) were nearly always consumed when the bonobos were observed feeding, alternated by Irvingia (6.0% δ15N), Gambeya (6.6% δ15N), and the leaves of an unidentified tree from April to July. Although feeding observations are incomplete and not all plant species could be identified, we found no evidence for seasonal isotopic fluctuation in food plants.
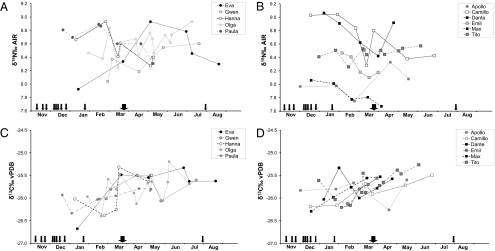
Fig. 3.
Individual bonobo hair isotope ratios plotted chronologically (December 2008–August 2009). The variation in δ15N for females (A) and males (B) is shown, as is the variation in δ13C for females (C) and males (D). Each hair sample was cut into sections, and each data point represents the mean point in time of the respective hair section. For 10 mm of sectioned hair, we calculated 28 d; a month here represents 30.5 d. Each arrow represents an observed event of faunivory (mammals, birds, and caterpillars), and its thickness indicates the relative amount of consumed vertebrate and nonvertebrate protein.
Differences in δ15N were expected in comparison with the savanna or woodland chimpanzees from Ugalla, Ishasha, and Fongoli, which have significantly lower δ15N values. In Ugalla and Fongoli, low mean δ15N values of 2.3 ± 0.8%, and 2.9 ± 0.3%, respectively, are the result of intense legume consumption (28, 29). For Ishasha, values with a mean of 5.9 ± 0.9% (1σ) were reported (two potentially breastfed individuals excluded), whereas the mean value for the Kibale chimpanzee sample was 6.4 ± 1.2% 1σ (28, 31). The nitrogen isotope ecology of the Salonga rainforest therefore differs significantly from other ape habitats.
Compared with the values obtained from sympatric animals and plant foods, bonobo isotope ratios were remarkably similar within and between individuals (Fig. 3 and Fig. 4). Moreover, on a community level, there was little evidence for 15N-enrichment that could be related to the consumption of fauna. We attempted to correlate observed incidences of faunivory—specifically the consumption of insects such as caterpillars—with increased hair nitrogen isotope values. Five faunivory events were observed in December 2008 and initially appear to be recorded as slightly elevated δ15N values (8.8–9.1%) in the hair of some individuals (e.g., Paula, Camillo, Dante). The female Eva had the lowest δ15N value in this time period, and she was not observed to be a primary consumer of vertebrate meat. Eva's isotope values show the strongest fluctuation in the group. Her δ15N values increased after a period of intense consumption of caterpillars in March 2009. Given the elevated δ15N values of 8.3% for the caterpillars, we suggest that the female Eva possibly shows a response to this significant intake of invertebrate protein (Fig. 3A). A similar pattern could be observed in the male Camillo. His δ15N values rose in the time he was observed eating caterpillars repeatedly (Fig. 3B). However, the isotope data of other individuals did not show the same increase, although they were also observed consuming insects (e.g., Dante, Tito). We found no evidence of isotopic fluctuation in the bonobos main plant diet, which might explain these short-term variations in the nitrogen isotope values in hair keratin; therefore, these could be related to faunivory events. However, the increase in nitrogen isotope values is very slight and not observed in all individuals. One limitation of our study derives from the unknown rate of hair growth in bonobos, which reduces our ability to align observed events of meat consumption to the hair. In addition, assessment of the amount of meat consumed by each individual is difficult and prone to errors. Finally, discontinuity of direct observations renders our knowledge of diet composition incomplete. However, despite these limitations, detecting peaks of faunivory or insectivory through the measurement of temporarily elevated δ15N values in small hair sections is likely and remains a promising approach, although it did not provide definitive results here.
No significant sex differences were detected in the isotope ratios measured in hair samples from female and male bonobos. This finding is in line with reports emphasizing the cohesiveness of community members and spatial associations of females and males (43) but contradicts reported sex differences in meat consumption. However, our data show that males differed: δ15N values significantly increased with male dominance status and were higher in adult males compared with adolescent males (Fig. 4 and Table S5). Evidence suggests a relation between dominance status and age, but at this stage we are not able to distinguish the effect of the two parameters. Adolescent males are subordinate to adult females and to adult males and are more often the target of aggression than other group members (44), and probably this also applies to the context of food competition. As a result, they are often excluded from accessing high-quality food patches such as ripe fruit or meat. From this perspective, the positive correlation between rank and δ15N can be explained by reduced feeding opportunities of subordinate males. Although the overall range of δ15N values is relatively small (7.7–9.1%), the interindividual variation indicates differences in access to food from higher trophic levels in the male bonobos. Dominance relations among female group members were unknown at the time of sample collection, and further studies are needed to explore the relation between status and δ15N in bonobos.
Conclusion
On the basis of the findings of this study, we conclude that overall, the amount of protein obtained from fauna does not make a significant contribution to the bonobo's tissue. Alternatively, it has been suggested that, rather than affecting the nutritional status, hunting and meat sharing may derive social rather than nutritional benefits (10, 45). Compared with the values obtained from sympatric animals and from plant foods, bonobo isotope ratios were remarkably similar within and between individuals. This finding is especially interesting for high-resolution carbon isotope studies on fossil hominins that found pronounced intra- and interindividual dietary variation (17). Even though physiological differences between the sample tissues of tooth enamel and hair should be taken into account, isotopic studies on extant apes can serve as a useful referential model for hominin paleoecological studies. Although direct evidence showed that LuiKotale bonobos do hunt and consume the meat of other vertebrates, the isotopic evidence indicated that the majority of dietary protein was derived from plant foods, which mainly revealed unexpectedly constant δ15N values. Although temporal changes in δ15N in the samples of some individuals did correlate with events of meat consumption, other observed events of faunivory and insectivory did not show the same effect. Given the large variation in hunting and the relative amounts of animal protein consumption for Pan populations across the African continent, detection of seasonal changes in faunivory and insectivory by temporarily elevated δ15N values in sectioned ape hair remains a promising approach.
Materials and Methods
Study Site and Subjects.
Data were collected from an ongoing field study at LuiKotale, Democratic Republic of Congo (46). The site is located at the western border of Salonga National Park and consists of tropical lowland forest with a closed canopy and small patches of grassland. At the time of data collection, the studied community consisted of 33–35 individuals, including 5 adult and 4 adolescent males and 11 adult and 5 adolescent females. Dominance status of males was based on behavioral interactions as described by Surbeck et al. (44) and was consistent throughout the study period.
Sample Material.
Hair samples from bonobos were collected systematically from fresh night nests. Samples of hair, feathers, and tissue from other animals were collected opportunistically whenever dead animals were found in the forest. It was not always possible to identify tissues on a species level so that some specimens are classified only as, e.g., bird or rodent. Plant samples were derived from an ongoing study (42). Observations of feeding behavior were recorded during focal scans and ad libitum (SI Materials and Methods). All samples were stored dry or were frozen. Dry matter of hair samples largely consists of protein (90–95%) and does not change its isotopic composition once it is formed (12, 36, 47). It therefore reflects the incorporated diet, however with a delay of ∼6–12 d (48). Human scalp hair has an average growth rate of 0.35 mm/day and about 10 mm/month (49). Because data on hair growth in Pan are not available, we used the value from human hair to align hair segments to a given time period. Each of the hair samples used in this study for sectioning consisted of 3–10 bonobo hairs of 2–7 cm in length and had complete hair roots Hence, hair samples could be aligned at the roots to a initial chronological point. Sectioning the hair with a scalpel gained several time-reverse samples covering a period of up to 9 mo (November 2008 to August 2009). See SI Materials and Methods for more details on the procedure.
All samples were cleaned and purified before isotopic analysis. Hair samples were classified and selected under a microscope. Complete hairs of a sample were aligned at the roots under the microscope and cut into 0.5-cm (= 2 wk) to 2-cm (= 2 mo) sections with a scalpel (Table S3). Each section of hair (≥0.4 mg) was transferred into tin capsules for isotopic measurement. Bulk samples of hair, feathers, and insects (≥0.5 mg) were weighed into tin capsules for combustion. Plant items were freeze-dried and homogenized. Following this, ∼2 mg of plant powder was weighed in for analysis (SI Materials and Methods). All measurements were performed in a Flash EA 2112 coupled to a DeltaXP mass spectrometer (Thermo-Finnigan) at the Max Planck-Institute for Evolutionary Anthropology in Leipzig, Germany. The analytical precision was better than 0.2% (1σ) for δ13C and δ15N.
Supplementary Material
Acknowledgments
We thank Roger Mundry for help with statistics, Bastiaan van der Veer for collecting hair samples from bonobo nests, and Andrew Fowler and Luke Ward for observational data. Sylvia Ortmann, Tobias Deschner, and especially the two anonymous reviewers made valuable comments on earlier drafts of the manuscript. The Institute Congolaise pour la Conservation de la Nature, the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) office in Kinshasa, and the Health Ministry of the Democratic Republic of the Congo kindly gave permission to export samples from the Democratic Republic of the Congo to Germany. This project was funded by the Max Planck Society and the Federal Ministry for Education and Science, Germany (BMBF).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018502108/-/DCSupplemental.
References
- 1.Stanford CB, Bunn HT. Meat Eating and Human Behaviour. Oxford University Press, Oxford; 2001. [Google Scholar]
- 2.Hublin J-J, Richards MP. The Evolution of Hominin Diets: Integrating Approaches to the Study of Palaeolithic Subsistence. Berlin: Springer; 2009. [Google Scholar]
- 3.Aiello LC, Wheeler P. The expensive-tissue hypothesis: The brain and the digestive system in human and primate evolution. Curr Anthropol. 1995;36:199–221. [Google Scholar]
- 4.Stanford CB. The hunting ecology of wild chimpanzees: Implications for the evolutionary ecology of Pliocene hominids. Am Anthropol. 1996;98:96–113. [Google Scholar]
- 5.McGrew WC. Chimpanzee Material Culture: Implications for Human Evolution. Cambridge, UK: Cambridge University Press; 1992. [Google Scholar]
- 6.Finch CE, Stanford CB. Meat-adaptive genes and the evolution of slower aging in humans. Q Rev Biol. 2004;79:3–50. doi: 10.1086/381662. [DOI] [PubMed] [Google Scholar]
- 7.Milton K. A hypothesis to explain the role of meat-eating in human evolution. Evol Anthropol. 1999;8:11–21. [Google Scholar]
- 8.McGrew WC. Dominance status, food sharing, and reproductive success in chimpanzees. In: Wiessner P, Schiefenhoevel W, editors. Food and the Status Quest: An Interdisciplinary Perspective. Oxford: Berghan Books; 1996. pp. 39–45. [Google Scholar]
- 9.Hohmann G, Fruth B. New records on prey capture and meat eating by bonobos at Lui Kotale, Salonga National Park, Democratic Republic of Congo. Folia Primatol (Basel) 2008;79:103–110. doi: 10.1159/000110679. [DOI] [PubMed] [Google Scholar]
- 10.Fruth B, Hohmann G. How bonobos handle hunts and harvests: Why share food? In: Boesch C, Hohmann G, Marchant LF, editors. Behavioural Diversity in Chimpanzees and Bonobos. Cambridge, UK: Cambridge University Press; 2002. pp. 231–243. [Google Scholar]
- 11.Kingdon J. The Kingdon Field Guide to African Mammals. Princeton, NJ: Princeton University Press; 1997. [Google Scholar]
- 12.Ambrose SH. Isotopic analysis of palaeodiets: Methodological and interpretative considerations. In: Sandford MK, editor. Investigations of Ancient Human Tissue: Chemical Analyses in Anthropology. Langhorne, PA: Gordon and Breach; 1993. pp. 59–130. [Google Scholar]
- 13.Schoeninger MJ, DeNiro MJ. Nitrogen and carbon isotopic composition of bone collagen from marine and terrestrial animals. Geochim Cosmochim Acta. 1984;48:625–639. [Google Scholar]
- 14.Schoeninger MJ. Trophic level effects on 15N/14N and 13C/12C ratios in bone collagen and strontium levels in bone mineral. J Hum Evol. 1985;14:515–525. [Google Scholar]
- 15.Wolf N, Carleton SA, Martínez de Rio C. Ten years of experimental animal isotope ecology. Funct Ecol. 2009;23:17–26. [Google Scholar]
- 16.Codron D, Lee-Thorp JA, Sponheimer M, de Ruiter D, Codron J. What insights can baboon feeding ecology provide for early hominin niche differentiation? Int J Primatol. 2008;29:757–772. [Google Scholar]
- 17.Lee-Thorp JA, Sponheimer M, Passey BH, de Ruiter DJ, Cerling TE. Stable isotopes in fossil hominin tooth enamel suggest a fundamental dietary shift in the Pliocene. Philos Trans R Soc Lond B Biol Sci. 2010;365:3389–3396. doi: 10.1098/rstb.2010.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwarcz HP, Schoeninger MJ. Stable isotope analyses in human nutritional ecology. Yearb Phys Anthropol. 1991;34:283–321. [Google Scholar]
- 19.Tieszen LL. Natural variations in the carbon isotope values of plants: Implications for archaeology, ecology and paleoecology. J Archaeol Sci. 1991;18:227–248. [Google Scholar]
- 20.Ambrose SH, DeNiro MJ. The isotopic ecology of East African mammals. Oecologia. 1986;69:395–406. doi: 10.1007/BF00377062. [DOI] [PubMed] [Google Scholar]
- 21.Medina E, Minchin P. Stratification of δ13C values of leaves in Amazonian rain forests. Oecologia. 1980;45:377–378. doi: 10.1007/BF00540209. [DOI] [PubMed] [Google Scholar]
- 22.van der Merwe NJ, Medina E. The canopy effect, carbon isotope ratios and foodwebs in Amazonia. J Archaeol Sci. 1991;18:249–259. [Google Scholar]
- 23.Ambrose SH. Effects of diet, climate and physiology on nitrogen isotope abundances in terrestrial foodwebs. J Archaeol Sci. 1991;18:293–317. [Google Scholar]
- 24.Heaton THE. The 15N/14N ratios of plants in South Africa and Namibia: Relationship to climate and coastal/saline environments. Oecologia. 1987;74:236–246. doi: 10.1007/BF00379365. [DOI] [PubMed] [Google Scholar]
- 25.Cerling TE, Hart JA, Hart TB. Stable isotope ecology in the Ituri Forest. Oecologia. 2004;138:5–12. doi: 10.1007/s00442-003-1375-4. [DOI] [PubMed] [Google Scholar]
- 26.Sponheimer M, et al. Using carbon isotopes to track dietary change in modern, historical, and ancient primates. Am J Phys Anthropol. 2009;140:661–670. doi: 10.1002/ajpa.21111. [DOI] [PubMed] [Google Scholar]
- 27.Schoeninger MJ, Iwaniec UT, Glander KE. Stable isotope ratios indicate diet and habitat use in New World monkeys. Am J Phys Anthropol. 1997;103:69–83. doi: 10.1002/(SICI)1096-8644(199705)103:1<69::AID-AJPA5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 28.Schoeninger MJ, Moore J, Sept JM. Subsistence strategies of two “savanna” chimpanzee populations: The stable isotope evidence. Am J Primatol. 1999;49:297–314. doi: 10.1002/(SICI)1098-2345(199912)49:4<297::AID-AJP2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 29.Sponheimer M, et al. Do “savanna” chimpanzees consume C4 resources? J Hum Evol. 2006;51:128–133. doi: 10.1016/j.jhevol.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 30.O'Regan HJ, et al. Modern macaque dietary heterogeneity assessed using stable isotope analysis of hair and bone. J Hum Evol. 2008;55:617–626. doi: 10.1016/j.jhevol.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Carter ML. Sensitivity of stable isotopes (13C, 15N and 18O) in bone to dietary specialization and niche separation among sympatric primates in Kibale National Park, Uganda. PhD dissertation (University of Chicago, Chicago) 2001 [Google Scholar]
- 32.Smith CC, Morgan ME, Pilbeam D. Isotopic ecology and dietary profiles of Liberian chimpanzees. J Hum Evol. 2010;58:43–55. doi: 10.1016/j.jhevol.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Martinelli LA, et al. Nitrogen stable isotopic composition of leaves and soil: Tropical versus temperate forests. Biogeochemistry. 1999;46:45–65. [Google Scholar]
- 34.Cerling TE, Harris JM, Passey BH. Diets of East African bovidae based on stable isotope analysis. J Mammal. 2003;84:456–470. [Google Scholar]
- 35.Högberg P. 15N natural abundance as a possible marker of the ectomycorrhizal habit of trees in mixed African woodlands. New Phytol. 1990;115:483–486. doi: 10.1111/j.1469-8137.1990.tb00474.x. [DOI] [PubMed] [Google Scholar]
- 36.Högberg P. Soil nutrient availability, root symbiosis and tree species composition in tropical Africa: A review. J Trop Ecol. 1986;2:359–372. [Google Scholar]
- 37.Schoeninger MJ, Iwaniec UT, Nash LT. Ecological attributes recorded in stable isotope ratios of arboreal prosimian hair. Oecologia. 1998;113:222–230. doi: 10.1007/s004420050372. [DOI] [PubMed] [Google Scholar]
- 38.Ambrose SH. Preparation and characterization of bone and tooth collagen for isotopic analysis. J Archaeol Sci. 1990;17:431–451. [Google Scholar]
- 39.Crowley BE, et al. Stable carbon and nitrogen isotope enrichment in primate tissues. Oecologia. 2010;164:611–626. doi: 10.1007/s00442-010-1701-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Connell TC, Hedges REM, Healey MA, Simpson AHRW. Isotopic comparison of hair, nail and bone: Modern analyses. J Archaeol Sci. 2001;28:1247–1255. [Google Scholar]
- 41.Friedli H, Lotscher H, Oeschger H, Siegenthaler U, Stauffer B. Ice core record of the C-13/C-12 ratio of atmospheric Co2 in the past 2 centuries. Nature. 1986;324:237–238. [Google Scholar]
- 42.Hohmann G, Fowler A, Sommer V, Ortmann S. Frugivory and gregariousness of Salonga bonobos and Gashak chimpanzees: The influence of abundance and nutritional quality of fruit. In: Hohmann G, Robbins M, Boesch C, editors. Feeding Ecology in Apes and Other primates: Ecological, Physiological and Behavioural Aspects. Cambridge, UK: Cambridge University Press; 2006. pp. 123–160. [Google Scholar]
- 43.Hohmann G, Fruth B. Dynamics in social organization of bonobos (Pan paniscus) In: Boesch C, Hohmann G, Marchant LF, editors. Behavioural Diversity in Chimpanzees and Bonobos. Cambridge, UK: Cambridge University Press; 2002. pp. 138–150. [Google Scholar]
- 44.Surbeck M, Mundry R, Hohmann G. Mothers matter! Maternal support, dominance status and mating success in male bonobos (Pan paniscus) Proc Biol Soc. 2011;278:590–598. doi: 10.1098/rspb.2010.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitani JC, Watts DP. Why do chimpanzees hunt and share meat? Anim Behav. 2001;61:915–924. [Google Scholar]
- 46.Hohmann G, Fruth B. Lui Kotale: A new site for field research on bonobos in the Salonga National Park. Pan African News. 2003;10:25–27. [Google Scholar]
- 47.Fuller BT, et al. Nitrogen balance and delta15N: Why you're not what you eat during pregnancy. Rapid Commun Mass Spectrom. 2004;18:2889–2896. doi: 10.1002/rcm.1708. [DOI] [PubMed] [Google Scholar]
- 48.Nakamura K, Schoeller DA, Winkler FJ, Schmidt HL. Geographical variations in the carbon isotope composition of the diet and hair in contemporary man. Biomed Mass Spectrom. 1982;9:390–394. doi: 10.1002/bms.1200090906. [DOI] [PubMed] [Google Scholar]
- 49.Tobin DJ. The biogenesis and growth of hair. In: Tobin DJ, editor. Hair in Toxicology: An Important Bio-Monitor. Cambridge, UK: The Royal Society of Chemistry; 2005. pp. 3–33. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.